Preparation of Nitrogen and Phosphorus Doped Porous Carbon from Watermelon Peel as Supercapacitor Electrode Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Materials
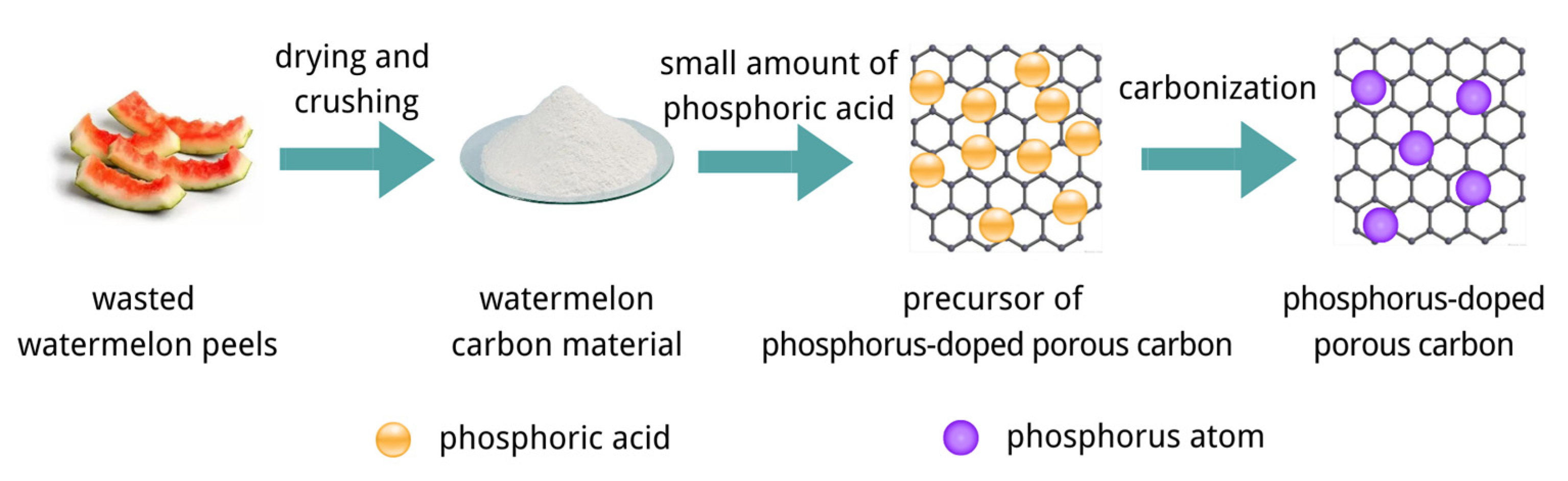
2.2. Preparation Method
3. Analytical Method
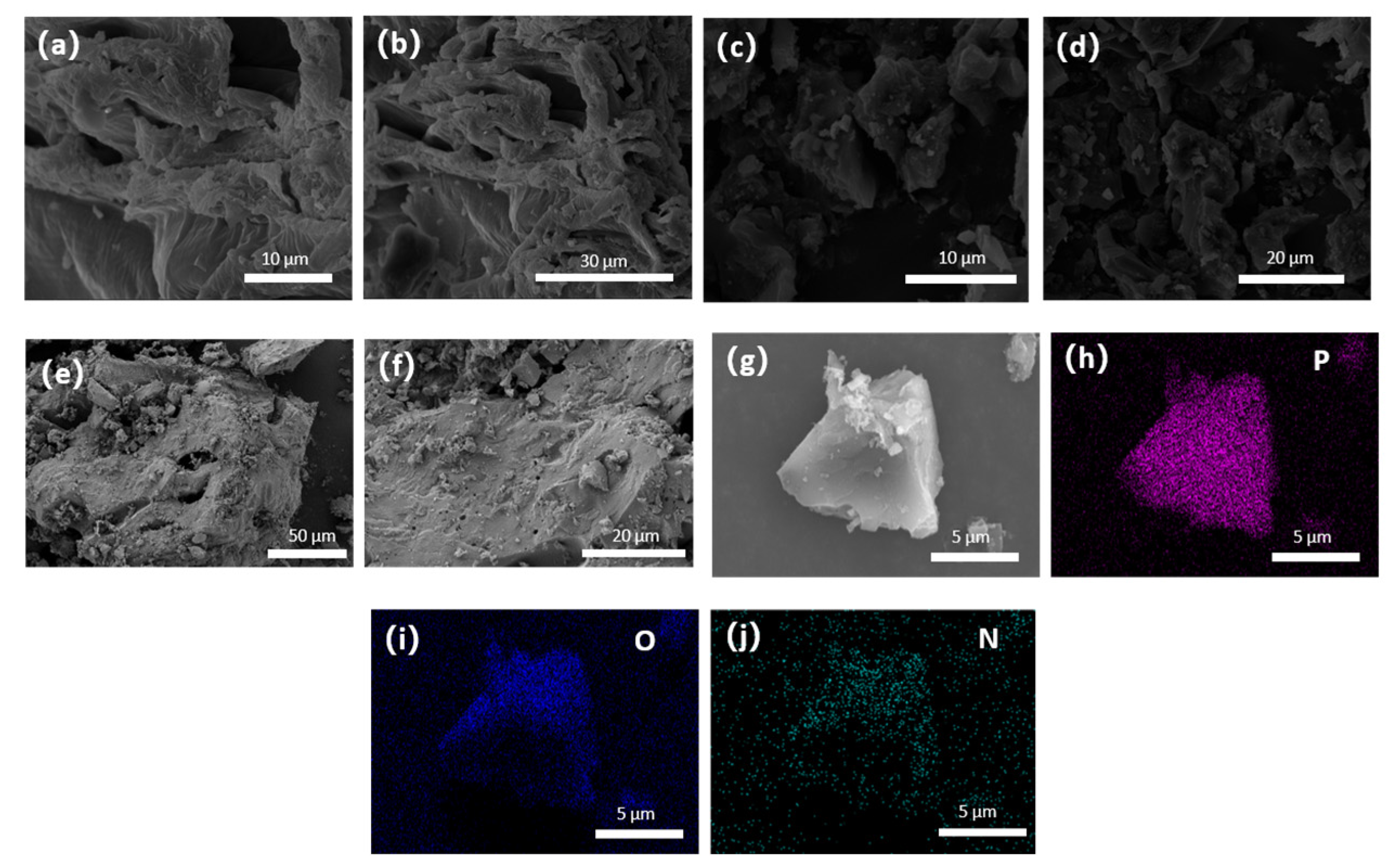
3.1. Scanning Electron Microscope Test Analysis (SEM)
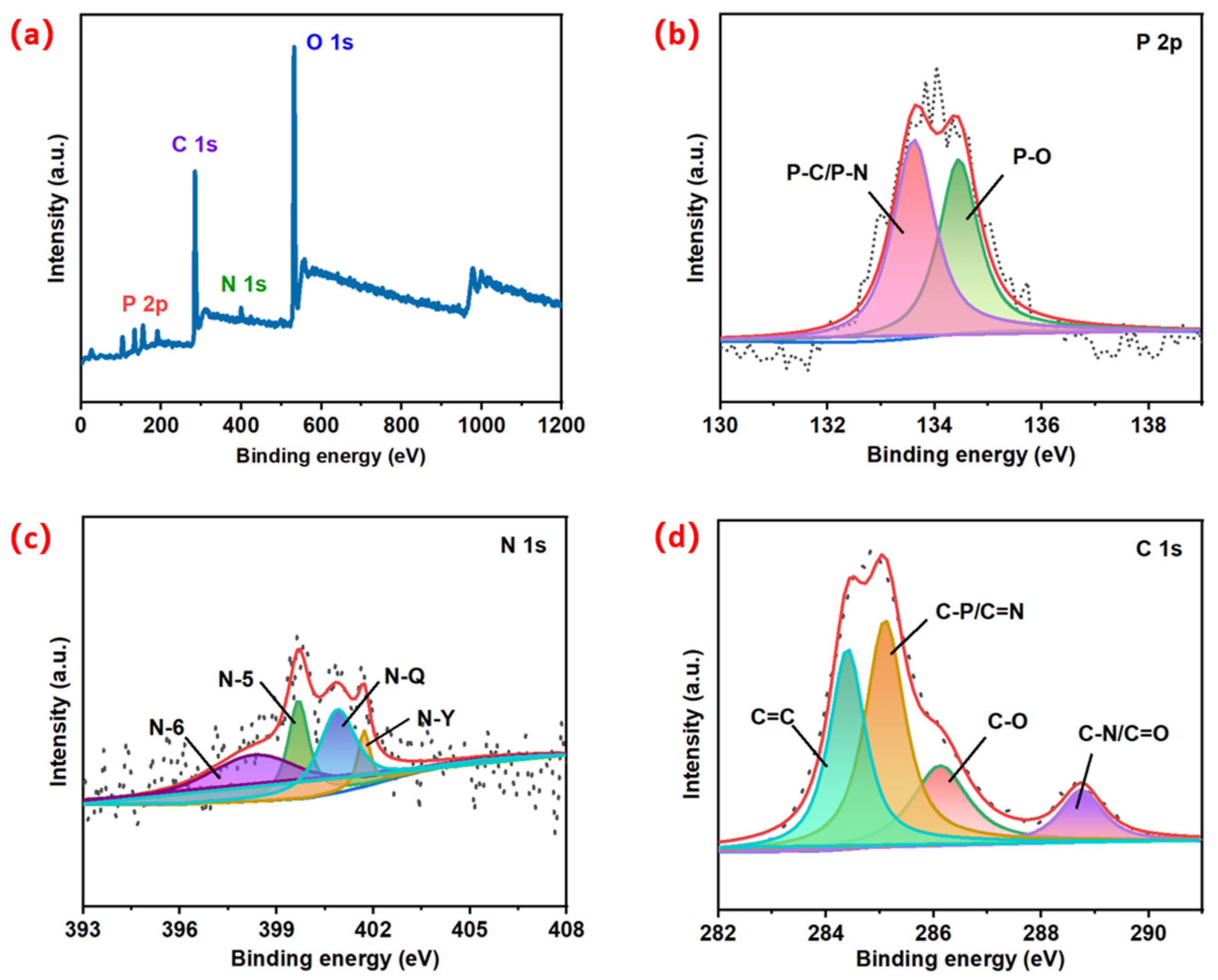
3.2. X-ray Photoelectron Spectroscopy
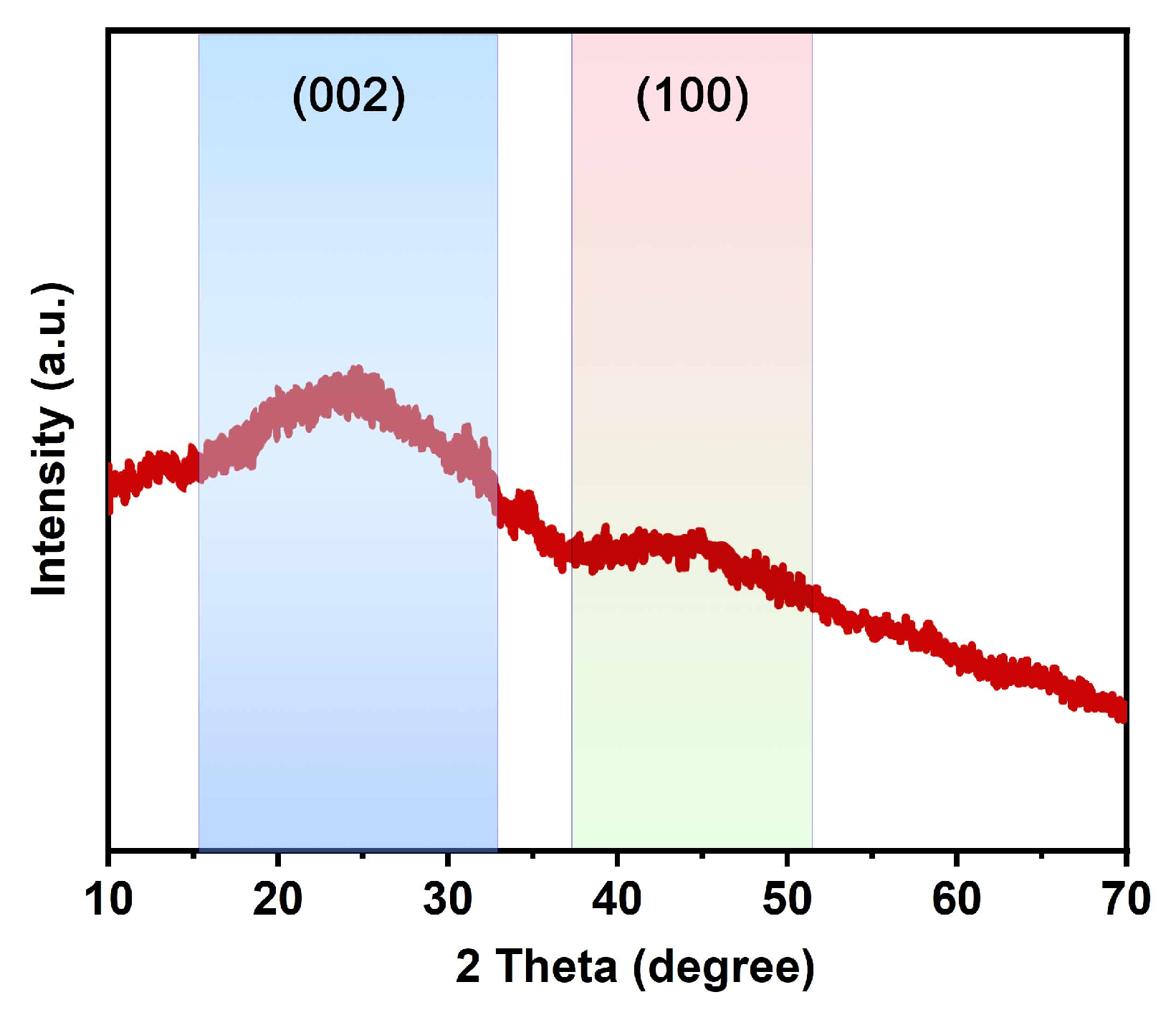
3.3. X-ray Diffraction Spectrum Analysis
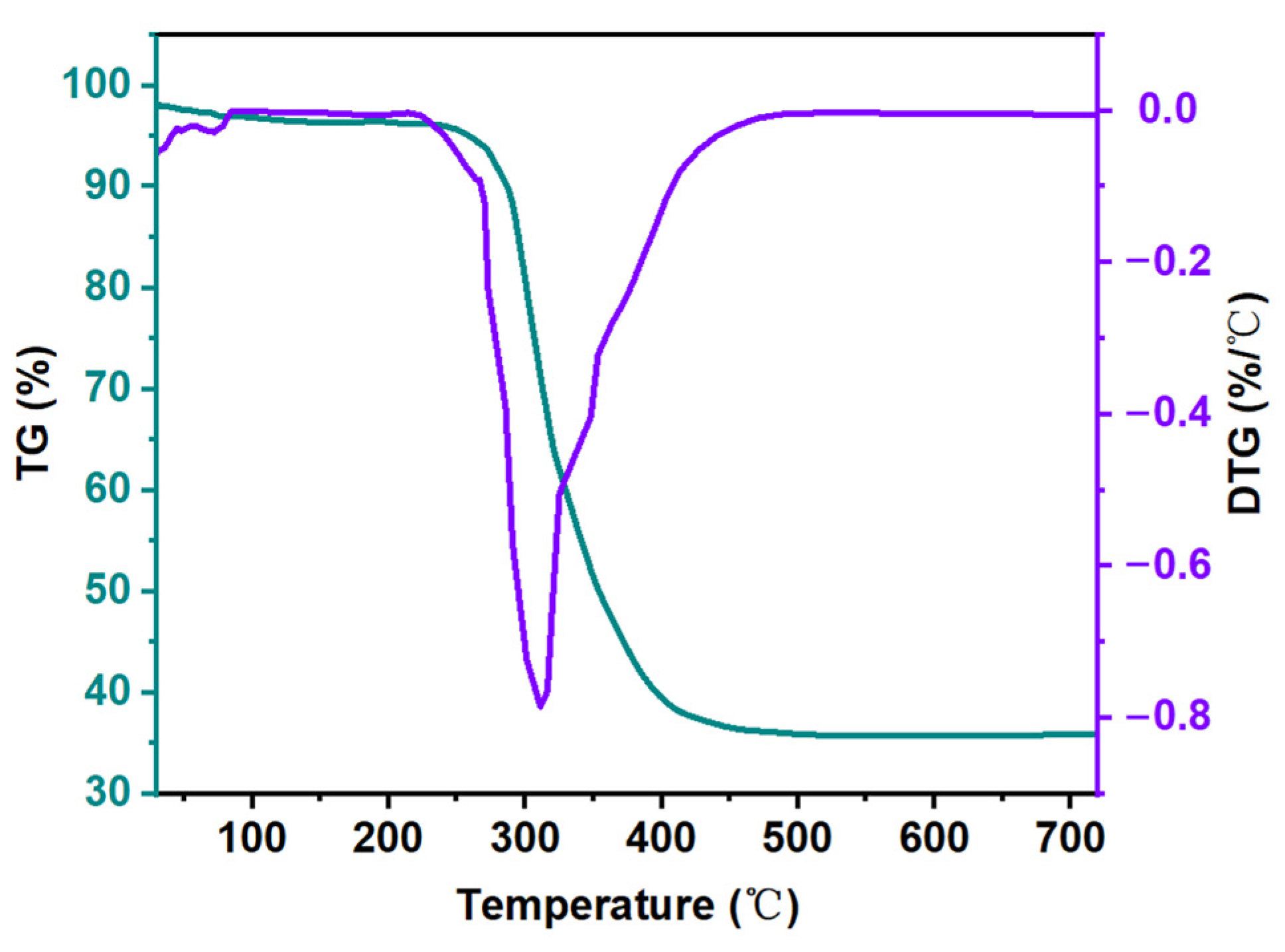
3.4. Thermogravimetric Analysis
3.5. Electrochemical Performance Test
3.5.1. Preparation of Working Electrode
3.5.2. Test Method
4. Results and Discussion
4.1. Morphology and Structure of MWC
4.2. Electrochemical Performance of MWC
| Biomass Precursor | Synthetic Method | Discharge Capacitance | Electrolyte | Current Density | Ref. |
|---|---|---|---|---|---|
| ZnO-AC nano-composite | sol-gel method | 38.4 F/g | 6 M KOH | 2 mA/cm2 | [56] |
| Chlorella | electrospun to nanofibrous material, carbonization and activation | 182 F/g | 1 M H2SO4 | 0.5 A/g | [57] |
| Potato | self-catalytic activation | 54 F/g | 6 M KOH | 0.5 A/g | [58] |
| Carbon nanofibers derived from polyimide and lignin precursors | electrospining and carbonization. | 83.6 F/g | 6 M KOH | 1 A/g | [59] |
| Waste cotton stalk | carbonization and activation | 111.1 F/g | 6 M KOH | 1 A/g | [60] |
| Activated carbon | - | 150.7 F/g | 1 M Na2SO4 | 0.1 A/g | [61] |
| Pleurotus eryngii | carbonization and KOH activation | 195 F/g | 6 M KOH | 0.2 A/g | [62] |
| Jackfruit peel | three-step approach consisting of KOH activation and CO2 activation at different temperatures | 191 F/g | 1 M H2SO4 | - | [63] |
| Watermelon peel | one-step carbonization | 135.2 F/g | 6 M KOH | 1 A/g | This work |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, D.; Li, Z.X.; Su, Q.; Wei, S.; Pang, S.F.; Zhao, X.F.; Liang, L.C.; Kang, L.H.; Cao, S.J. Preparation of boron/sulfur-codoped porous carbon derived from biological wastes and its application in a supercapacitor. Nanomaterials 2022, 12, 1182. [Google Scholar] [CrossRef]
- Wang, J.M.; Huang, Y.; Han, X.P.; Li, Z.Y.; Zhang, S.; Zong, M. A flexible Zinc-ion hybrid supercapacitor constructed by porous carbon with controllable structure. Appl. Surf. Sci. 2022, 579, 152247. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; da Silva, E.L.; Oliveira, A.P.S.; Matsushima, J.T.; Cuna, A.; Marcuzzo, J.S.; Goncalves, E.S.; Baldan, M.R. High-performance supercapacitor electrode based on activated carbon fiber felt/iron oxides. Mater. Today Commun. 2019, 21, 100553. [Google Scholar] [CrossRef]
- Yadav, M.S.; Sinha, A.K.; Singh, M.N.; Kumar, A. Electrochemical study of copper oxide and activated charcoal based nanocomposite electrode for supercapacitor. Mater. Today 2021, 46, 5722–5729. [Google Scholar]
- Jangir, H.; Pandey, M.; Jha, R.; Dubey, A.; Verma, S.; Philip, D.; Sarkar, S.; Das, M. Sequential entrapping of Li and S in a conductivity cage of N-doped reduced graphene oxide supercapacitor derived from silk cocoon: A hybrid Li-S-silk supercapacitor. Appl. Nanosci. 2018, 8, 379–393. [Google Scholar] [CrossRef]
- Pour, G.B.; Aval, L.F.; Mirzaee, M. CNTs supercapacitor based on the PVDF/PVA gel electrolytes. Recent. Pat. Nanotech. 2020, 14, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Kim, Y.R.; Jeong, H.T. All-solid-state supercapacitor composed of reduced graphene oxide (rGO)/activated carbon (AC) composite and polymer electrolyte. Carbon. Lett. 2020, 30, 107–113. [Google Scholar] [CrossRef]
- Jain, D.; Kanungo, J.; Tripathi, S.K. Performance enhancement approach for supercapacitor by using mango kernels derived activated carbon electrode with p-hydroxyaniline based redox additive electrolyte. Mater. Chem. Phys. 2019, 229, 66–77. [Google Scholar] [CrossRef]
- Lin, T.Q.; Chen, I.W.; Liu, F.X.; Yang, C.Y.; Bi, H.; Xu, F.F.; Huang, F.Q. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Wang, C.L. Liquid-based exfoliation of black phosphorus into phosphorene and its application for energy storage devices. Small Struct. 2021, 2, 2000148. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Wang, X.Y.; Xu, K.; Zhong, J.H. Biomass-derived activated carbon nanoarchitectonics with hibiscus flowers for high-performance supercapacitor electrode applications. Chem. Eng. Technol. 2022, 45, 649–657. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Sankar, A.B.; Rohita, D.S.; Rao, T.N.; Karthik, M. Conversion of biomass waste into high performance supercapacitor electrodes for real-time supercapacitor applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.M.; Nazari, M.; Shah, A.T.; Zhou, X.M.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green. Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Lim, C.H.; How, B.S.; Ng, W.P.Q.; Lam, H.L. Debottlenecking of biomass element deficiency in a multiperiod supply chain system via element targeting approach. J. Clean. Prod. 2019, 230, 751–766. [Google Scholar] [CrossRef]
- Jiang, G.C.; Liu, L.; Xiong, J.J.; Luo, Y.M.; Cai, L.C.; Qian, Y.; Wang, H.; Mu, L.W.; Feng, X.; Lu, X.H.; et al. Advanced material-oriented biomass precise reconstruction: A review on porous carbon with inherited natural structure and created artificial structure by post-treatment. Macromol. Biosci. 2022, 22, 2100479. [Google Scholar] [CrossRef] [PubMed]
- Quan, G.X.; Yan, J.L.; Ding, C. Preparation and characterization of biomass carbon adsorbent from rice husk and its adsorption properties on p-chlorophenol. Adv. Mater. 2012, 356–360, 510–513. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.Q.; Balasubramanian, R. Synthesis, formation mechanisms and applications of biomass-derived carbonaceous materials: A critical review. J. Mater. Chem. A 2021, 9, 24759–24802. [Google Scholar] [CrossRef]
- Shen, F.; Xiong, X.N.; Fu, J.Y.; Yang, J.R.; Qiu, M.; Qi, X.H.; Tsang, D.C.W. Recent advances in mechanochemical production of chemicals and carbon materials from sustainable biomass resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Hai, A.B.; Luo, S.H.; Liao, K.; Abu Haija, M.; Banat, F.; Naushad, M. Development of watermelon peel derived activated carbon/manganese ferrite nanocomposite for cleaner desalination by capacitive deionization. J. Clean. Prod. 2020, 272, 122626. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Li, Y.J.; Zhu, G.; Shi, J.Y.; Lu, T.; Pan, L.K. Waste fruit grain orange-derived 3D hierarchically porous carbon for high-performance all-solid-state supercapacitor. Ionics 2019, 25, 3935–3944. [Google Scholar] [CrossRef]
- Zhang, R.J.; Zhang, W.; Shi, M.P.; Li, H.; Ma, L.N.; Niu, H.J. Morphology controllable synthesis of heteroatoms-doped carbon materials for high-performance flexible supercapacitor. Dyes. Pigments 2022, 199, 109968. [Google Scholar] [CrossRef]
- Ciftyurek, E.; Bragg, D.; Oginni, O.; Levelle, R.; Singh, K.; Sivanandan, L.; Sabolsky, E.M. Performance of activated carbons synthesized from fruit dehydration biowastes for supercapacitor applications. Environ. Prog. Sustain. 2019, 38, e13030. [Google Scholar] [CrossRef]
- Chu, D.W.; Nashalian, A.; Zhou, Y.H.; Zhao, X.; Guo, D.X.; He, R.R.; Chen, W.W.; Chen, J. Low-cost and nature-friendly hierarchical porous carbon for enhanced capacitive electrochemical energy storage. ACS Appl. Energy. Mater. 2020, 3, 7246–7250. [Google Scholar] [CrossRef]
- Muhammad Jawad, U.; Gao, L.; Gebremeskel, H.; Bin Safdar, L.; Yuan, P.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Liu, W. Expression pattern of sugars and organic acids regulatory genes during watermelon fruit development. Sci. Hortic. 2020, 265, 109102. [Google Scholar] [CrossRef]
- Rico, X.; Gullon, B.; Alonso, J.L.; Yanez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food. Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef]
- Du, X.F.; Davila, M.; Ramirez, J.; Williams, C. Free amino acids and volatile aroma compounds in watermelon peel, flesh, and three peel-flesh juices. Molecules 2022, 27, 2536. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Razuan, R.; Appaturi, J.N.; Wilson, L.D. Adsorption and mechanism study for methylene blue dye removal with carbonized watermelon (Citrullus lanatus) peel prepared via one-step liquid phase H2SO4 activation24. Surf. Interfaces 2019, 16, 76–84. [Google Scholar] [CrossRef]
- Zhong, K.Q.; Li, M.; Yang, Y.; Zhang, H.G.; Zhang, B.P.; Tang, J.F.; Yan, J.; Su, M.H.; Yang, Z.Q. Nitrogen-doped biochar derived from watermelon peel as oxygen reduction catalyst in air cathode microbial fuel cells. Appl. Energy 2019, 242, 516–525. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Yusuf, B.A.; Zhou, C.S.; Xu, Y.G.; Ji, Q.H.; Xie, J.M.; Ma, H.L. Simplistic two-step fabrication of porous carbon-based biomass-derived electrocatalyst for efficient hydrogen evolution reaction. Energy Convers. Manag. 2021, 227, 113628. [Google Scholar] [CrossRef]
- Omar, N.; Abdullah, E.C.; Numan, A.; Mubarak, N.M.; Khalid, M.; Aid, S.R.; Agudosi, E.S. Facile synthesis of a binary composite from watermelon peel using response surface methodology for supercapacitor electrode material. J. Energy Storage 2022, 49, 104147. [Google Scholar] [CrossRef]
- Zhang, P.; Mu, J.H.; Guo, Z.Y.; Wong, S.I.; Sunarso, J.; Zhao, Y.; Xing, W.; Zhou, J.; Zhuo, S.P. Watermelon peel-derived heteroatom-doped hierarchical porous carbon as a high-performance electrode material for supercapacitors. Chemelectrochem 2021, 8, 1196–1203. [Google Scholar] [CrossRef]
- Tu, P.M.; Phat, L.N.; Lin, T.H.; Nguyen, H.C.; Tram, T.D.T.; Lam, H.D.N.; Khoa, B.D.D.; Cong, C.Q.; Son, N.T.; Phong, M.T. Effects of activation conditions on the characteristics, adsorption capacity, and energy storage of carbon aerogel from watermelon peel. Chemnanomat 2022, 8, e202200426. [Google Scholar] [CrossRef]
- Lin, X.Q.; Yang, N.; Lu, Q.F.; Liu, R. Self-Nitrogen-doped porous biocarbon from watermelon peel: A high-performance supercapacitor electrode and its improved electrochemical performance using redox additive electrolyte. Energy. Technol. 2019, 7, 1800628. [Google Scholar] [CrossRef]
- Komolov, A.S.; Lazneva, E.F.; Gerasimova, N.B.; Panina, Y.A.; Sobolev, V.S.; Koroleva, A.V.; Pshenichnyuk, S.A.; Asfandiarov, N.L.; Modelli, A.; Handke, B.; et al. Conduction band electronic states of ultrathin layers of thiophene/phenylene co-oligomers on an oxidized silicon surface. J. Electron. Spectrosc. 2019, 235, 40–45. [Google Scholar] [CrossRef]
- Kumar, A. Sol gel synthesis of zinc oxide nanoparticles and their application as nano-composite electrode material for supercapacitor. J. Mol. Struct. 2020, 1220, 128654. [Google Scholar] [CrossRef]
- Hasan, M.F.; Mantripragada, S.; Gbewonyo, S.; Xiu, S.N.; Shahbazi, A.; Zhang, L.F. Carbon nanofibrous electrode material from electrospinning of chlorella (microalgae) with polyacrylonitrile for practical high-performance supercapacitor. Int. J. Energy Res. 2022, 46, 22867–22882. [Google Scholar] [CrossRef]
- Wang, A.; Sun, K.; Xu, R.T.; Sun, Y.J.; Jiang, J.C. Cleanly synthesizing rotten potato-based activated carbon for supercapacitor by self-catalytic activation. J. Clean. Prod. 2021, 283, 125385. [Google Scholar] [CrossRef]
- Komolov, A.S.; Zhukov, Y.M.; Lazneva, E.F.; Aleshin, A.N.; Pshenichnyuk, S.A.; Gerasimova, N.B.; Panina, Y.A.; Zashikhin, G.D.; Baramygin, A.V. Thermally induced modification of the graphene oxide film on the tantalum surface. Mater. Design. 2016, 113, 319–325. [Google Scholar] [CrossRef]
- Park, G.T.; Jeon, H.B.; Kim, S.Y.; Gang, H.E.; Jeong, Y.G. Flexible and self-standing polyimide/lignin-derived carbon nanofibers for high-performance supercapacitor electrode material applications. Mater. Sci. Eng. B 2022, 283, 115530. [Google Scholar] [CrossRef]
- Yan, X.D.; Yu, Y.H.; Yang, X.P. Effects of electrolytes on the capacitive behavior of nitrogen/phosphorus co-doped nonporous carbon nanofibers: An insight into the role of phosphorus groups. RSC Adv. 2014, 4, 24986–24990. [Google Scholar] [CrossRef]
- Tian, J.S.; Zhang, T.; Talifu, D.; Abulizi, A.; Ji, Y.J. Porous carbon materials derived from waste cotton stalk with ultra-high surface area for high performance supercapacitors. Mater. Res. Bull. 2021, 143, 111457. [Google Scholar] [CrossRef]
- Islam, M.A.; Ong, H.L.; Villagracia, A.; Halim, K.A.A.; Ganganboina, A.B.; Doong, R.E.A. Biomass-derived cellulose nanofibrils membrane from rice straw as sustainable separator for high performance supercapacitor. Ind. Crop. Prod. 2021, 170, 13694. [Google Scholar] [CrossRef]
- Yuan, Y.D.; Yi, R.W.; Sun, Y.; Zeng, J.Q.; Li, J.Q.; Hu, J.H.; Zhao, Y.C.; Sun, W.; Zhao, C.; Yang, L. Porous activated carbons derived from pleurotus eryngii for supercapacitor applications. J. Nanomater. 2018, 2018, 7539509. [Google Scholar] [CrossRef]
- Awitdrus, A.; Yusra, D.A.; Taer, E.; Agustino, A.; Apriwandi, A.; Farma, R.; Taslim, R. Biomass conversion into activated carbon as a sustainable energy material for the development of supercapacitor devices. Energy Source Part A 2022, 44, 3349–3359. [Google Scholar] [CrossRef]
- Cheng, J.J.; Wang, Z.J.; Song, H.J.; Zhong, X.L.; Wang, J.B. Effects of physical properties of N-doped carbon on carbon/N-doped carbon/sulfur composite cathodes. Ionics 2021, 27, 3271–3279. [Google Scholar] [CrossRef]
- Zhao, H.J.; Wu, R.J.; Zhang, J.; Ma, F.; Zhao, W.X.; Han, Y.W. Preparation of FeS/iron oxide composite and flax stem-based porous carbon and its Cr(VI) removal ability. J. Chin. Chem. Soc. 2019, 66, 1296–1299. [Google Scholar] [CrossRef]
- Li, F.Q.; Liu, W.W.; Lai, Y.Q.; Qin, F.R.; Zou, L.; Zhang, K.; Li, J. Nitrogen and sulfur co-doped hollow carbon nanofibers decorated with sulfur doped anatase TiO2 with superior sodium and lithium storage properties. J. Alloy. Compd. 2017, 695, 1743–1752. [Google Scholar] [CrossRef]
- Lin, G.F.; Wang, Q.; Yang, X.; Cai, Z.H.; Xiong, Y.Z.; Huang, B. Preparation of phosphorus-doped porous carbon for high performance supercapacitors by one-step carbonization. RSC Adv. 2020, 10, 17768–17776. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.Y.; Li, G.S.; Xiong, X.L.; Ji, L.; Xu, Q.; Zou, X.L.; Lu, X.G. Molten salt synthesis of porous carbon and its application in supercapacitors: A review. J. Energy Chem. 2021, 61, 622–640. [Google Scholar] [CrossRef]
- Nirosha, B.; Selvakumar, R.; Jeyanthi, J.; Vairam, S. Elaeocarpus tectorius derived phosphorus-doped carbon as an electrode material for an asymmetric supercapacitor. New J. Chem. 2020, 44, 181–193. [Google Scholar] [CrossRef]
- Rabbani, M.A.; Oladipo, A.A.; Kusaf, M. N and P co-doped green waste derived hierarchical porous carbon as a supercapacitor electrode for energy storage: Electrolyte effects. Chemistryselect 2023, 8, e202204288. [Google Scholar]
- Li, N.; Yang, Q.Y.; Wei, Y.X.; Rao, R.C.; Wang, Y.P.; Sha, M.L.; Ma, X.H.; Wang, L.L.; Qian, Y.T. Phosphorus-doped hard carbon with controlled active groups and microstructure for high-performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 20486–20492. [Google Scholar] [CrossRef]
- Yang, J.J.; Tan, X.Y.; Qian, Y.; Li, L.; Xue, Y.; Dai, Z.; Wang, H.T.; Qu, W.L.; Chu, Y.Y. Methanol oxidation on Pt/CeO2@C–N electrocatalysts prepared by the in-situ carbonization of polyvinylpyrrolidone. J. Appl. Electrochem. 2016, 46, 779–789. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.J.; Jin, C.; Tian, J.H.; Yang, R.Z. Ternary doping of phosphorus, nitrogen, and sulfur into porous carbon for enhancing electrocatalytic oxygen reduction. Carbon 2015, 92, 327–338. [Google Scholar] [CrossRef]
- Zhao, C.C.; Gao, Y.J.; Zhang, Z.Y.; Ma, D. Functions of phytic acid in fabricating metal-free carbocatalyst for oxidative coupling of benzylamines. Chin. J. Chem. 2020, 38, 1292–1298. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin, C.H.; Park, J.H.; Jin, W.Z.; Bae, K.H.; Jung, M.J. Preparation of highly carbonized material from nanoparticle impregnated biomass. J. Ind. Eng. Chem. 2012, 18, 1828–1835. [Google Scholar] [CrossRef]
- Chen, X.J.; Lin, Q.M.; He, R.D.; Zhao, X.R.; Li, G.T. Hydrochar production from watermelon peel by hydrothermal carbonization. Bioresour. Technol. 2017, 241, 236–243. [Google Scholar] [CrossRef]
- Kruse, A.; Kirchherr, M.; Gaag, S.; Zevaco, T.A. Hydrothermal carbonization. 4. thermal properties of the products. Chem. Ing. Tech. 2015, 87, 1707–1712. [Google Scholar] [CrossRef]
- Andrea, K.; Monika, K.; Stefanie, G.; Thomas, A.Z. Hydrothermale Karbonisierung. 4. Thermische Eigenschaften der Produkte. Chem. Ing. Tech. 2015, 87, 1707–1712. [Google Scholar]
- Fu, G.S.; Li, Q.; Ye, J.L.; Han, J.J.; Wang, J.Q.; Zhai, L.; Zhu, Y.W. Hierarchical porous carbon with high nitrogen content derived from plant waste (pomelo peel) for supercapacitor. J. Mater. Sci. Mater. Electron. 2018, 29, 7707–7717. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Li, P.; Wei, Y.; Wang, Y.; Jiang, B.; Wu, W. Preparation of Nitrogen and Phosphorus Doped Porous Carbon from Watermelon Peel as Supercapacitor Electrode Material. Micromachines 2023, 14, 1003. https://doi.org/10.3390/mi14051003
Yang C, Li P, Wei Y, Wang Y, Jiang B, Wu W. Preparation of Nitrogen and Phosphorus Doped Porous Carbon from Watermelon Peel as Supercapacitor Electrode Material. Micromachines. 2023; 14(5):1003. https://doi.org/10.3390/mi14051003
Chicago/Turabian StyleYang, Chi, Penghui Li, Yumeng Wei, Yanting Wang, Bo Jiang, and Wenjuan Wu. 2023. "Preparation of Nitrogen and Phosphorus Doped Porous Carbon from Watermelon Peel as Supercapacitor Electrode Material" Micromachines 14, no. 5: 1003. https://doi.org/10.3390/mi14051003
APA StyleYang, C., Li, P., Wei, Y., Wang, Y., Jiang, B., & Wu, W. (2023). Preparation of Nitrogen and Phosphorus Doped Porous Carbon from Watermelon Peel as Supercapacitor Electrode Material. Micromachines, 14(5), 1003. https://doi.org/10.3390/mi14051003






