Particle Size-Dependent Component Separation Using Serially Arrayed Micro-Chambers
Abstract
1. Introduction
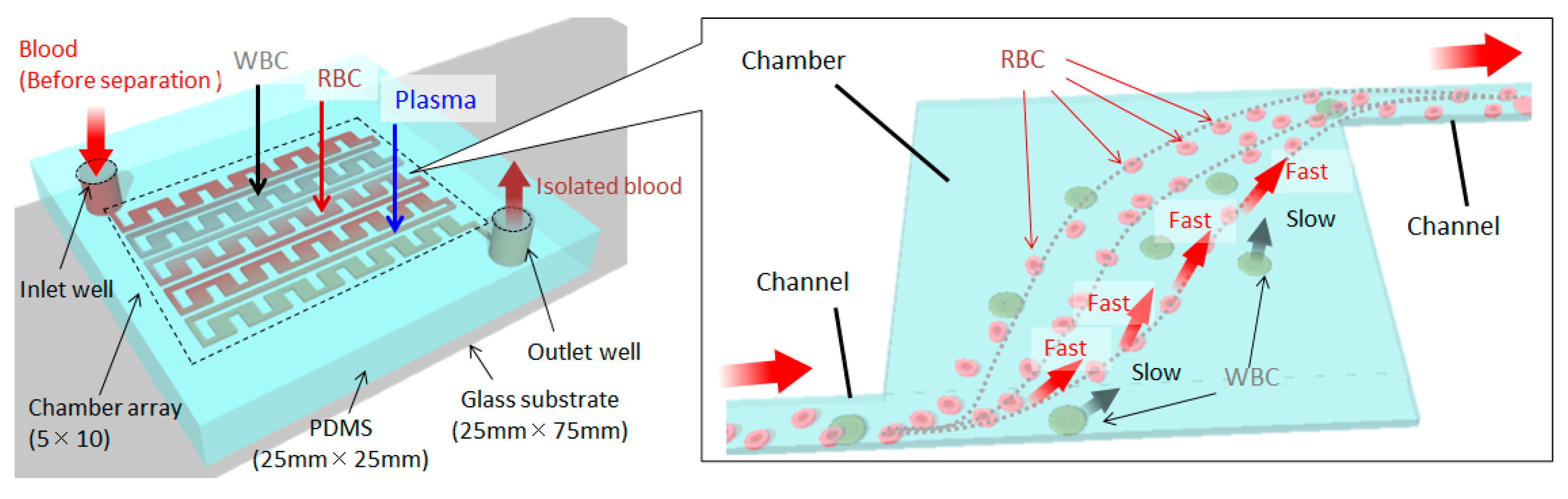
2. Materials and Methods
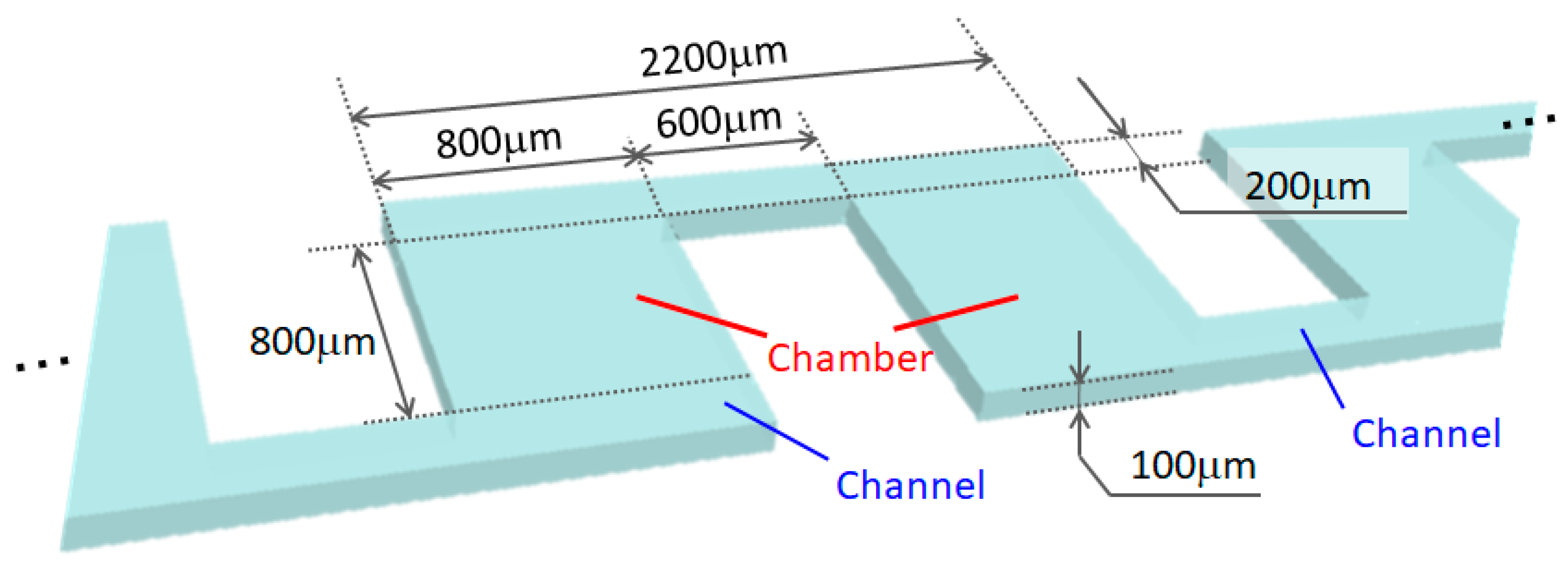
2.1. Design Guidelines
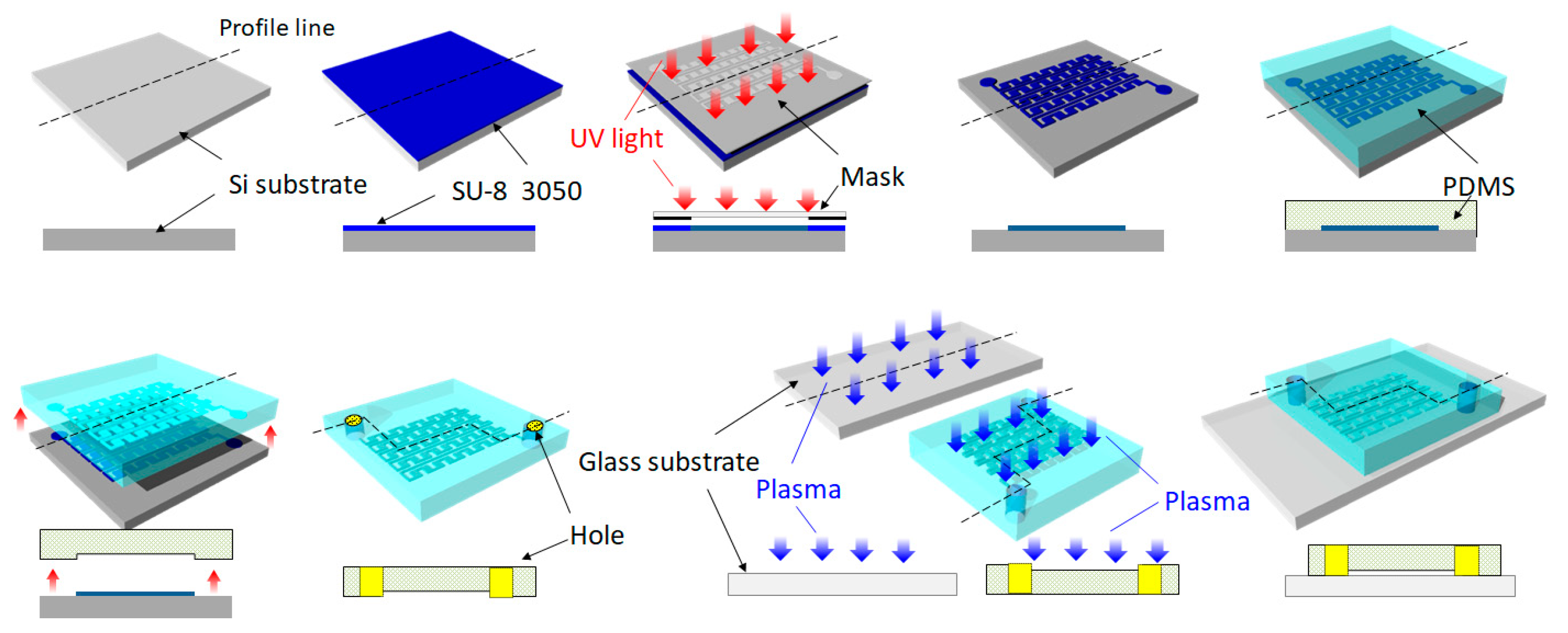
2.2. Fabrication Process
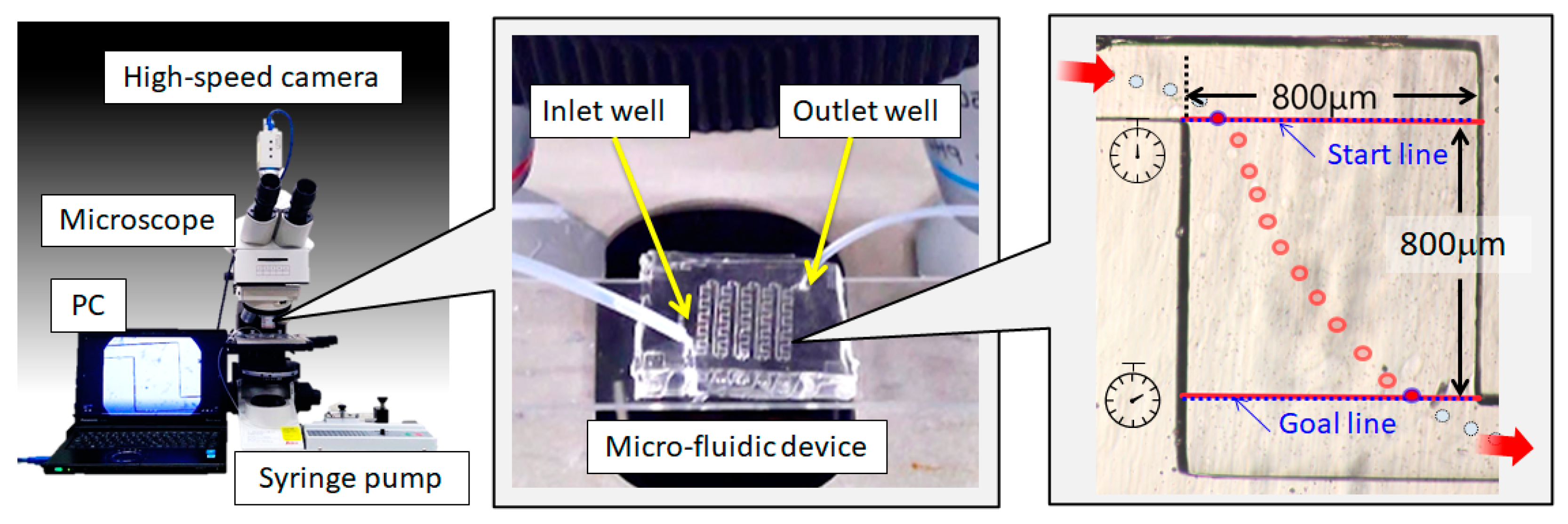
2.3. Microscopic Observation
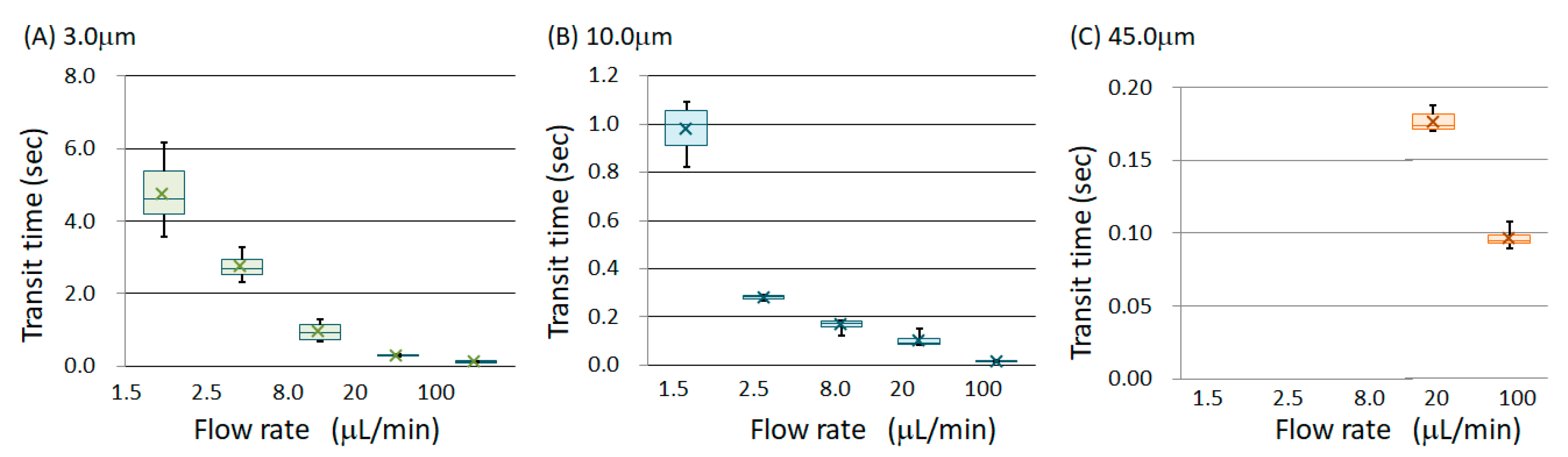
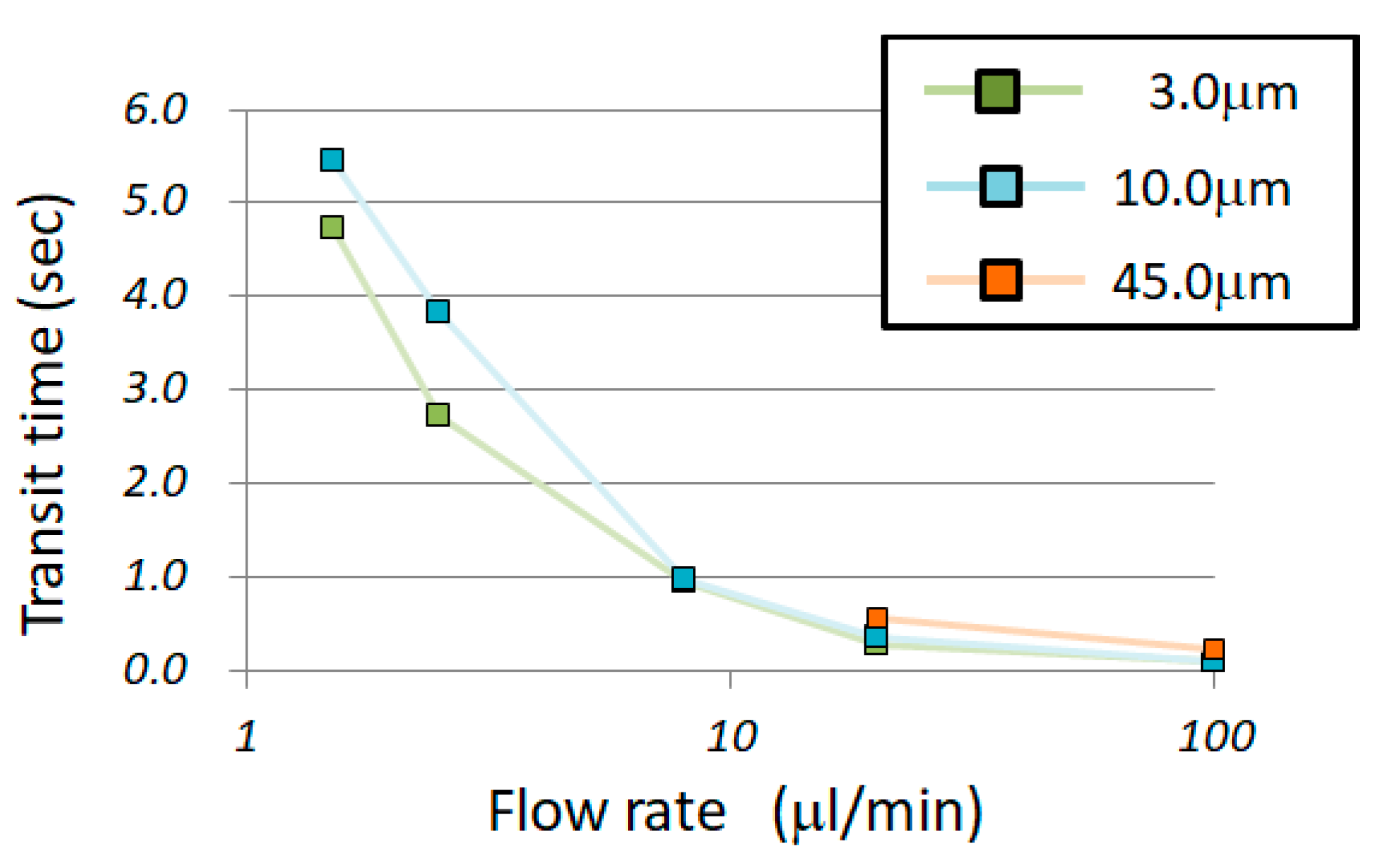
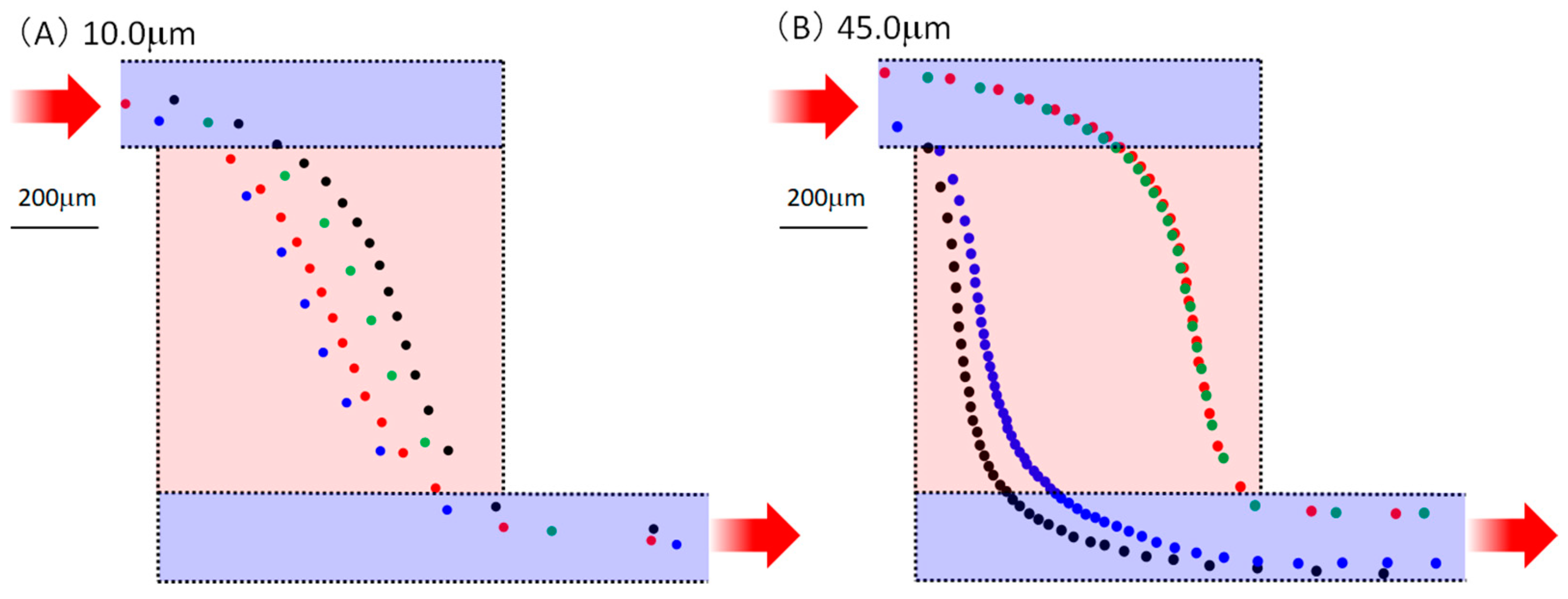
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chiu, R.W.; Poon, L.L.; Lau, T.K.; Leung, T.N.; Wong, E.M.; Lo, Y.D. Effects of blood-processing protocols on fetal and total DNA quantification in maternal plasma. Clin. Chem. 2001, 47, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Pei, B.; Jiang, M.; Wang, B.; Xu, D.; Chen, Y. Numerical analysis of forces exerted on particles in cyclone separators. Powder Technol. 2016, 294, 437–448. [Google Scholar] [CrossRef]
- Brouwers, J.J.H. Phase separation in centrifugal fields with emphasis on the rotational particle separator. Exp. Therm. Fluid. Sci. 2002, 26, 325–334. [Google Scholar] [CrossRef]
- Jiao, Y.; He, Y.; Jiao, F. Two-dimensional Simulation of Motion of Red Blood Cells with Deterministic Lateral Displacement Devices. Micromachines 2019, 10, 393. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Wadbro, E.; Liu, Z. Topology optimization of passive cell traps. Micromachines 2021, 12, 809. [Google Scholar] [CrossRef]
- Veerapen, J.P.; Lowry, B.J.; Couturier, M.F. Design methodology for the swirl separator. Aquac. Eng. 2005, 33, 21–45. [Google Scholar] [CrossRef]
- Joensson, H.N.; Uhlén, M.; Svahn, H.A. Droplet size based separation by deterministic lateral displacement—Separating droplets by cell-induced shrinking. Lab. A Chip 2011, 11, 1305–1310. [Google Scholar] [CrossRef]
- Chen, P.C.; Chen, C.C.; Young, K.C. Characterization of thermoplastic microfiltration chip for the separation of blood plasma from human blood. Biomicrofluidics 2016, 10, 54112. [Google Scholar] [CrossRef]
- Kang, T.G.; Yoon, Y.J.; Ji, H.; Lim, P.Y.; Chen, Y. A continuous flow micro filtration device for plasma/blood separation using submicron vertical pillar gap structures. J. Micromech. Microeng. 2014, 24, 87001. [Google Scholar] [CrossRef]
- Singh, A.K.; Ko, D.H.; Vishwakarma, N.K.; Jang, S.; Min, K.I.; Kim, D.P. Micro-total envelope system with silicon nanowire separator for safe carcinogenic chemistry. Nat. Commun. 2016, 7, 10741. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Lieberthal, T.J.; del Río Hernández, A.E. Exosomes as a platform for ‘liquid biopsy’ in pancreatic cancer. Converg. Sci. Phys. Oncol. 2017, 3, 13005. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Faley, S.; Zagnoni, M.; Wikswo, J.P.; Cooper, J.M. Microfluidic single-cell array cytometry for the analysis of tumor apoptosis. Anal. Chem. 2009, 81, 5517–5523. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.W.; Zhang, Q.; Naler, L.B.; Ma, S.; Lu, C. Recent advances in the use of microfluidic technologies for single cell analysis. Analyst 2018, 143, 60–80. [Google Scholar] [CrossRef] [PubMed]
- Totani, M.; Kojima, M.; Horade, M.; Mae, Y.; Ogura, T.; Kaneko, M.; Arai, T. Microfluidic device for applying a mechanical stimulus to a large number of cellular nuclei. In Proceedings of the IEEE 2017 International Symposium on Micro-NanoMechatronics and Human Science (MHS), Nagoya, Japan, 3–6 December 2017. [Google Scholar] [CrossRef]
- Sobecki, C.; Zhang, J.; Wang, C. Numerical study of paramagnetic elliptical microparticles in curved channels and uniform magnetic fields. Micromachines 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Prentice, H.; Oliveira, J.D.; Madziva, N.; Warkiani, M.E.; Hamel, J.F.P.; Han, J. Microfluidic cell retention device for perfusion of mammalian suspension culture. Sci. Rep. 2017, 7, 6703. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, J.; Ang, S.S. A low temperature co-fired ceramic mesofluidic separator. J. Phys. Conf. Ser. 2006, 34, 734. [Google Scholar] [CrossRef]
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef]
- Alnaimat, F.; Mathew, B.; Hilal-Alnaqbi, A. Modeling a Dielectrophoretic Microfluidic Device with Vertical Interdigitated Transducer Electrodes for Separation of Microparticles Based on Size. Micromachines 2020, 11, 563. [Google Scholar] [CrossRef]
- De Pastina, A.; Maillard, D.; Villanueva, L.G. Fabrication of suspended microchannel resonators with integrated piezoelectric transduction. Microelectron. Eng. 2018, 192, 83–87. [Google Scholar] [CrossRef]
- Kuhn, S.; Noël, T.; Gu, L.; Heider, P.L.; Jensen, K.F. A Teflon microreactor with integrated piezoelectric actuator to handle solid forming reactions. Lab. A Chip 2011, 11, 2488–2492. [Google Scholar] [CrossRef]
- Orbay, S.; Ozcelik, A.; Bachman, H.; Huang, T.J. Acoustic actuation of in situ fabricated artificial cilia. J. Micromech. Microeng. 2018, 28, 25012. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Ahn, Y. Influence of electrode groove geometry on the passive control of the depletion layer in microfluidic fuel cells. J. Micromech. Microeng. 2015, 25, 127001. [Google Scholar] [CrossRef]
- Dong, T.; Su, Q.; Yang, Z.; Zhang, Y.; Egeland, E.B.; Gu, D.D.; Calabrese, P.; Kapiris, M.J.; Karlsen, F.; Minh, N.T.; et al. A smart fully integrated micromachined separator with soft magnetic micro-pillar arrays for cell isolation. J. Micromech. Microeng. 2010, 20, 115021. [Google Scholar] [CrossRef]
- Destgeer, G.; Sung, H.J. Recent advances in microfluidic actuation and micro-object manipulation via surface acoustic waves. Lab. A Chip 2015, 15, 2722–2738. [Google Scholar] [CrossRef]
- Yousuff, C.M.; Ho, E.T.W.; Hussain, K.I.; Hamid, N.H.B. Microfluidic platform for cell isolation and manipulation based on cell properties. Micromachines 2017, 8, 15. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Zhu, R. Computer-Vision-Based Dielectrophoresis Mobility Tracking for Characterization of Single-Cell Biophysical Properties. Anal. Chem. 2022, 94, 14331–14339. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Fan, L.; Zhu, R.; Sun, D. Microfluidic single-cell manipulation and analysis: Methods and applications. Micromachines 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Horade, M.; Tsai, C.H.D.; Kaneko, M. On-chip cell incubator for simultaneous observation of culture with and without periodic hydrostatic pressure. Micromachines 2019, 10, 133. [Google Scholar] [CrossRef]
- Akai, T.; Ito, H.; Kaneko, M. Deep learning assisted analysis of multiple individual red blood cells in blood flow. In Proceedings of the 22nd International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS2018), Kaohsiung, Taiwan, 11–15 November 2018. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horade, M.; Okumura, R.; Yamawaki, T.; Yashima, M.; Murakami, S.; Saiki, T. Particle Size-Dependent Component Separation Using Serially Arrayed Micro-Chambers. Micromachines 2023, 14, 919. https://doi.org/10.3390/mi14050919
Horade M, Okumura R, Yamawaki T, Yashima M, Murakami S, Saiki T. Particle Size-Dependent Component Separation Using Serially Arrayed Micro-Chambers. Micromachines. 2023; 14(5):919. https://doi.org/10.3390/mi14050919
Chicago/Turabian StyleHorade, Mitsuhiro, Ryuusei Okumura, Tasuku Yamawaki, Masahito Yashima, Shuichi Murakami, and Tsunemasa Saiki. 2023. "Particle Size-Dependent Component Separation Using Serially Arrayed Micro-Chambers" Micromachines 14, no. 5: 919. https://doi.org/10.3390/mi14050919
APA StyleHorade, M., Okumura, R., Yamawaki, T., Yashima, M., Murakami, S., & Saiki, T. (2023). Particle Size-Dependent Component Separation Using Serially Arrayed Micro-Chambers. Micromachines, 14(5), 919. https://doi.org/10.3390/mi14050919




