Abstract
Rechargeable metal-ion batteries (RMIBs) are prospective highly effective and low-cost devices for energy storage. Prussian blue analogues (PBAs) have become a subject of significant interest for commercial applications owing to their exceptional specific capacity and broad operational potential window as cathode materials for rechargeable metal-ion batteries. However, the limiting factors for its widespread use are its poor electrical conductivity and stability. The present study describes the direct and simple synthesis of 2D nanosheets of MnFCN (Mn3[Fe(CN)6]2·nH2O) on nickel foam (NF) via a successive ionic layer deposition (SILD) method, which provided more ion diffusion and electrochemical conductivity. MnFCN/NF exhibited exceptional cathode performance for RMIBs, delivering a high specific capacity of 1032 F/g at 1 A/g in an aqueous 1M NaOH electrolyte. Additionally, the specific capacitance reached the remarkable levels of 327.5 F/g at 1 A/g and 230 F/g at 0.1 A/g in 1M Na2SO4 and 1M ZnSO4 aqueous solutions, respectively.
1. Introduction
Over the last few years, significant attention has been directed towards developing diverse categories of metal-ion batteries. Sodium- and potassium-ion batteries have gained significant attention in light of their extensive utilization and cost-effective nature as well as their superior safety during operation when contrasted with lithium-ion batteries. In addition, recently, metal-ion batteries that use two-charge cations (Zn2+, Mg2+, Ca2+, etc.) have attracted much attention since they have a higher volume energy density due to the amount of charge that is transferred [1,2,3]. Of particular interest are zinc-ion batteries with aqueous electrolytes since they can be used to create compact, safe, and inexpensive autonomous power sources with large specific capacities.
The rechargeable zinc-ion batteries demonstrate the potential to satisfy the criteria of contemporary electric energy storage systems owing to abundant zinc-based resources, economic feasibility, and their substantial theoretical specific capacity (up to 820 mAh/g [4]). Therefore, these batteries have emerged as a promising substitute for lithium-ion batteries. Recently, certain achievements have been observed that improved the electrochemical parameters of electrode materials for such devices, but researches have not identified suitable cathode materials that can withstand multiple cycles of intercalation/deintercalation of zinc ions [5]. The stability of a zinc-ion battery’s structure depends greatly on the cathode material’s ability to chemically bond with Zn2+ ions quickly and efficiently while maintaining a strong and durable structure. Therefore, features such as a crystal framework that has appropriate crystallographic sites for the placement of Zn2+, pathways for ion diffusion that require minimal energy, and efficient electronic conductivity for the transport of charges are favorable for the cathode. Often, compounds with a layered tunnel structure and redox components meet these requirements. Consideration should also be given to the porosity of the cathode, which enables the infiltration of the electrolytic solution, thereby intensifying the number of active sites and the maximum rate of charge transfer [6]. From this point of view, Prussian blue analogues and oxides of manganese, vanadium, and cobalt as well as some organic compounds can be used as cathode materials. Among these materials, Prussian blue analogues (hexacyanoferrates) are the most promising for further commercialization due to their wide operating potential window and high specific capacity. Additionally, their synthesis can be achieved by employing comparatively cost-effective and readily available precursors [7,8,9]. It was previously discovered that manganese (II) hexacyanoferrates (III) Mn3[Fe(CN)6]2 with the elimination of interstitial water gave an increased reversible capacity and enhanced cycle stability [10]. However, the practical values of the specific capacitance are often far from the theoretical values due to the low electronic conductivity of these cathode materials. The use of two-dimensional nanocrystals with a “nanosheets”-like morphology, which have a set of distinctive physical and chemical characteristics, will solve this problem. The planar two-dimensional morphology of these materials affords a suitable quantity of effective adsorption sites. One salient attribute of two-dimensional (2D) materials pertains to their exceedingly slender dimensions, typically spanning a few nanometers. This property enables charge carriers to traverse minuscule distances from the material volume to its surface, leading to a substantial enhancement of electronic conductivity [11]. As noted in the above-mentioned works, a significant challenge in the development of cathode materials for hybrid batteries lies in the intricacy of attaining ultrathin nanocrystals exhibiting the graphene-like morphology of “nanosheets” through existing synthesis techniques. Such a material morphology holds potential for mitigating electroactive material degradation and enhancing electronic conductivity.
To solve the above-mentioned problems in the synthesis of electrode materials with 2D morphologies, the most promising approach, from our point of view, is the use of layer-by-layer synthesis methods, also known as successful ionic layer adsorption and reaction (SILAR) or successive ionic layer deposition (SILD) [12]. The SILD method allows the regulation of a wide range of synthesis conditions (the number of processing cycles, the processing sequence, the processing time in reagent solutions, the concentration, the anionic composition and pH of reagent solutions, the heating of the obtained samples, etc.) to influence the morphology, composition, and structure of the synthesized compounds as well as to obtain various 1D, 2D, and 3D nanomaterials, which is important for the creation of highly effective electroactive materials [13,14,15,16]. A considerable body of research exists on the subject of the layer-by-layer synthesis of transition metal oxides for application in electrode materials, including those intended for utilization in zinc-ion batteries [17,18,19,20,21].
In this work, we describe a simple and direct technique for producing 2D Mn3[Fe(CN)6]2·nH2O nanosheets using successive ionic layer deposition (SILD) from an aqueous solution of MnSO4 and K3[Fe(CN)6] and an investigation of its electrochemical performances in different types of electrolytes. The obtained Mn3[Fe(CN)6]2·nH2O nanosheets are examined as cathode materials for metal-ion batteries and supercapacitors as a proof-of-concept application.
2. Materials and Methods
2.1. Materials
Highly pure analytical-grade reagents were used for synthesis: manganese sulfate pentahydrate (MnSO4·5H2O), potassium ferrocyanide (K3[Fe(CN)6]), hydrochloric acid (HCl), and potassium hydroxide (KOH). All reagents were produced by Vecton (Russia). Nickel foam (110 PPI; size: 20 × 30 mm) was used as a carrier.
2.2. Synthesis of Mn3[Fe(CN)6]2 Nanosheets
The nickel foam underwent pre-treatment with acetone, 3 M hydrochloric acid, and deionized water. For the preparation of a coating of Mn3[Fe(CN)6]2·nH2O (denoted further as MnFCN) on the Ni foam using the SILD method, two aqueous precursor solutions were used. The optimal synthesis conditions (the concentration of the precursor solutions (0.01 M), the processing time in the precursor solutions (30 s), and the number of treatment cycles (50 cycles)) were determined in accordance with the methodology [12]. Under these conditions, the formation of a uniform coating of two-dimensional structures of Mn3[Fe(CN)6]2·nH2O on the surface of the nickel foam occurred.
The cationic precursor was a 0.01 M MnSO4 solution, and a 0.01 M K3[Fe(CN)6] solution was used as the anionic precursor. The SILD cycle consisted of four stages: the nickel foam was immersed in the cationic precursor for 30 s (1); the excess reagent was removed by rinsing the nickel foam with deionized water for 30 s (2); after that, the nickel foam was immersed in the anionic precursor for 60 s (3); and the excess reagent was removed again by rinsing the nickel foam with deionized water for 30 s (4). This SILD cycle was repeated 50 times. Reactions between adsorbed Mn2+ cations and adsorbed [Fe(CN)6]3− anions led to the formation of a MnFCN thin film. After SILD synthesis, the nickel foam was air-dried at an ambient temperature.
2.3. Material Characterization
The MnFCN thin film was characterized using scanning electron microscopy (SEM) on a Tescan Vega 3 SBH microscope (Tescan, Brno, Czech Republic) to reveal its surface morphology, using an energy-dispersive X-ray (EDX) spectrometer attached to an Oxford INCA 350 (Oxford Instruments, Abingdon, UK) to detect the elements and the surface distribution of Mn and Fe, and using X-ray diffraction (XRD) with Cu Kα radiation (λ = 0.1542 nm) on a Rigaku SmartLab 3 Powder X-ray diffractometer (Rigaku, Tokyo, Japan) to display the crystalline phases. The qualitative and quantitative analysis of the samples was characterized via X-ray photoelectron spectroscopy (XPS) using an ESCALAB 250Xi electron spectrometer (ThermoFisher Scientific, Waltham, MA, USA).
2.4. Electrochemical Characterization
The electrochemical activity of the prepared MnFCN was tested in a three-electrode system containing MnFCN, which was deposited on the Ni foam (with a mass loading of 1.2 mg/cm2) and served as the working electrode; Ag/AgCl (3 M KCl) or Hg/HgO (1 M NaOH), which were used as reference electrodes; and a graphite rod, which was used as the counter electrode. Cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charging/discharging (GCD) measurements were performed using an Elins P-45X electrochemical workstation with an FRA24M module of electrochemical impedance (Chernogolovka, Russia) in different electrolytes (1 M NaOH, 1 M Na2SO4, and 1 M ZnSO4 aqueous solutions). CV measurements were obtained in the potential windows from 0.0 V to 1.2 V (vs. Ag/AgCl) or from 0.0 V to 0.5 V (vs. Hg/HgO) at different scan rates (2–20 mV/s), and GCD measurements were obtained at various current densities (0.1–5 A/g). EIS measurements were obtained at the open-circuit potential (0.224 V vs. Ag/AgCl) within a frequency range from 50 kHz to 0.1 Hz and a sinusoidal wave with a 5 mV amplitude. The specific capacitance (C, F/g) of the prepared MnFCN was calculated from the GCD measurements using Equation (1):
where dV is the potential window (V), m is the mass of the MnFCN deposited on the Ni foam (g), I is the applied current (A), and dt is the discharge time (s) [22]. The mass of the MnFCN (2.8 mg) electroactive materials was measured using a JOANLAB FA1204 microbalance (China).
3. Results and Discussion
Figure 1a–c show the surface of a nickel foam carrier at different magnifications. Figure 1d–f show MnFCN nanostructures grown on nickel foam. As can be seen, individual globules (2–6 µm) are formed by a set of 2D nanosheets (10–20 nm) (Figure 1d). The morphology of MnFCN in the form of 2D nanosheets has the potential to enhance the properties of the electrode surface, facilitate charge transport, and improve the consistency of the electrochemical performance of the electrode specimen. The MnFCN’s nanostructure, consisting of multiple layers, facilitates more efficient electrochemical reactions and promotes a greater active electrode surface area, thereby enhancing the overall performance of the material. The energy-dispersive X-ray spectroscopy (EDX) elemental mapping results are shown in the inset of Figure 1f. The elemental analysis results indicate the existence of the elements Mn, Fe, C, and N. A homogenous distribution of Mn and Fe atoms is observable over the entirety of the surface of the nickel foam (Figure 1f inset).
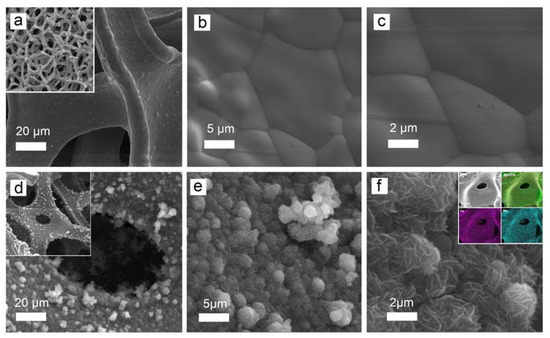
Figure 1.
SEM images of nickel foam carrier (a–c) and MnFCN coating (d–f) with different magnifications (EDX elemental mapping of MnFCN coating is shown in inset of (f)).
The crystalline phase structure of MnFCN was analyzed using X-ray diffraction (XRD), as depicted in Figure 2. The XRD pattern of MnFCN contains characteristic peaks at 17.12°, 24.43°, 35.02°, 39.40°, 43.56°, 50.52°, 53.91°, 57.07°, 66.03°, and 68.92° associated with planes (002), (022), (004), (024), (224), (044), (244), (026), (046), and (246), respectively. The observed characteristic peaks are in full agreement with the structure of Mn3[Fe(CN)6]2·nH2O (ICSD card #24-00929 [23]).
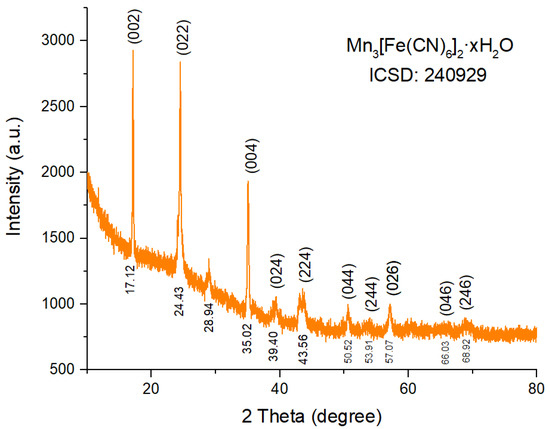
Figure 2.
XRD pattern of MnFCN.
X-ray photoelectron spectroscopy (XPS) was conducted to analyze the chemical compounds and their corresponding oxidation states. The Mn 2p XPS spectrum of MnFCN is shown in Figure 3a. The Mn 2p spectrum showed two peaks at 642.0 and 653.8 eV, which corresponded to the Mn 2p2/3 and Mn 2p1/2 orbitals and could be fitted to the peaks ascribed to the Mn2+ state [24]. Figure 3b shows the XPS spectrum of Fe 2p. The Fe 2p peaks of MnFCN were observed at 712.4 and 726.2 eV and were attributed to Fe 2p3/2 and Fe 2p1/2. This revealed that Fe exists in the Fe3+ state in MnFCN [24]. These results were in good agreement with the EDX data. Therefore, the molecular formula of the final compound was established as Mn3[Fe(CN)6]2·nH2O.
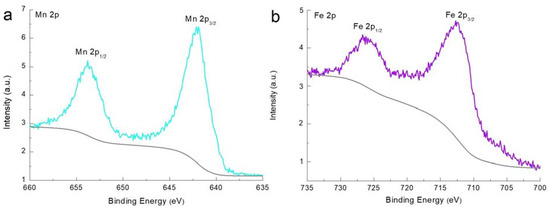
Figure 3.
XPS spectra of (a) Mn 2p and (b) Fe 2p of MnFCN.
The electrochemical characteristics of an NF electrode that comprised nanolayers of Mn3[Fe(CN)6]2·nH2O (MnFCN/NF) were investigated via various methods such as CVA, GCD, and EIS using different types of electrolytes.
Electrochemical measurements of MnFCN/NF as a cathode for a pseudocapacitor were obtained using 1 M NaOH. Figure 4a shows CVA curves of MnFCN/NF in the range from 0.0 V to 0.5 V vs. Hg/HgO. On the CVA curves, one pair of wide redox peaks, which possibly originated from the fast redox reactions of Mn2+ → Mn4+ and Mn4+ → Mn2+ on the electrode/electrolyte interface, suggests the capacitive behavior of this material. Figure 4d depicts the galvanostatic charge/discharge profiles of the MnFCN/NF electrode at varying current densities (1, 2, and 5 A/g). The MnFCN/NF electrode’s specific capacitance was determined using Equation (1) and was found to be 1032 F/g at 1 A/g in an alkaline medium (1M NaOH).
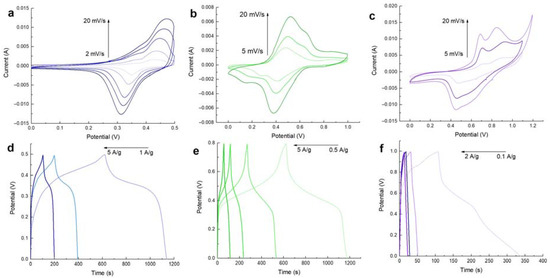
Figure 4.
CVA and CD curves of MnFCN/NF in (a;d) 1M NaOH, (b;e) 1M Na2SO4, and (c;f) 1M ZnSO4 electrolytes, respectively.
The cyclic voltammetry curves (CVA) of MnFCN/NF electrodes, which were obtained at varying scan rates within a 1 M Na2SO4 electrolyte, are displayed in Figure 4b. The obtained results indicate the presence of two sets of clearly observable redox peaks. The initial set of redox peaks noted at 0.55 V and 0.4 V vs. Ag/AgCl are indicative of redox manganese reactions involving Mn2+ → Mn3+ and Mn3+ → Mn2+, respectively. The second set of redox peaks, occurring at potential values of 0.72 V and 0.23 V vs. Ag/AgCl, respectively, were identified to correspond to the processes of oxidation (Fe2+ → Fe3+) and reduction (Fe3+ → Fe2+). The suggestion is that the capacitance of MnFCN electrodes is a result of the presence of both Mn and Fe ions. The GCD curve’s configuration confirms the course of the redox reactions on the surfaces of the fabricated electrodes. According to the results obtained through the GCD analysis, the specific capacitance was determined to be 327.5 F/g at 1 A/g (Figure 4e).
Electrochemical measurements of MnFCN/NF as a cathode for a hybrid zinc-ion supercapacitor were obtained using a 1 M ZnSO4 solution as an electrolyte. In Figure 4c, the CVA curves show two pairs of peaks in the range from 0.0 V to 1.2 V vs. Ag/AgCl. The CVA curves exhibited negligible deformation, even when examined at multiple scan rates, which suggests that MnFCN/NF displayed exceptional reaction kinetics when undergoing rapid charging and discharging. Figure 4f shows the GCD curves of a MnFCN/NF hybrid zinc-ion supercapacitor at different currents. The analysis of the discharge curves demonstrates that at a current density of 0.1 A/g, the specific capacity values amounted to 230 F/g.
Electrochemical impedance spectroscopy (EIS) was employed for the purpose of investigating the charge transfer behavior of the MnFCN electrode material. Figure 5 shows the EIS spectra of the MnFCN/NF electrode in 1 M NaOH (a), 1 M Na2SO4 (b), and 1 M ZnSO4 (c) aqueous solutions. The findings indicate that the decreased semicircle size, reduced intercept, and greater vertical line towards the low-frequency side of the samples imply a more prominent mass transfer limit and diminished interfacial charge resistance. These observations suggest that the sample exhibited enhanced conductivity and mass transport characteristics in all electrolyte types.
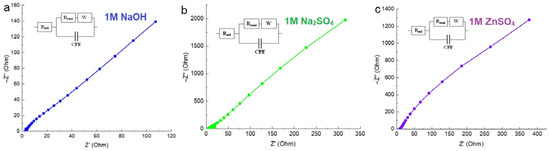
Figure 5.
EIS spectra of MnFCN/NF in (a) 1M NaOH, (b) 1M Na2SO4, and (c) 1M ZnSO4 electrolytes.
A comparison of the specific capacities of electrodes based on PBAs in different electrolytes synthesized using different methods is shown in Table 1. The results obtained in this study demonstrate the proximity and, in certain instances, superiority of the presented values in comparison to the characteristics of samples produced through alternative synthetic methods. The superior performance exhibited by the MnFCN cathode may be attributed to its layered structure, which greatly facilitated the adsorption/desorption and diffusion of electrolyte ions when subjected to rapid charging–discharging cycles. Furthermore, the agglomerates with spherical morphologies, which were acquired through the incorporation of two-dimensional nanosheets, offered a greater number of active sites for the purpose of electrochemical reactions. Consequently, this enhanced the specific capacitance value.

Table 1.
Comparative table of electrochemical characteristics of electrodes based on PBAs.
4. Conclusions
The present investigation represents a novel achievement, as it reports the successful synthesis of a Mn3[Fe(CN)6]2·nH2O thin film composed of a set of two-dimensional nanosheets utilizing the SILD technique. A notable improvement in the specific capacitance was observed, reaching 1032 F/g when operating at 1 A/g with an aqueous alkaline 1M NaOH electrolyte. Moreover, the discharge capacitance values of 327.5 F/g at 1 A/g and 230 F/g at 0.1 A/g were obtained in 1M Na2SO4 and 1M ZnSO4 aqueous solutions, respectively. These results show that Mn3[Fe(CN)6]2·nH2O is a prospective candidate for practical applications as a supercapacitor in both alkaline and neutral media and, in particular, as a cathode material for a hybrid Zn-ion supercapacitor.
Author Contributions
Conceptualization and methodology, A.A.L. and M.V.K.; investigation, M.I.T. and M.V.K.; writing—original draft preparation, A.A.L. and M.V.K.; writing—review and editing, A.A.L.; visualization, M.I.T.; supervision and project administration, V.I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Russian Science Foundation (RSF) (grant number 22-23-20138).
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Centers for Physical Methods of Surface Investigation and the Centers for Nanotechnology of Saint Petersburg State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [PubMed]
- Wang, M.; Zhang, F.; Lee, C.-S.; Tang, Y. Low-Cost Metallic Anode Materials for High Performance Rechargeable Batteries. Adv. Energy Mater. 2017, 7, 1700536. [Google Scholar]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar]
- Jin, J.; Geng, X.; Chen, Q.; Ren, T.L. A Better Zn-Ion Storage Device: Recent Progress for Zn-Ion Hybrid Supercapacitors. Nano-Micro Lett. 2022, 14, 64. [Google Scholar]
- Liu, P.; Lv, R.; He, Y.; Na, B.; Wang, B.; Liu, H. An integrated, flexible aqueous Zn-ion battery with high energy and power densities. J. Power Sources 2019, 410, 137–142. [Google Scholar]
- Wu, X.; Ru, Y.; Bai, Y.; Zhang, G.; Shi, Y.; Pang, H. PBA composites and their derivatives in energy and environmental applications. Coord. Chem. Rev. 2022, 451, 214260. [Google Scholar]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian Blue Analogs as Battery Materials. Joule 2018, 2, 1950–1960. [Google Scholar]
- Zeng, Y.X.; Lu, X.F.; Zhang, S.L.; Luan, D.Y.; Li, S.; Lou, X.W. Construction of Co-Mn Prussian Blue Analog Hollow Spheres for Efficient Aqueous Zn-ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 22189–22194. [Google Scholar]
- Fan, L.; Guo, X.; Hang, X.; Pang, H. Synthesis of truncated octahedral zinc-doped manganese hexacyanoferrates and lowtemperature calcination activation for lithium-ion battery. J. Colloid Interface Sci. 2022, 607, 1898–1907. [Google Scholar]
- Lobinsky, A.; Popkov, V. Ultrathin 2D nanosheets of transition metal (hydro)oxides as prospective materials for energy storage devices: A short review. Electrochem. Mater. Technol. 2022, 1, 20221008. [Google Scholar] [CrossRef]
- Tolstoy, V.P. Successive ionic layer deposition. An application in nanotechnology. Russ. Chem. Rev. 2006, 75, 161. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.; Colusso, E.; Guglielmi, M.; Martucci, A.; Della Gaspera, E. SILAR Deposition of Metal Oxide Nanostructured Films. Small 2021, 17, 2101666. [Google Scholar] [CrossRef] [PubMed]
- Soonmin, H. Recent advances in the growth and characterizations of SILAR-deposited thin films. Appl. Sci. 2022, 12, 8184. [Google Scholar] [CrossRef]
- Ghos, B.C.; Farhad, S.F.U.; Patwary, M.A.M.; Majumder, S.; Hossain, M.A.; Tanvir, N.I.; Rahman, M.A.; Tanaka, T.; Guo, Q. Influence of the substrate, process conditions, and postannealing temperature on the properties of ZnO thin films grown by the successive ionic layer adsorption and reaction method. ACS Omega 2021, 6, 2665–2674. [Google Scholar] [CrossRef]
- Farhad, S.F.U.; Majumder, S.; Hossain, M.A.; Tanvir, N.I.; Akter, R.; Patwary, M.A. Effect of solution pH and post-annealing temperatures on the optical bandgap of the copper oxide thin films grown by modified SILAR method. MRS Adv. 2019, 4, 937–944. [Google Scholar] [CrossRef]
- Lobinsky, A.A.; Popkov, V.I. Direct SILD synthesis of efficient electroactive materials based on ultrathin nanosheets of amorphous CoCr-LDH. Mater. Lett. 2022, 322, 132472. [Google Scholar] [CrossRef]
- Kodintsev, I.A.; Martinson, K.D.; Lobinsky, A.A.; Popkov, V.I. Successive ionic layer deposition of co-doped Cu(OH)2 nanorods as electrode material for electrocatalytic reforming of ethanol. Nanosyst. Phys. Chem. Math. 2019, 10, 573–578. [Google Scholar] [CrossRef]
- Lobinsky, A.A.; Kaneva, M.V. Synthesis Ni-doped CuO nanorods via successive ionic layer deposition method and their capacitive performance. Nanosyst. Phys. Chem. Math. 2020, 11, 608–614. [Google Scholar] [CrossRef]
- Lobinsky, A.A.; Kodintsev, I.A.; Tenevich, M.I.; Popkov, V.I. A novel oxidation–reduction route for the morphology-controlled synthesis of manganese oxide Nanocoating as highly Effective Material for Pseudocapacitors. Coatings 2023, 13, 361. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Raja, M.; Bahadur, S.A.; Manikandan, A. Preparation and characterization studies of nanostructured CdO thin films by SILAR method for photocatalytic applications. J. Inorg. Organomet. Polym. Mater. 2017, 27, 692–699. [Google Scholar] [CrossRef]
- Eswaran, M.; Swamiappan, S.; Chokkiah, B.; Dhanusuraman, R.; Bharathkumar, S.; Ponnusamy, V.K. A green and economical approach to derive nanostructured hydroxyapatite from Garra mullya fish scale waste for biocompatible energy storage applications. Mater. Lett. 2021, 302, 130341. [Google Scholar] [CrossRef]
- Deng, W.; Li, Z.; Ye, Y.; Zhou, Z.; Li, Y.; Zhang, M.; Yuan, X.; Hu, J.; Zhao, W.; Huang, Z.; et al. Zn2+ Induced Phase Transformation of K2MnFe(CN)6 Boosts Highly Stable Zinc-Ion Storage. Adv. Energy Mater. 2021, 11, 2003639. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first-row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Yang, T.; Gao, J.; Zhou, L.; He, Q.; Chen, S.; He, P.; Zhang, H. Tailored manganese hexacyanoferrate/graphene oxide nanocomposites: One-pot facile synthesis and favorable capacitance behavior for supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 2720–2728. [Google Scholar] [CrossRef]
- Wang, J.-G.; Ren, L.; Hou, Z.; Shao, M. Flexible reduced graphene oxide/prussian blue films for hybrid supercapacitors. Chem. Eng. J. 2020, 397, 125521. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Wang, H.; Zhang, Z.; Yuan, R.; Lu, J.; Fu, Q. Self-Templating Synthesis of Cobalt Hexacyanoferrate Hollow Structures with Superior Performance for Na-Ion Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 29496–29504. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Xu, X.; Liu, Y.; Song, R.; Lu, G.; Li, Y. Cobalt Hexacyanoferrate Nanoparticles as a High-Rate and Ultra-Stable Supercapacitor Electrode Material. ACS Appl. Mater. Interfaces 2014, 6, 11007–11012. [Google Scholar] [CrossRef]
- Sun, X.; Li, S.; Liu, R.; Sun, X.; Liu, X.; Li, A.; Li, W. A three-dimensional heterogeneous ZnCo-PBA@α-Co(OH)2 nanostructure for high-performance supercapacitors. J. Nanoparticle Res. 2020, 22, 1–12. [Google Scholar] [CrossRef]
- Ren, J.; Meng, Q.; Xu, Z.; Zhang, X.; Chen, J. CoS2 hollow nanocubes derived from Co-Co Prussian blue analogue: High-performance electrode materials for supercapacitors. J. Electroanal. Chem. 2019, 836, 30–37. [Google Scholar] [CrossRef]
- Xu, P.; Wang, G.; Wang, H.; Li, Y.; Miao, C.; Qu, J.; Zhang, X. K2.25Ni0.55Co0.37Fe(CN)6 nanoparticle connected by cross-linked carbon nanotubes conductive skeletons for high-performance energy storage. Chem. Eng. J. 2017, 328, 834–843. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).