Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Vitrification
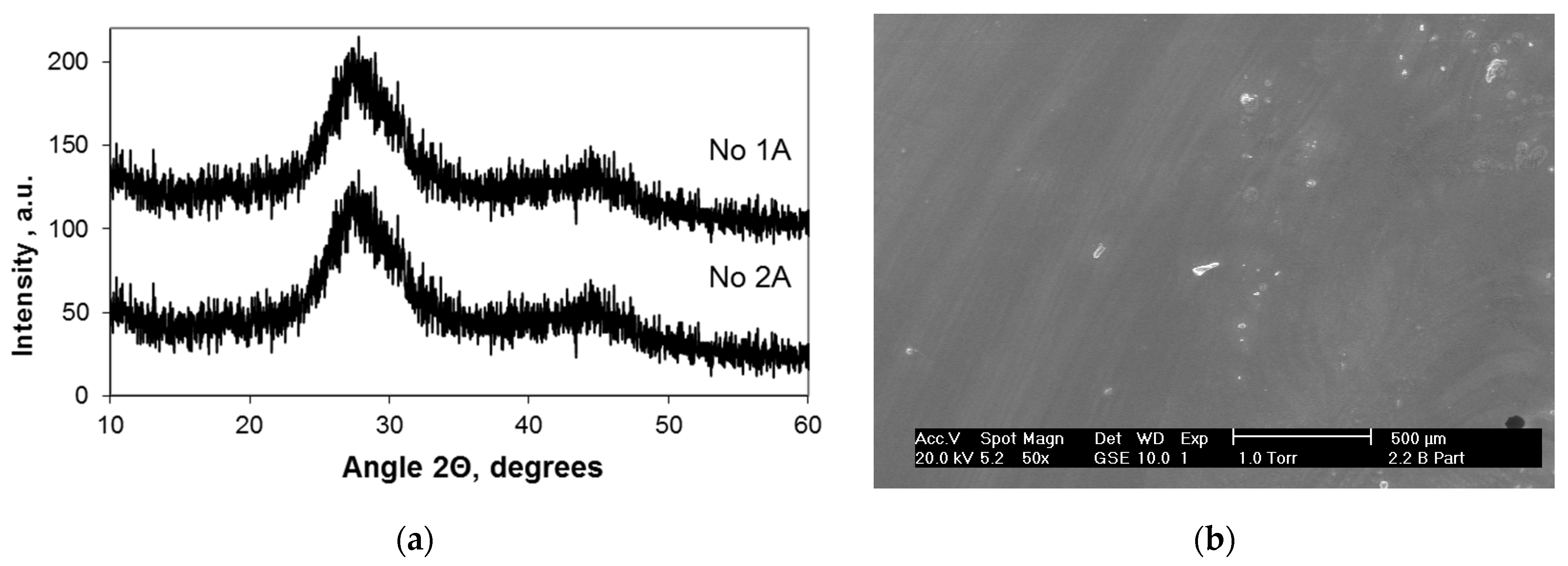
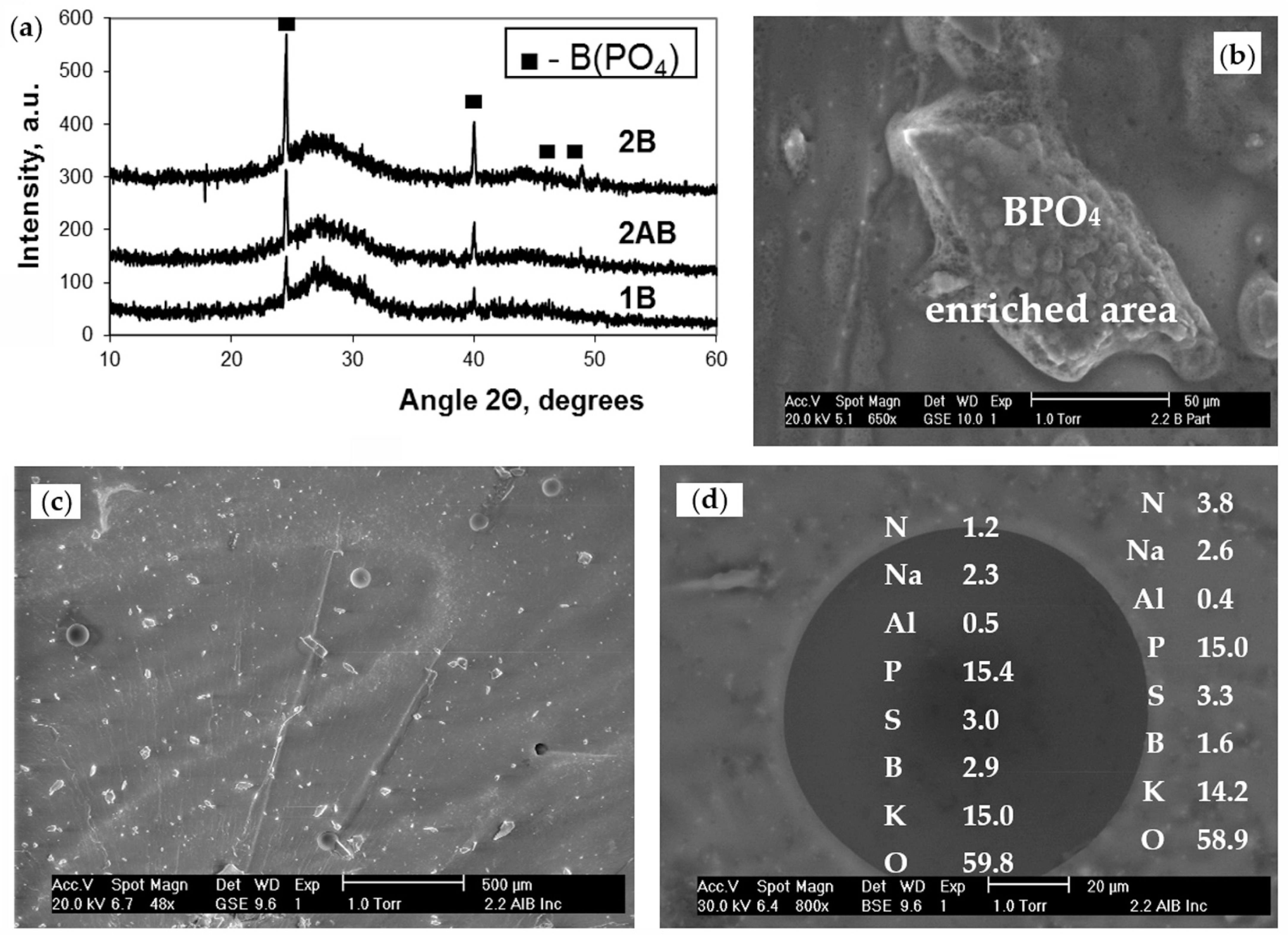
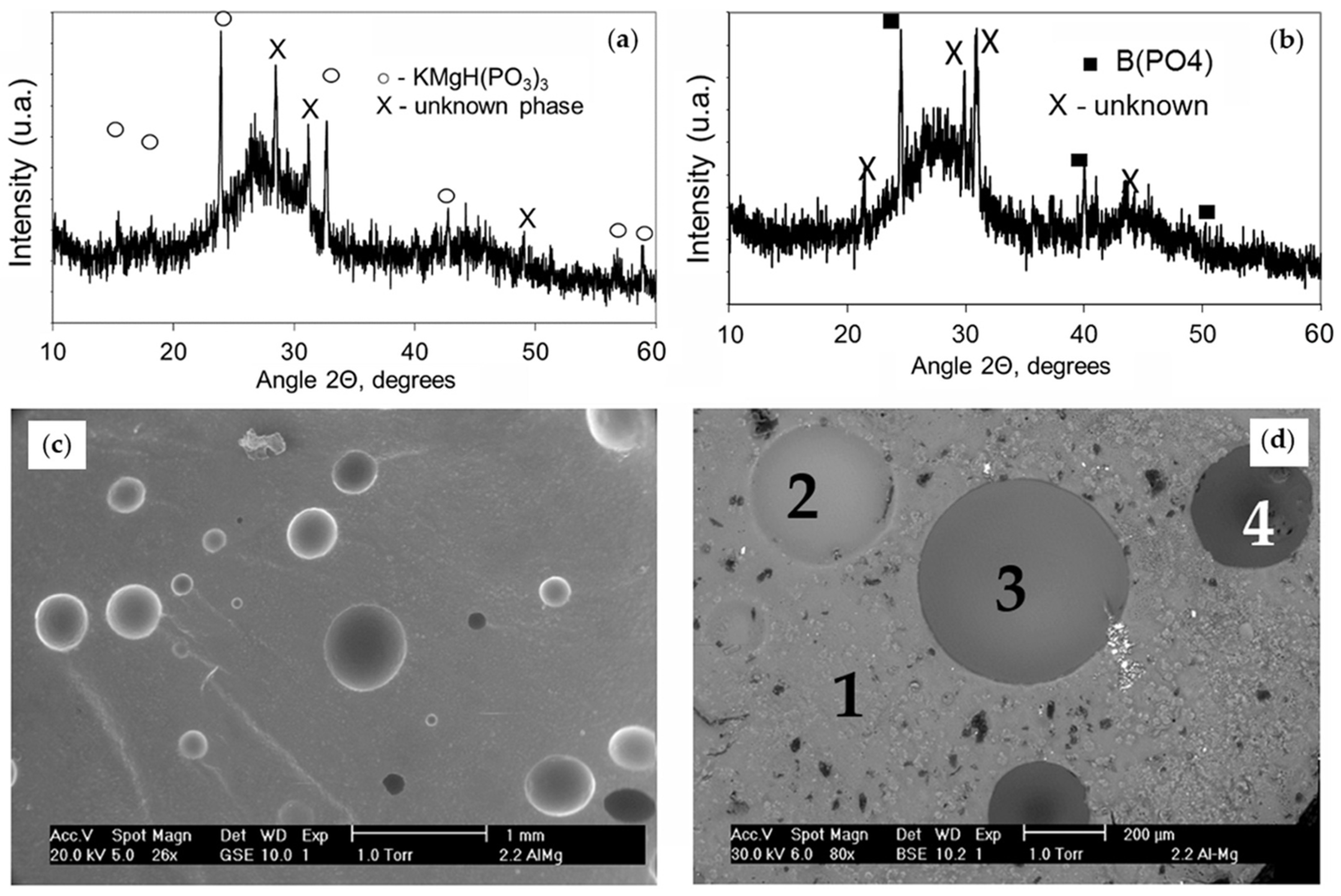
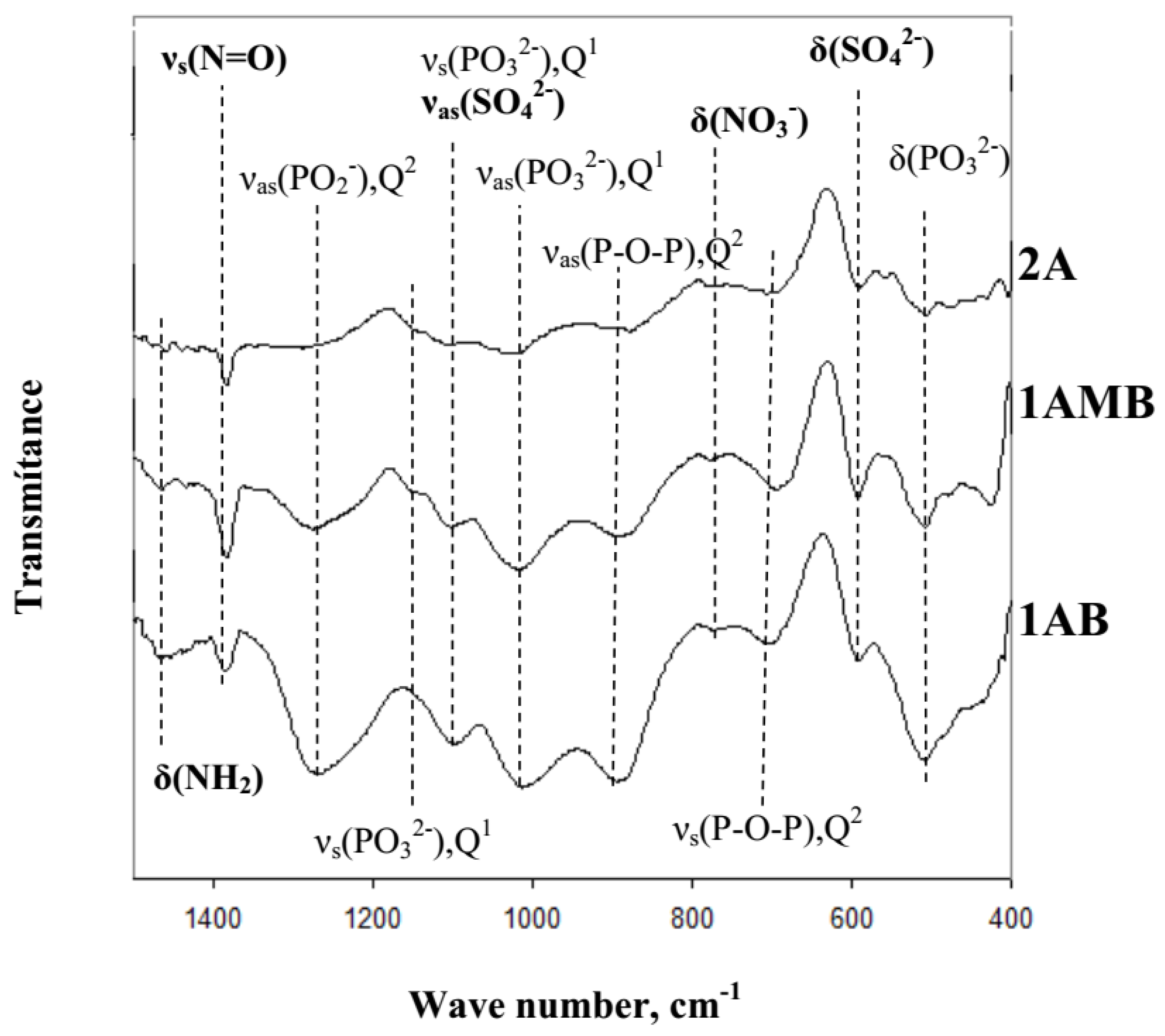
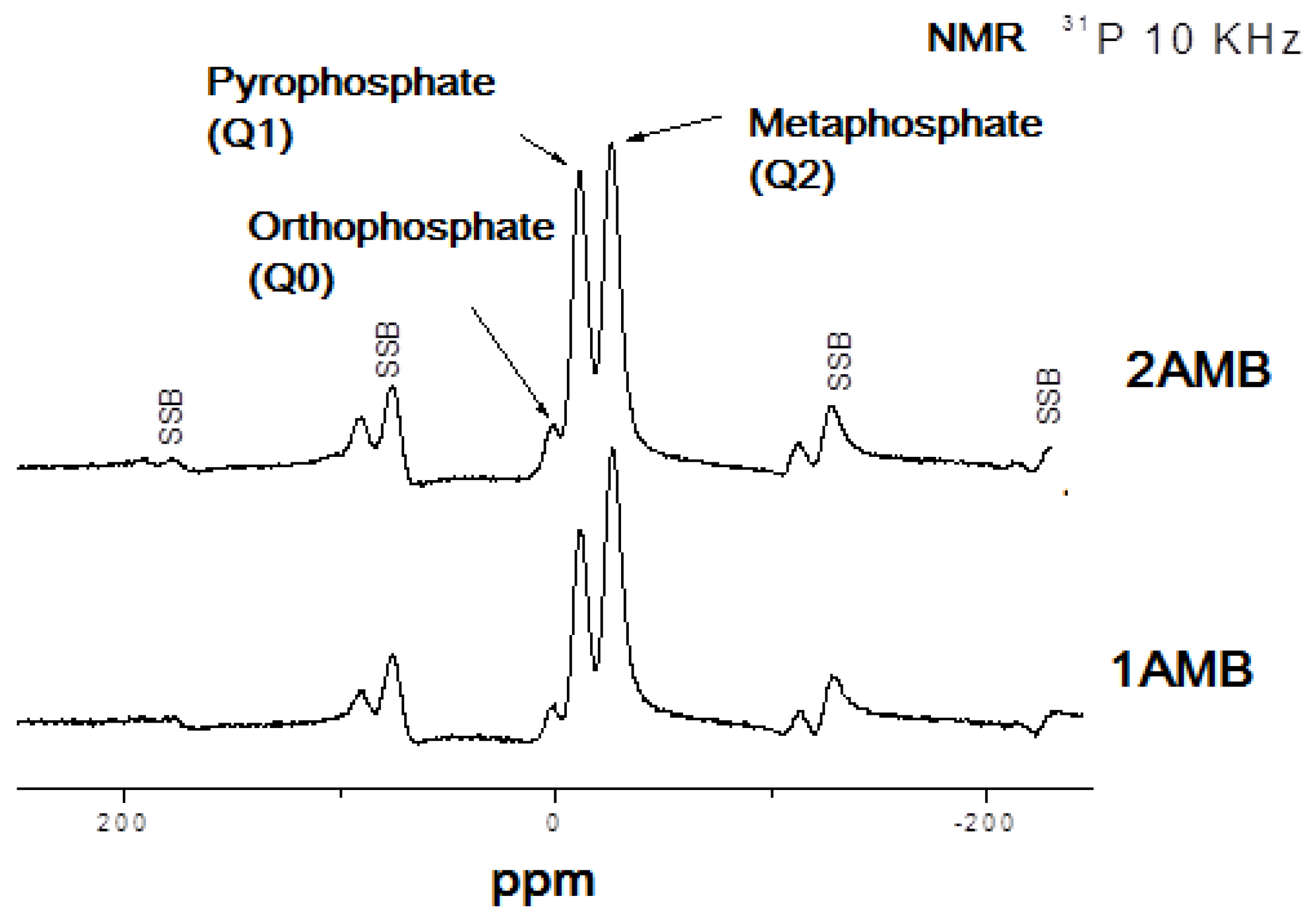
3.2. Structure
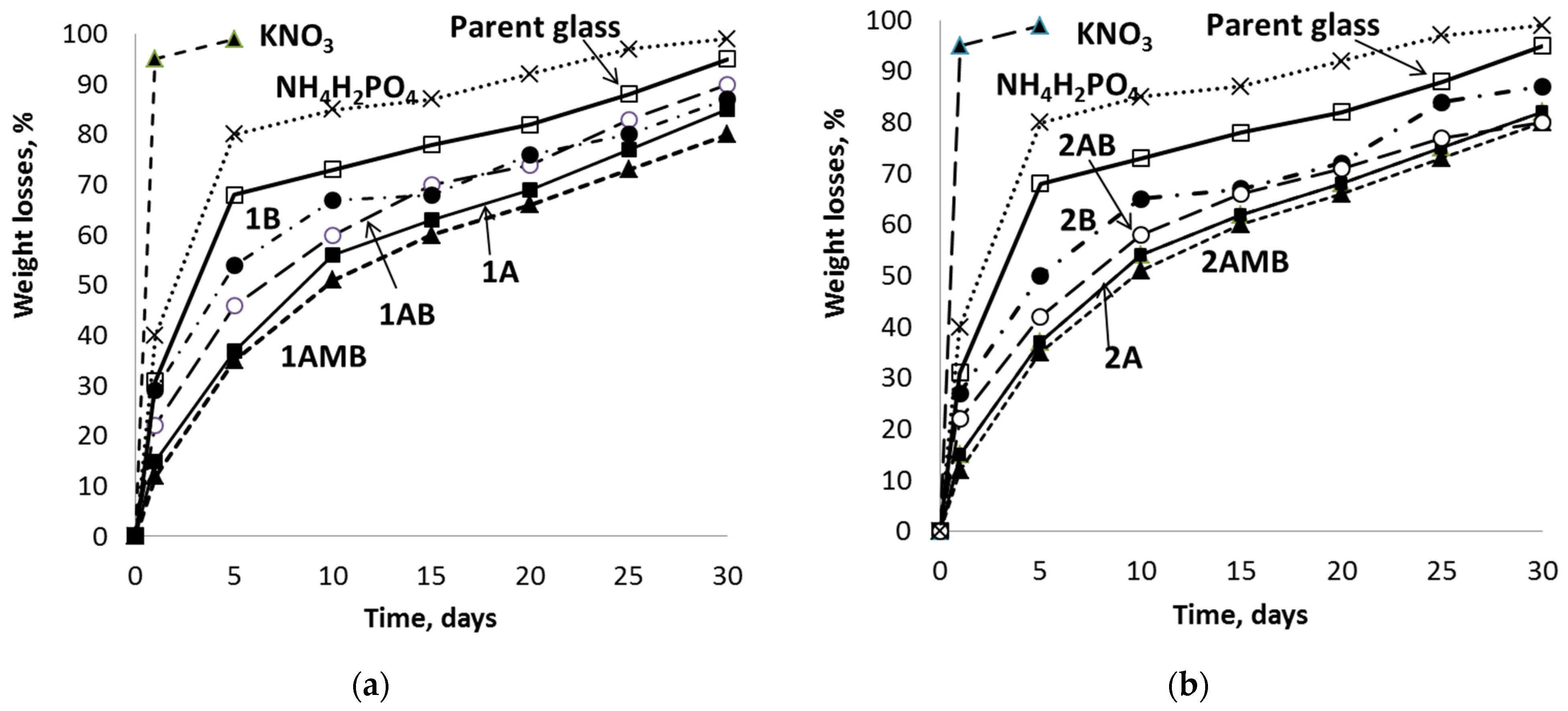
3.3. Chemical Durability
4. Discussion

5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hazra, G.; Das, T. A Review on Controlled Release Advanced Glassy Fertilizer. Glob. J. Sci. Front. Res. B Chem. 2014, 14, 33–44. [Google Scholar]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally Friendly Fertilizers: A Review of Materials Used and Their Effects on the Environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Sempeho, S.I.; Kim, H.T.; Egid, M.; Askwar, H. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef]
- The International Organization for Standardization. ISO 18644 Fertilizers and Soil Conditioners, Controlled-Release Fertilizer, General Requirements, 1st ed.; ISO: Geneva, Switzerland, 2016; pp. 2–4. [Google Scholar]
- Barba, M.F.; Callejas, P.; Arzabe, J.O.; Ajò, D. Characterization of two frit ceramic materials in low cost fertilizers. J. Eur. Ceram. Soc. 1998, 18, 1313–1317. [Google Scholar] [CrossRef]
- Ouis, M.A.; Abd-Eladl, M.; Abou-Baker, N.H. Evaluation of agriglass as an environment friendly slow release fertilizer. Silicon 2018, 10, 293–299. [Google Scholar] [CrossRef]
- D’Amato, R.; De Feudis, M.; Troni, E.; Gualtieri, S.; Soldati, R.; Famiani, F.; Businelli, D. Agronomic potential of two different glass-based materials as novel inorganic slow-release iron fertilizers. J. Sci. Food Agric. 2022, 102, 1660–1664. [Google Scholar] [CrossRef]
- Karapetyan, G.; Karapetyan, K.; Maksimov, L. Glassy Environmentally Friendly Fertilizers of Prolonged Action. Phosphorus Res. Bull. 2004, 15, 60–67. [Google Scholar] [CrossRef]
- Labbilta, T.; Ait-El-Mokhtar, M.; Abouliatim, Y.; Khouloud, M.; Meddich, A.; Mesnaoui, M. Innovative Formulations of Phosphate Glasses as Controlled-Release Fertilizers to Improve Tomato Crop Growth, Yield and Fruit Quality. Molecules 2021, 26, 3928. [Google Scholar] [CrossRef]
- Militaru, B.A.; Vancea, C.; Pode, R. Glass Fertilizers Obtained Using Sewage Sludge Ash Wastes. Rev. Chim. 2019, 70, 3824–3829. [Google Scholar] [CrossRef]
- Perez-Medina, J.C.; Gorokhovsky, A.; Escalante-Garcia, J.I.; Peña-Cabriales, J.J. Synthesis and characterization of nitrate sulfate phosphate glasses. Glass Technol. 2005, 46, 183–186. [Google Scholar]
- Brow, R.K. Review: The structure of simple phosphate glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Downs, A.J. Chemistry of Aluminium, Gallium, Indium, and Thallium, 1st ed.; Chapman & Hall: London, UK, 1993; pp. 153–156. [Google Scholar]
- Donald, I.W.; Metcalfe, B.L.; Fong, S.K.; Gerrard, L.A. The influence of Fe2O3 and B2O3 additions on the thermal properties, crystallization kinetics and durability of a sodium aluminum phosphate glass. J. Non-Cryst. Solids 2006, 352, 2993–3001. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Chapter 13: The Group 13 Elements/Inorganic Chemistry, 3rd ed.; Pearson: London, UK, 2008; p. 340. [Google Scholar]
- Paulik, F.; Paulik, J.; Arnold, M.; Naumann, R. Investigation on the thermal behaviour of Mg(NO3)2∙6H2O. The decomposition behavior. J. Therm. Anal. Calorim. 1988, 34, 627–635. [Google Scholar] [CrossRef]
- Jlassi, I.; Elhouichet, H.; Ferida, M. Influence of MgO on structure and optical properties of alumino-lithium-phosphate glasses. Phys. E 2016, 81, 219–225. [Google Scholar] [CrossRef]
- Paryab, A.H.; Abdollahi, S.; Khalilifard, R.; Hosseini, H.R.M. Porous Slow Release Silicate-Phosphate Glasses Synthesized by Polymer Derived Ceramics Method Appropriate for Plants Nourishment. Iran. J. Mater. Sci. Eng. 2021, 18, 81–90. [Google Scholar]
- Calahoo, C.; Wondraczek, L. Ionic glasses: Structure, properties and classification. J. Non-Cryst. Solids X 2020, 8, 100054. [Google Scholar] [CrossRef]
- Łączka, M.; Ciecinska, M. Preparation, structure and properties of silicate-phosphate glasses obtained by means of sol-gel method. J. Sol-Gel Sci. Technol. 1994, 3, 219–227. [Google Scholar] [CrossRef]
- de Jager, H.J.; Prinsloo, L.C. The dehydration of the phosphates monitored by DSC/TGA and in situ Raman spectroscopy. Thermochim. Acta 2001, 376, 187–196. [Google Scholar] [CrossRef]
- Achary, S.N.; Tyagi, A.K. Strong anisotropic thermal expansion in cristobalite-type BPO4. J. Solid State Chem. 2004, 177, 3918–3926. [Google Scholar] [CrossRef]
- Moustafa, Y.M.; El-Eligi, K. Infrared spectra of sodium phosphate glasses. J. Non-Cryst. Solids 1998, 340, 144–153. [Google Scholar] [CrossRef]
- Peak, D.R.; Ford, G.D.; Sparks, L. An In Situ ATR-FTIR Investigation of Sulfate Bonding Mechanisms on Goethite. J. Colloid Interface Sci. 1999, 218, 289–299. [Google Scholar] [CrossRef] [PubMed]
- de los Arada Perez, M.A.; Perez Marin, L.; Calvo Quintana, J.; Yazdani-Pedram, M. Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination. Sens. Actuators B 2003, 89, 262–268. [Google Scholar] [CrossRef]
- Huayashi, S.; Hayamizu, K. High-resolution solid state 31PNMR of alkali phosphates. Bull. Chem. Soc. Jpn 1989, 62, 3061–3068. [Google Scholar] [CrossRef]
- Brow, R.K.; Kirkpatrick, R.J.; Turner, G.L. Nature of alumina in phosphate glass: II Structure of sodium aluminophosphate glass. J. Am. Ceram. Soc. 1993, 76, 919–928. [Google Scholar] [CrossRef]
- Thilo, E.; Blumenthal, G. Zur chemie der kondensierten phosphate und arsenate über sulfatophosphate. Z. Anorg. Allg. Chem. 1966, 348, 77–88. [Google Scholar] [CrossRef]
- Nepomiluev, A.M.; Pletnev, R.N.; Lapina, O.B.; Kozlova, S.G.; Bamburov, V.G. Structure of Glasses in the Na2SO4–P2O5–H2O System. Glass Phys. Chem. 2002, 28, 1–4. [Google Scholar] [CrossRef]
- Greaves, G.N.; Sen, S. Inorganic glasses, glass-forming liquids and amorphizing solids. Adv. Phys. 2007, 56, 1–166. [Google Scholar] [CrossRef]
- Sirotkin, S.; Meszaros, R.; Wondraczek, L. Chemical Stability of ZnO-Na2O-SO3-P2O5 Glasses. Int. J. Appl. Glass Sci. 2012, 3, 44–52. [Google Scholar] [CrossRef]
- Le Sauze, A.; Montagne, L.; Palavit, G.; Fayon, F.; Marchand, R. X-ray photoelectron spectroscopy and nuclear magnetic resonance structural study of phosphorus oxynitride glasses, LiNaPON. J. Non-Cryst. Solids 2000, 263–264, 139–145. [Google Scholar] [CrossRef]
- Wang, Y.B.; Ryan, D.H.; Altounian, Z. Structural and thermal studies of nitrate glasses. J. Non-Cryst. Solids 1996, 205–207, 221–224. [Google Scholar] [CrossRef]
- Malugani, J.P.; Mercier, R.; Fahys, B.; Robert, G. Ionic conductivity of and Raman spectroscopy investigation binary oxosalts (1 − x)AgPO3−xAg2SO4 glasses. J. Solid State Chem. 1982, 45, 309–316. [Google Scholar] [CrossRef]
- Reibstein, S.; Da, N.; Simon, J.P.; Spiecker, E.; Wondraczek, L. Phase separation and crystal precipitation in supercooled sulphophosphate ionic melts. Phys. Chem. Glass-Eur. J. Glass Sci. Technol. Part B 2012, 53, 61–67. [Google Scholar]
| No | Content of the Component (wt.%) | ||||
|---|---|---|---|---|---|
| NH4H2PO4 | KNO3 | NaNO3 | KHSO4 | {H3BO3 + Al(NO3)3 + Mg(NO3)2} | |
| 0 | 67.7 | 21.3 | 7.4 | 4.9 | - |
| 1 | 64.8 | 20.4 | 7.1 | 3.5 | 4.2 |
| 2 | 62.1 | 19.4 | 6.7 | 3.4 | 8.4 |
| Group | Admixtures (Glass Composition Number) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Mg | B | Al + Mg | Al + B | Mg + B | Al + Mg + B | |
| 1 | Transparent glass (1A) | Non (1M) | White glass (1B) | White glass (1AM) | White glass (1AB) | Non (1BM) | White glass (1AMB) |
| 2 | Transparent glass (2A) | Non (2M) | White glass (2B) | Non (2AM) | White glass (2AB) | Non (2MB) | White glass (2AMB) |
| No | Composition (Chemical Elements, at.%) | |||||||||
| H | N | S | K | Na | P | Al | B | Mg | O | |
| 0 | 8.2 | 8.1 | 4.4 | 14.0 | 2.8 | 14.7 | - | - | 47.7 | |
| 1AMB | 6.9 | 7.8 | 4.9 | 14.1 | 3.1 | 15.5 | 2.1 | 1.8 | 0.2 | 44.6 |
| 2AMB | 6.4 | 7.3 | 4.6 | 14.6 | 2.7 | 14.6 | 2.7 | 1.9 | 0.4 | 44.8 |
| Composition (oxides, mol.%) | ||||||||||
| H2O | N2O5 | SO3 | K2O | Na2O | P2O5 | Al2O3 | B2O3 | MgO | ||
| 0 | 12.2 | 14.1 | 15.4 | 24.3 | 4.9 | 29.1 | - | - | - | |
| 1AMB | 11.5 | 12.8 | 15.5 | 23.0 | 4.8 | 25.1 | 3.6 | 3.0 | 0.7 | |
| 2AMB | 10.5 | 12.2 | 15.0 | 23.6 | 4.6 | 25.6 | 4.3 | 3.3 | 0.9 | |
| Chemical Elements | Relative Contents, at.% | |||
|---|---|---|---|---|
| Point 1 | Point 2 | Point 3 | Point 4 | |
| N | 4.1 | 2 | 1.6 | 2.5 |
| B | 0.8 | 1.4 | 0.3 | 0.2 |
| Na | 2.6 | 2.8 | 2.6 | 3 |
| Al | 0.2 | 7.9 | 4.6 | 0.4 |
| P | 14.6 | 11.7 | 12.7 | 14.9 |
| S | 4.4 | 4.1 | 5.4 | 6 |
| K | 14.3 | 10.4 | 13 | 14 |
| Mg | 0.2 | 0.6 | 0.3 | - |
| O | 58.8 | 59.1 | 59.5 | 59 |
| Configuration | Content, mol.% | |
|---|---|---|
| 2AMB | 1AMB | |
| Orthophosphate | 9.4 | 4.5 |
| Pyrophosphate | 48.2 | 41.7 |
| Metaphisphate | 42.4 | 53.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorokhovsky, A.; Burmistrov, I.; Kuznetsov, D.; Gusev, A.; Khaydarov, B.; Kiselev, N.; Boychenko, E.; Kolesnikov, E.; Prokopovich, K. Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives. Micromachines 2023, 14, 851. https://doi.org/10.3390/mi14040851
Gorokhovsky A, Burmistrov I, Kuznetsov D, Gusev A, Khaydarov B, Kiselev N, Boychenko E, Kolesnikov E, Prokopovich K. Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives. Micromachines. 2023; 14(4):851. https://doi.org/10.3390/mi14040851
Chicago/Turabian StyleGorokhovsky, Alexander, Igor Burmistrov, Denis Kuznetsov, Alexander Gusev, Bekzod Khaydarov, Nikolay Kiselev, Elena Boychenko, Evgeny Kolesnikov, and Ksenia Prokopovich. 2023. "Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives" Micromachines 14, no. 4: 851. https://doi.org/10.3390/mi14040851
APA StyleGorokhovsky, A., Burmistrov, I., Kuznetsov, D., Gusev, A., Khaydarov, B., Kiselev, N., Boychenko, E., Kolesnikov, E., & Prokopovich, K. (2023). Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives. Micromachines, 14(4), 851. https://doi.org/10.3390/mi14040851











