Stopped Flow of Glycerol Induces the Enhancement of Adsorption and Aggregation of HRP on Mica
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Protein
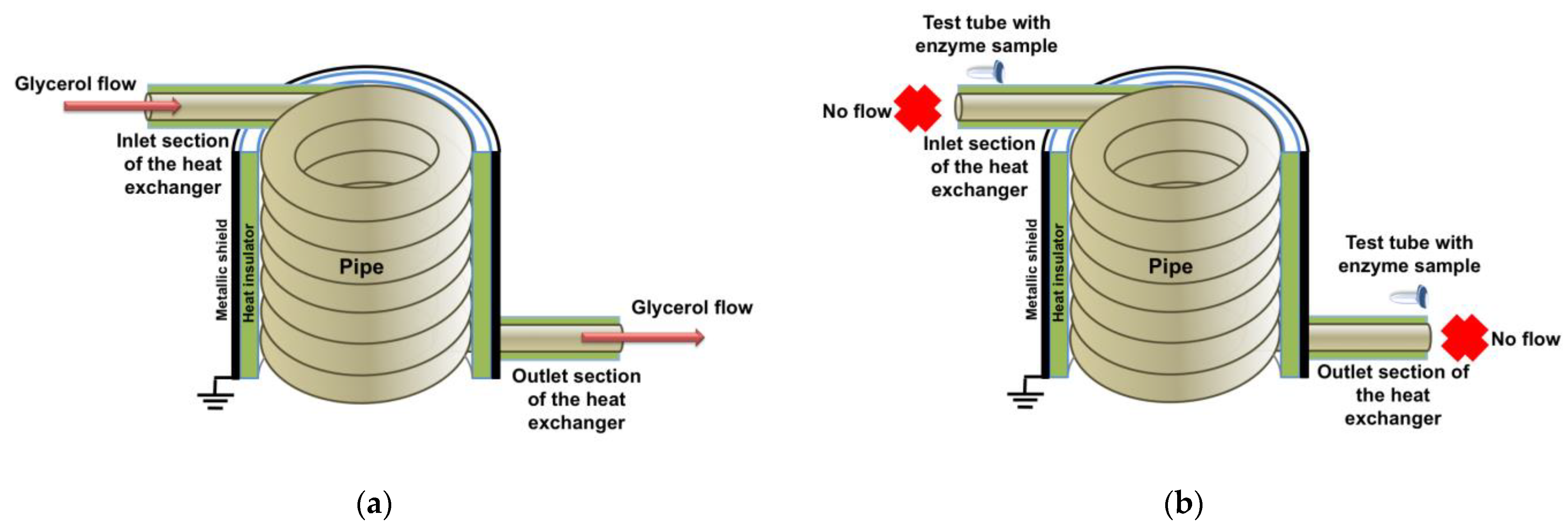
2.2. Experimental Setup
2.3. AFM Measurements
2.4. Spectrophotometric Measurements
3. Results
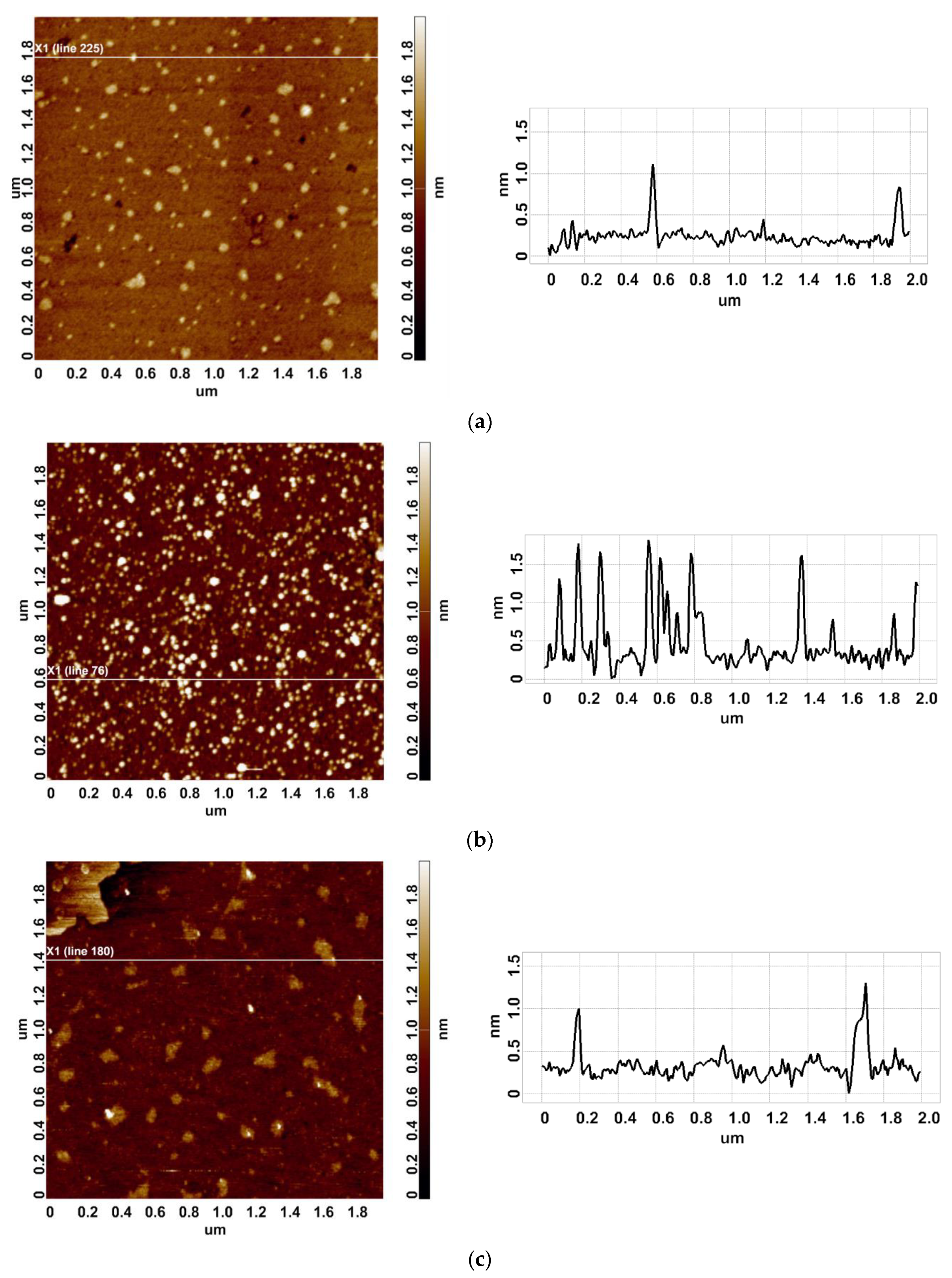
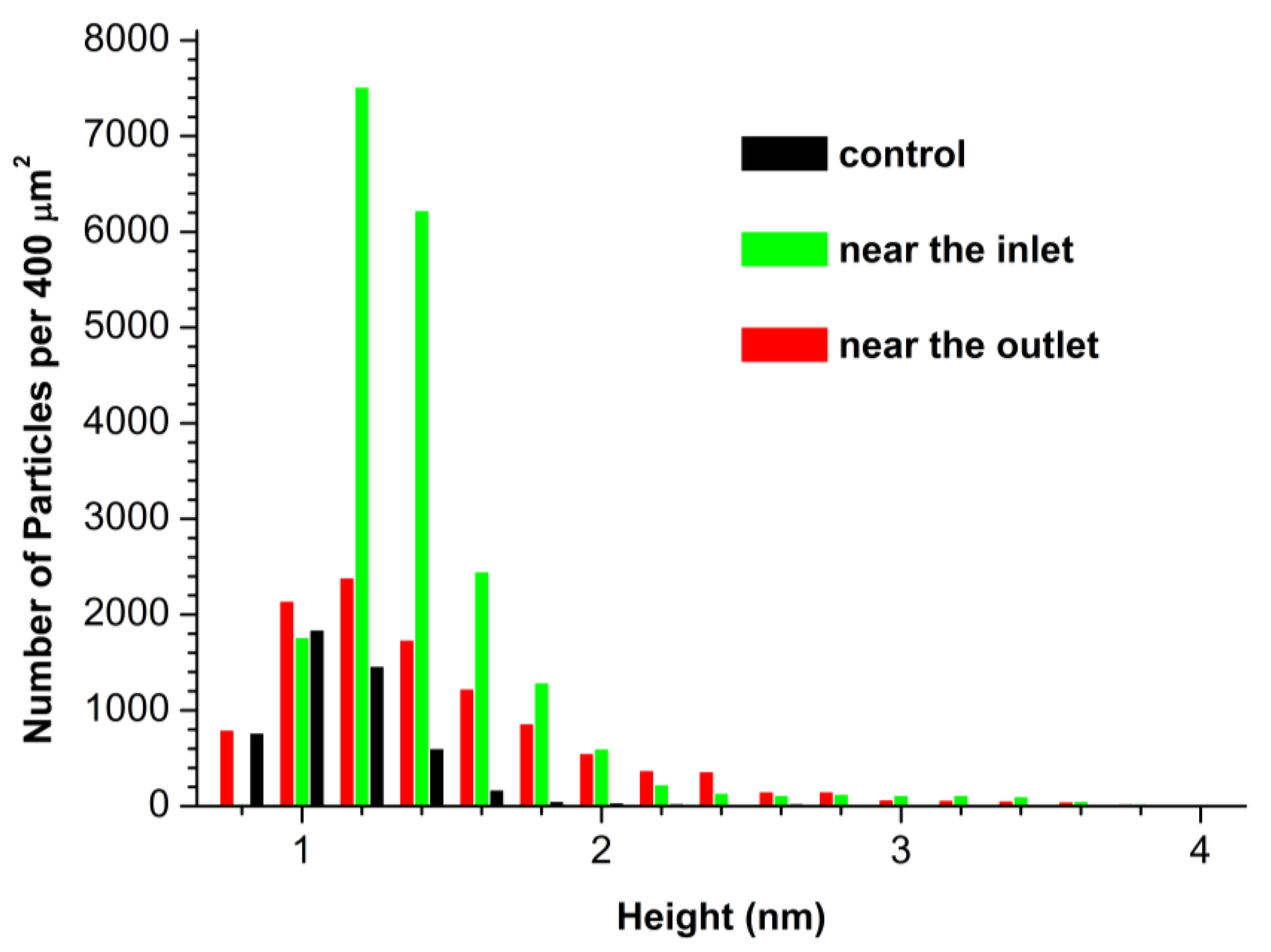
3.1. Atomic Force Microscopy
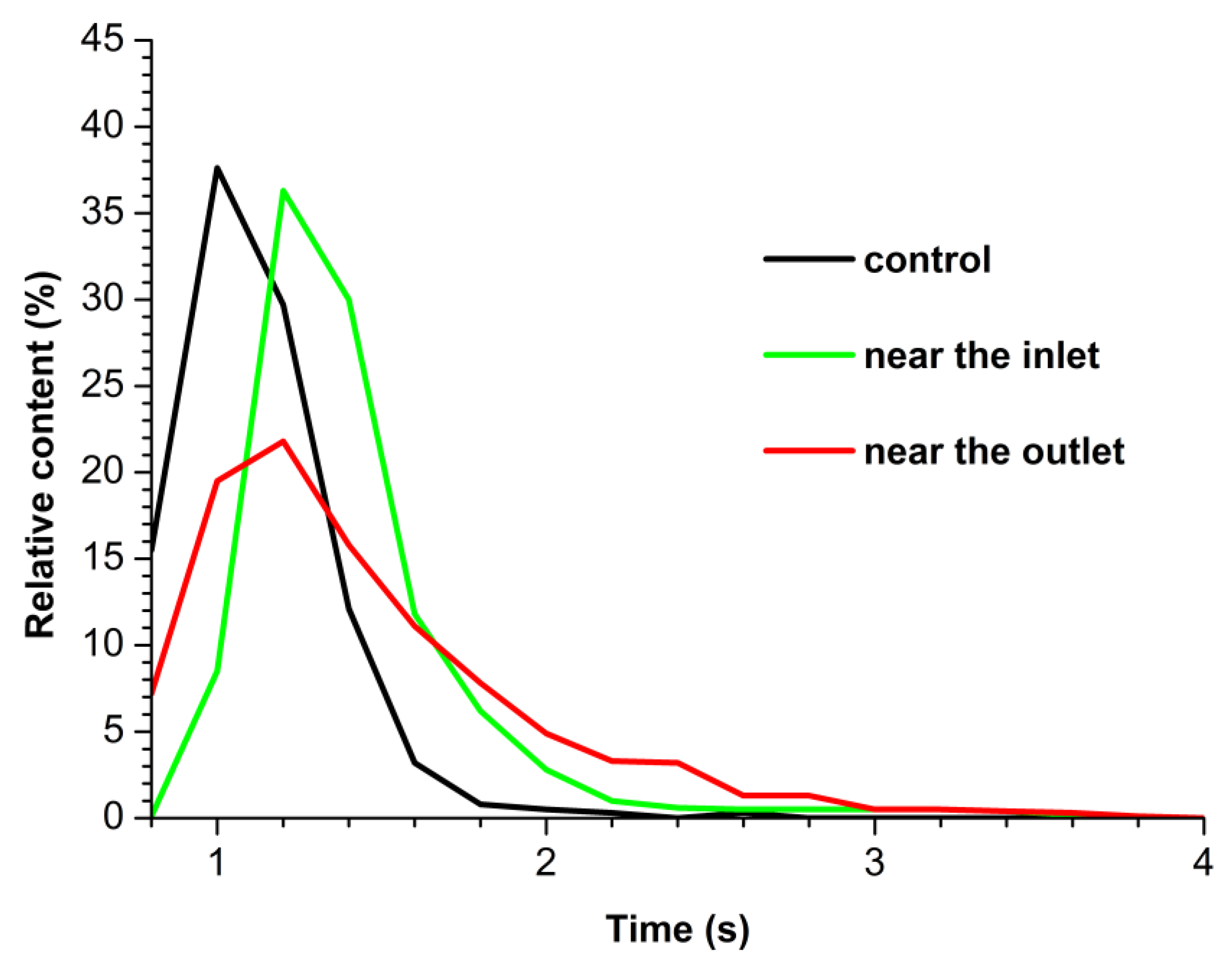
3.2. Spectrophotometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pauling, L. General Chemistry, 3rd ed.; W.H. Freeman and Company: San Francisco, CA, USA, 1970. [Google Scholar]
- Yang, B.; Sarafraz, M.M.; Arjomandi, M. Marangoni effect on the thermal performance of glycerol/water mixture in Microchannel. Appl. Therm. Eng. 2019, 161, 114142. [Google Scholar] [CrossRef]
- Jacobsen, D.; McMartin, K.E. Alcohols and glycols. In Human Toxicology; Descotes, J., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; pp. 623–648. [Google Scholar] [CrossRef]
- Vijayendran, R.A.; Ligler, F.S.; Leckband, D.E. A computational reaction-diffusion model for the analysis of transport-limited kinetics. Anal. Chem. 1998, 71, 5405–5412. [Google Scholar] [CrossRef] [PubMed]
- Papaneophytou, C.P.; Grigoroudis, A.I.; McInnes, C.; Kontopidis, G. Quantification of the Effects of Ionic Strength, Viscosity, and Hydrophobicity on Protein−Ligand Binding Affinity. ACS Med. Chem. Lett. 2014, 5, 931–936. [Google Scholar] [CrossRef] [PubMed]
- ProteOn XPR36 Experimental Design and Application Guide; Bulletin 6414 Rev. B; Bio-Rad Laboratories, Inc.: Hercules, CA, USA, 2019.
- Stern, E.; Vacic, A.; Rajan, N.K.; Criscione, J.M.; Park, J.; Ilic, B.R.; Mooney, D.J.; Reed, M.A.; Fahmy, T.M. Label-free biomarker detection from whole blood. Nat. Nanotechnol. 2010, 5, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Malsagova, K.A.; Pleshakova, T.O.; Galiullin, R.A.; Kozlov, A.F.; Romanova, T.S.; Shumov, I.D.; Popov, V.P.; Tikhonenko, F.V.; Glukhov, A.V.; Smirnov, A.Y.; et al. SOI-Nanowire Biosensor for the Detection of Glioma-Associated miRNAs in Plasma. Chemosensors 2020, 8, 95. [Google Scholar] [CrossRef]
- Singupuram, R.; Alam, T.; Ali, M.A.; Shaik, S.; Gupta, N.K.; Akkurt, N.; Kumar, M.; Eldin, S.M.; Dobrotă, D. Numerical analysis of heat transfer and fluid flow in microchannel heat sinks for thermal management. Case Stud. Therm. Eng. 2023, 45, 102964. [Google Scholar] [CrossRef]
- Akkurt, N.; Shedd, T.; Memon, A.A.; Usman; Ali, M.R.; Bouye, M. Analysis of the forced convection via the turbulence transport of the hybrid mixture in three-dimensional L-shaped channel. Case Stud. Therm. Eng. 2023, 45, 102558. [Google Scholar] [CrossRef]
- Lin, Y.; Rehman, S.; Akkurt, N.; Shedd, T.; Kamran, M.; Qureshi, M.I.; Botmart, T.; Alharbi, A.N.; Farooq, A.; Khan, I. Free convective trickling over a porous medium of fractional nanofluid with MHD and heat source/sink. Sci. Rep. 2022, 12, 20778. [Google Scholar] [CrossRef]
- Choi, D.; Lee, H.; Im, D.J.; Kang, I.S.; Lim, G.; Kim, D.S.; Kang, K.H. Spontaneous electrical charging of droplets by conventional pipetting. Sci. Rep. 2013, 3, 2037. [Google Scholar] [CrossRef]
- Cheedarala, R.K.; Song, J.I. Harvesting of flow current through implanted hydrophobic PTFE surface within silicone-pipe as liquid nanogenerator. Sci. Rep. 2022, 12, 3700. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Wang, Z.L. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.I.; Arafat, A.; Briand, D. Triboelectric effect to harness fluid flow energy. J. Phys. Conf. Ser. 2019, 1407, 012084. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Yang, X.; Hong, H.; Yang, Q.; Wang, J.; Tang, Q. Cumulative charging behavior of water droplets driven freestanding triboelectric nanogenerator toward hydrodynamic energy harvesting. J. Mater. Chem. A 2020, 8, 7880–7888. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Romanova, T.S.; Valueva, A.A.; Tatur, V.Y.; Stepanov, I.N.; Ziborov, V.S. Investigation of the Influence of Liquid Motion in a Flow-based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Water Flow. Appl. Sci. 2020, 10, 4560. [Google Scholar] [CrossRef]
- Chung, J.; Heo, D.; Shin, G.; Chung, S.-H.; Hong, J. Water behavior based electric generation via charge separation. Nano Energy 2021, 82, 105687. [Google Scholar] [CrossRef]
- Tanasescu, F.; Cramariuc, R. Electroststica în Technica; Editura Technica: Bucuresti, Romania, 1977. [Google Scholar]
- Balmer, R. Electrostatic Generation in Dielectric Fluids: The Viscoelectric Effect. In Proceedings of the WTC2005 World Tribology Congress III, Washington, DC, USA, 12–16 September 2005; Paper No. WTC2005-63806. pp. 521–527. [Google Scholar] [CrossRef]
- Shafer, M.R.; Baker, D.W.; Benson, K.R. Electric Currents and Potentials Resulting from the Flow of Charged Liquid Hydrocarbons Through Short Pipes. J. Res. Natl. Bur. Stand. C Eng. Instrum. 1965, 69, 307–317. [Google Scholar] [CrossRef]
- Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Negodailov, A.N.; Lukyanitsa, A.A.; Ivanov, Y.D. Investigation of the Influence of Liquid Motion in a Flow-Based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Glycerol Flow. Appl. Sci. 2020, 10, 4825. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Ershova, M.O.; Tatur, V.Y.; Ziborov, V.S. AFM Study of the Influence of Glycerol Flow on Horseradish Peroxidase near the in/out Linear Sections of a Coil. Appl. Sci. 2021, 11, 1723. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.-J.; Cheng, J.-H.; Sun, D.-W. Effects of electric fields and electromagnetic wave on food protein structure and functionality: A review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Bendicho, S.; Estela, C.; Giner, J.; Barbosa-Canovas, G.V.; Martin, O. Effects of High Intensity Pulsed Electric Field and Thermal Treatments on a Lipase from Pseudomonas fluorescens. J. Dairy Sci. 2002, 85, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Tamura, T.; Sato, M. Influence of pulsed electric field on various enzyme activities. J. Elstat. 2007, 65, 156–161. [Google Scholar] [CrossRef]
- Ohshima, T.; Tanino, T.; Guionet, A.; Takahashi, K.; Takaki, K. Mechanism of pulsed electric field enzyme activity change and pulsed discharge permeabilization of agricultural products. Jpn. J. Appl. Phys. 2021, 60, 060501. [Google Scholar] [CrossRef]
- Poojary, M.M.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Passamonti, P.; Jambrak, A.R.; Oey, I.; Greiner, R. Impact of Pulsed Electric Fields on Enzymes. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Queirós, M.; Pereira, G.; Leite, A.C.; Leal, R.; Rodrigues, R.; Teixeira, J.A.; Pereira, R.N. Tunning pectinase activity under the effects of electric fields in the enhanced clarification of wine must. Front. Sustain. Food Syst. 2023, 7, 1053013. [Google Scholar] [CrossRef]
- Yang, R.-J.; Li, S.-Q.; Zhang, Q.H. Effects of Pulsed Electric Fields on the Activity of Enzymes in Aqueous Solution. J. Food Sci. 2004, 69, FCT241–FCT248. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. The influence of rotating magnetic field on bio-catalytic dye degradation using the horseradish peroxidase. Biochem. Eng. J. 2019, 147, 81–88. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. Rotating magnetic field as tool for enhancing enzymes properties—Laccase case study. Sci. Rep. 2019, 9, 3707. [Google Scholar] [CrossRef]
- Emamdadi, N.; Gholizadeh, M.; Housaindokht, M.R. Investigation of static magnetic field effect on horseradish peroxidase enzyme activity and stability in enzymatic oxidation process. Int. J. Biol. Macromol. 2021, 170, 189–195. [Google Scholar] [CrossRef]
- Sun, J.; Sun, F.; Xu, B.; Gu, N. The quasi-one-dimensional assembly of horseradish peroxidase molecules in presence of the alternating magnetic field. Coll. Surf. A Physicochem. Eng. Asp. 2010, 360, 94–98. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Jin, Y.; Wang, M.; Gu, N. Magnetically enhanced dielectrophoretic assembly of horseradish peroxidase molecules: Chaining and molecular monolayers. Chem. Phys. Chem. 2008, 9, 1847–1850. [Google Scholar] [CrossRef]
- Mohamed-Ali, H.; Kolkenbrock, H.; Ulbrich, N.; Sörensen, H.; Kramer, K.D.; Merker, H.-J. Influence of Electromagnetic Fields on the Enzyme Activity of Rheumatoid Synovial Fluid Cells in vitro. Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.A.; Wu, B.-I.; Klibanov, A.M. Radio Frequency Radiation Causes No Nonthermal Damage in Enzymes and Living Cells. Biotechnol. Prog. 2010, 26, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.E.; Bonelli, P.R.; Rojas, A.M.; Gerschenson, L.N. Microwave inactivation of red beet (Beta vulgaris L. var. conditiva) peroxidase and polyphenoloxidase and the effect of radiation on vegetable tissue quality. J. Food Eng. 2012, 10, 676–684. [Google Scholar] [CrossRef]
- Lopes, L.C.; Barreto, M.T.; Gonçalves, K.M.; Alvarez, H.M.; Heredia, M.F.; De Souza, R.O.M.; Cordeiro, Y.; Dariva, C.; Fricks, A.T. Stability and structural changes of horseradish peroxidase: Microwave versus conventional heating treatment. Enzym. Microb. Technol. 2015, 69, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Caliga, R.; Maniu, C.L.; Mihăşan, M. ELF-EMF exposure decreases the peroxidase catalytic efficiency in vitro. Open Life Sci. 2016, 11, 71–77. [Google Scholar] [CrossRef]
- Kastenios, N.; Bilalis, D.; Efthimiadou, A.; Aivalakis, G.; Nikolopoulou, A.-E.; Karkanis, A.; Travlos, I. Role of pulsed electromagnetic field on enzyme activity, germination, plant growth and yield of durum wheat. Biocatal. Agric. Biotechnol. 2016, 6, 152–158. [Google Scholar] [CrossRef]
- Hamedi, N.; Saviz, M.; Banaei, A.; Shabani, S.; Qafari, M.; Moosavi-Movahedi, A.A.; Faraji-Dana, R. Effects of circularly-polarized electromagnetic fields on solvated hemoglobin structure. J. Mol. Liq. 2020, 312, 113283. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Pang, H.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. The effect of radio frequency heating on the inactivation and structure of horseradish peroxidase. Food Chem. 2023, 398, 133875. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Ravera, S.; Panfoli, I.; Pepe, I.M. Effects of extremely low frequency electromagnetic fields on membrane-associated enzymes. Arch. Biochem. Biophys. 2005, 441, 191–198. [Google Scholar] [CrossRef]
- Thumm, S.; Löschinger, M.; Glock, S.; Hämmerle, H.; Rodemann, H.P. Induction of cAMP-dependent protein kinase A activity in human skin fibroblasts and rat osteoblasts by extremely low-frequency electromagnetic fields. Radiat. Environ. Biophys. 1999, 38, 195–199. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Tatur, V.Y.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Repnikov, V.V.; et al. Effect of Spherical Elements of Biosensors and Bioreactors on the Physicochemical Properties of a Peroxidase Protein. Polymers 2021, 13, 1601. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Frantsuzov, P.A.; Zöllner, A.; Medvedeva, N.V.; Archakov, A.I.; Reinle, W.; Bernhardt, R. Atomic Force Microscopy Study of Protein–Protein Interactions in the Cytochrome CYP11A1 (P450scc)-Containing Steroid Hydroxylase System. Nanoscale Res. Lett. 2011, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.D.; Bukharina, N.S.; Frantsuzov, P.A.; Pleshakova, T.O.; Kanashenko, S.L.; Medvedeva, N.V.; Argentova, V.V.; Zgoda, V.G.; Munro, A.W.; Archakov, A.I. AFM study of cytochrome CYP102A1 oligomeric state. Soft Matter 2012, 8, 4602–4608. [Google Scholar] [CrossRef]
- Kiselyova, O.I.; Yaminsky, I.; Ivanov, Y.D.; Kanaeva, I.P.; Kuznetsov, V.Y.; Archakov, A.I. AFM study of membrane proteins, cytochrome P450 2B4, and NADPH–Cytochrome P450 reductase and their complex formation. Arch. Biochem. Biophys. 1999, 371, 1–7. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef]
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef]
- Enzymatic Assay of Peroxidase (EC 1.11.1.7) 2,20-Azino-Bis(3-Ethylbenzthiazoline-6-Sulfonic Acid) as a Substrate Sigma Prod. No. P-6782. Available online: https://www.sigmaaldrich.com/RU/en/technical-documents/protocol/protein-biology/enzymeactivity-assays/enzymatic-assay-of-peroxidase-abts-as-substrate (accessed on 18 February 2022).
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Tatur, V.Y.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; Lukyanitsa, A.A.; et al. The Effect of a Dodecahedron-Shaped Structure on the Properties of an Enzyme. J. Funct. Biomater. 2022, 13, 166. [Google Scholar] [CrossRef]
- Davies, P.F.; Rennke, H.G.; Cotran, R.S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J. Cell Sci. 1981, 49, 69–86. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Bolotskova, P.N.; Bondarchuk, E.V.; Gryaznov, V.G.; Gudkov, S.V.; Kozlov, V.A.; Okuneva, M.A.; Ovchinnikov, O.V.; Smoliy, O.P.; Turkanov, I.F. Long-Term Effect of Low-Frequency Electromagnetic Irradiation in Water and Isotonic Aqueous Solutions as Studied by Photoluminescence from Polymer Membrane. Polymers 2021, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering; Krieger: Malabar, FL, USA, 1990. [Google Scholar]
- Bunkin, N.F.; Bunkin, F.V. Bubston structure of water and electrolyte aqueous solutions. Phys. Usp. 2016, 59, 846–865. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Shkirin, A.V.; Suyazov, N.V.; Babenko, V.A.; Sychev, A.A.; Penkov, N.V.; Belosludtsev, K.N.; Gudkov, S.V. Formation and dynamics of ion-stabilized gas nanobubbles in the bulk of aqueous NaCl solutions. J. Phys. Chem. B 2016, 120, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, S.O.; Shkirin, A.V.; Ninham, B.W.; Sychev, A.A.; Babenko, V.A.; Penkov, N.V.; Kryuchkov, N.P.; Bunkin, N.F. Ion-specific and thermal effects in the stabilization of the gas nanobubble phase in bulk aqueous electrolyte solutions. Langmuir 2016, 32, 11245–11255. [Google Scholar] [CrossRef] [PubMed]
- Schramm, F.D.; Schroeder, K.; Jonas, K. Protein aggregation in bacteria. FEMS Microbiol. Rev. 2020, 44, 54–72. [Google Scholar] [CrossRef]
- Fogarty, A.C.; Laage, D. Water Dynamics in Protein Hydration Shells: The Molecular Origins of the Dynamical Perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef]
- Verma, P.K.; Rakshit, S.; Mitra, R.K.; Pal, S.K. Role of hydration on the functionality of a proteolytic enzyme α-chymotrypsin under crowded environment. Biochimie 2011, 93, 1424–1433. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Shipov, G.I. The effect of the stopped environment on the biological system. Zametki Uchenogo (Scientist’s Notes) 2021, 10, 294–297. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Shumov, I.D.; Kozlov, A.F.; Ershova, M.O.; Valueva, A.A.; Ivanova, I.A.; Tatur, V.Y.; Lukyanitsa, A.A.; Ivanova, N.D.; Ziborov, V.S. Stopped Flow of Glycerol Induces the Enhancement of Adsorption and Aggregation of HRP on Mica. Micromachines 2023, 14, 1024. https://doi.org/10.3390/mi14051024
Ivanov YD, Shumov ID, Kozlov AF, Ershova MO, Valueva AA, Ivanova IA, Tatur VY, Lukyanitsa AA, Ivanova ND, Ziborov VS. Stopped Flow of Glycerol Induces the Enhancement of Adsorption and Aggregation of HRP on Mica. Micromachines. 2023; 14(5):1024. https://doi.org/10.3390/mi14051024
Chicago/Turabian StyleIvanov, Yuri D., Ivan D. Shumov, Andrey F. Kozlov, Maria O. Ershova, Anastasia A. Valueva, Irina A. Ivanova, Vadim Y. Tatur, Andrei A. Lukyanitsa, Nina D. Ivanova, and Vadim S. Ziborov. 2023. "Stopped Flow of Glycerol Induces the Enhancement of Adsorption and Aggregation of HRP on Mica" Micromachines 14, no. 5: 1024. https://doi.org/10.3390/mi14051024
APA StyleIvanov, Y. D., Shumov, I. D., Kozlov, A. F., Ershova, M. O., Valueva, A. A., Ivanova, I. A., Tatur, V. Y., Lukyanitsa, A. A., Ivanova, N. D., & Ziborov, V. S. (2023). Stopped Flow of Glycerol Induces the Enhancement of Adsorption and Aggregation of HRP on Mica. Micromachines, 14(5), 1024. https://doi.org/10.3390/mi14051024






