Swirl-like Acoustofluidic Stirring Facilitates Microscale Reactions in Sessile Droplets
Abstract
1. Introduction
2. Materials and Methods
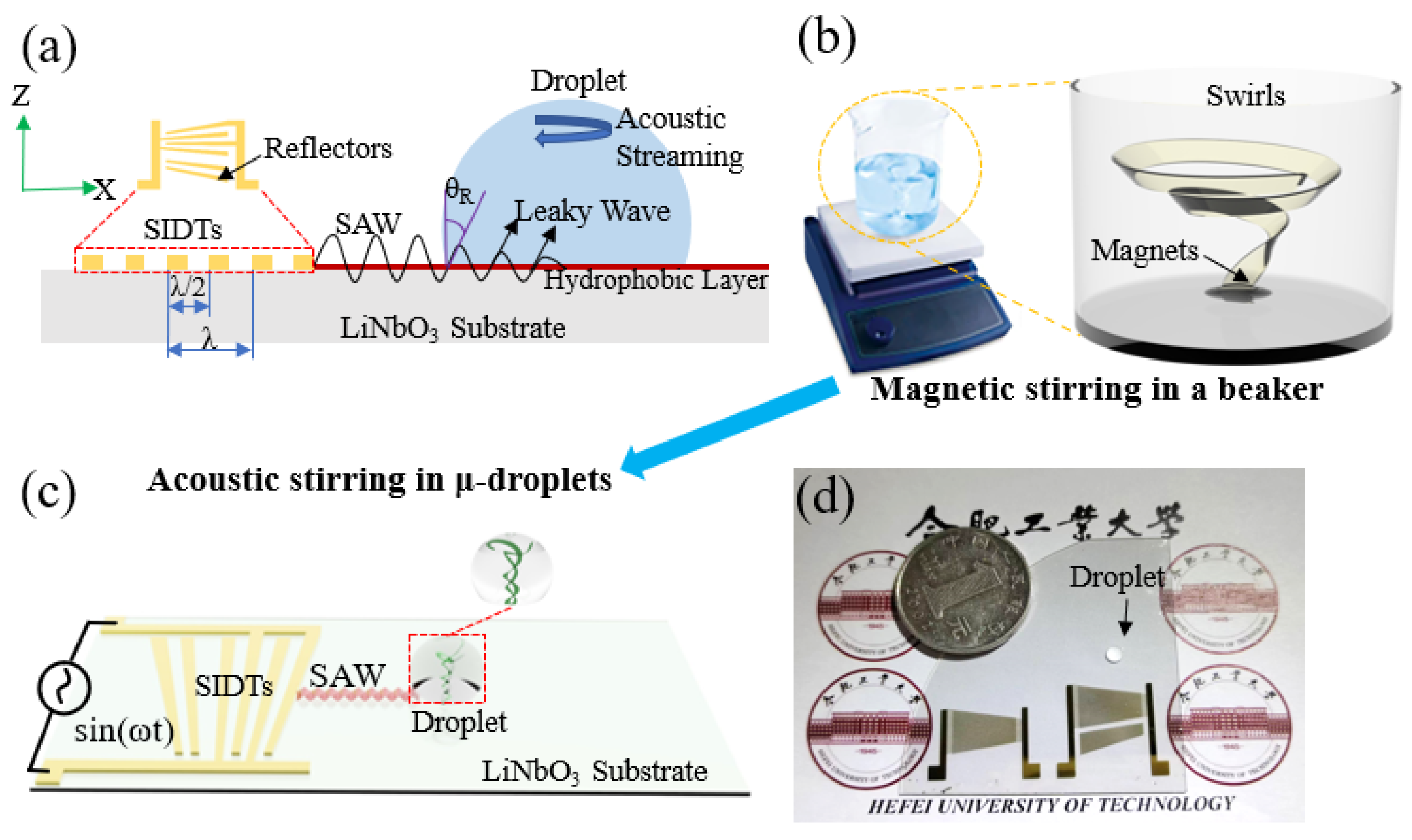
2.1. Acoustic Microdevices Fabrication
2.2. Experimental Setup
2.3. Sample Preparation
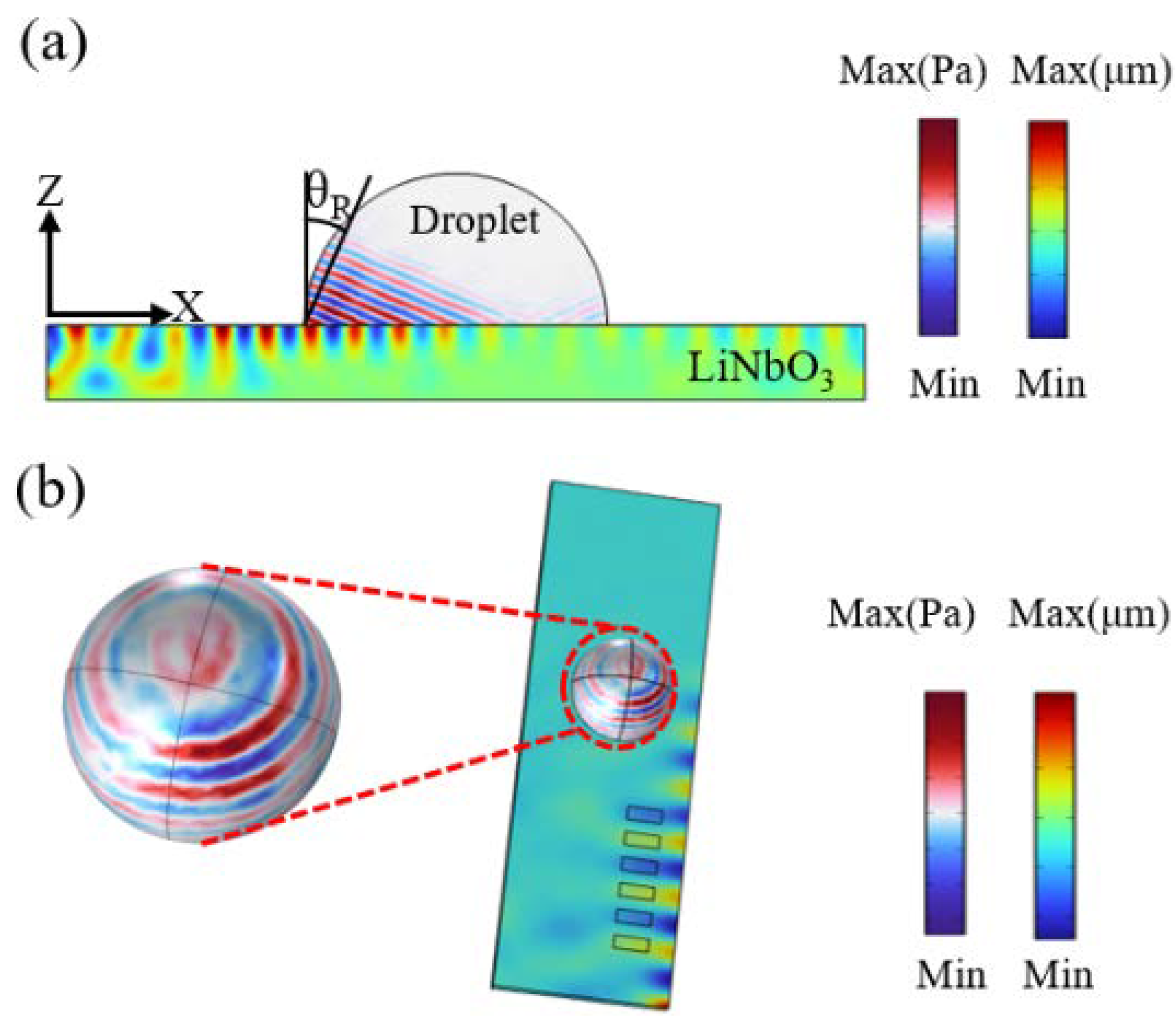
2.4. Numerical Simulation and Theoretical Analysis
3. Results
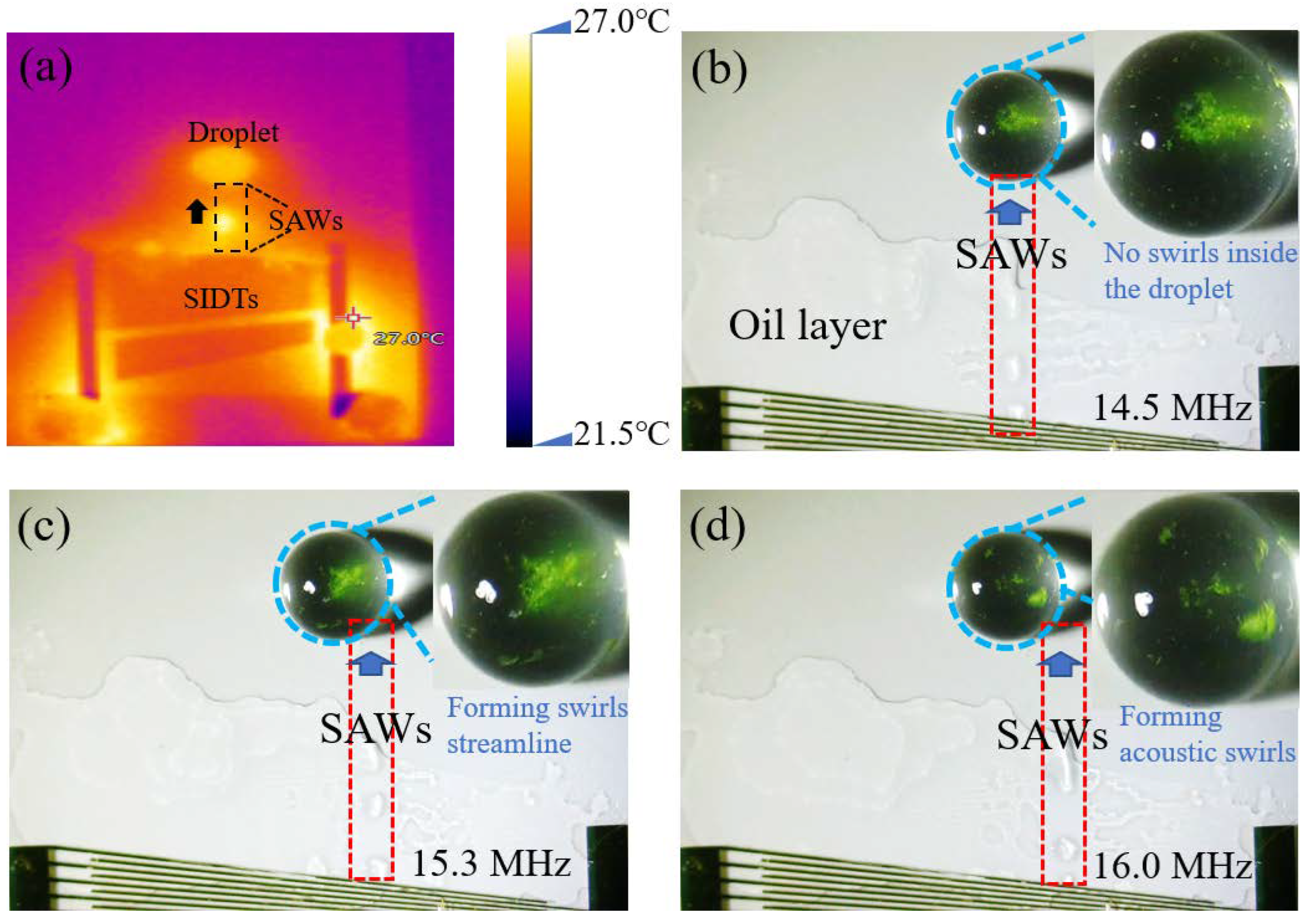
3.1. Optimization Analysis of the Location for SAWs to Form Acoustic Swirls in the Droplet
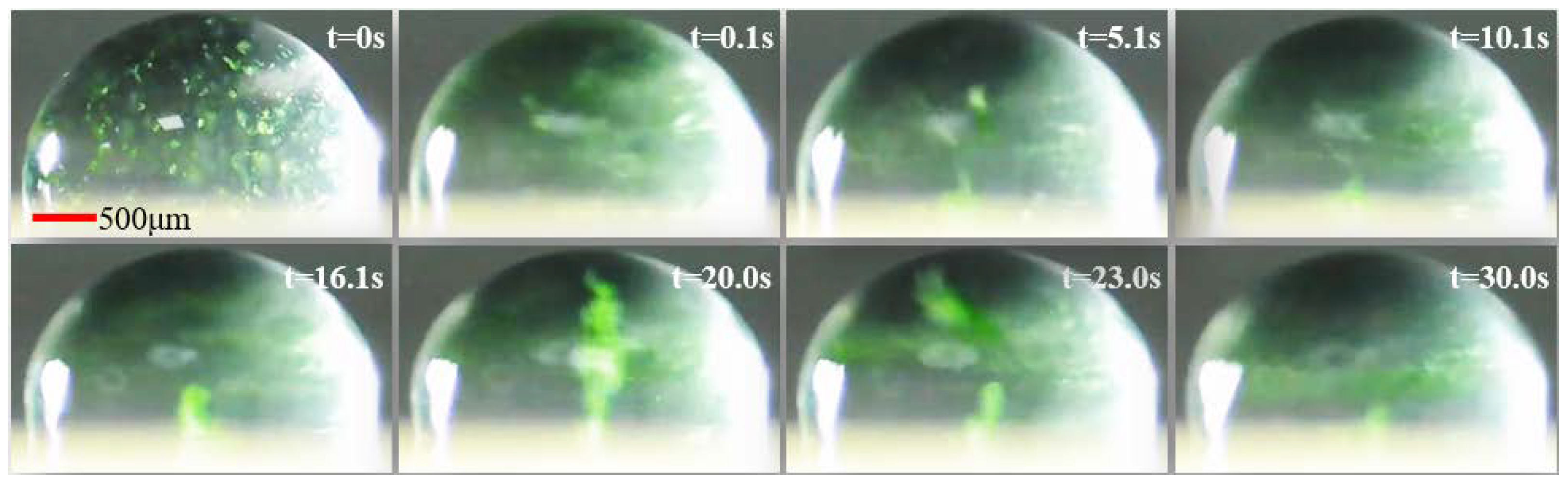
3.2. The 9 μm PS Particles in Droplet Driven by SAWs to Form Acoustic Swirls
3.3. Testing of the Stirring Abilities of Acoustic Swirls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Juang, D.S.; Lang, J.M.; Beebe, D.J. Volumeless reagent delivery: A liquid handling method for adding reagents to microscale droplets without increasing volume. Lab Chip 2022, 22, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Rich, J.; Hao, N.; Gu, Y.; Chen, C.; Yang, S.; Zhang, P.; Huang, T.J. Acoustofluidics for simultaneous nanoparticle-based drug loading and exosome encapsulation. Microsyst. Nanoeng. 2022, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- De Vlaminck, I.; Dekker, C. Recent advances in magnetic tweezers. Annu. Rev. Biophys. 2012, 41, 453–472. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, H.; Mei, D.; Xu, C.; Weng, W. Programmable motion control and trajectory manipulation of microparticles through tri-directional symmetrical acoustic tweezers. Lab Chip 2022, 22, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gunaseelan, M.; Vaippully, R.; Kumar, A.; Ajith, M.; Vaidya, G.; Dutta, S.; Roy, B. Pitch-rotational manipulation of single cells and particles using single-beam thermo-optical tweezers. Biomed. Opt. Express 2020, 11, 3555–3566. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Li, P.; Liu, D.; Kojima, M.; Huang, Q.; Arai, T. Efficient Single-Cell Mechanical Measurement by Integrating a Cell Arraying Microfluidic Device with Magnetic Tweezer. IEEE Robot. Autom. Lett. 2021, 6, 2978–2984. [Google Scholar] [CrossRef]
- Jung, J.H.; Destgeer, G.; Ha, B.; Park, J.; Sung, H.J. On-demand droplet splitting using surface acoustic waves. Lab Chip 2016, 16, 3235–3243. [Google Scholar] [CrossRef]
- Zhang, S.P.; Lata, J.; Chen, C.; Mai, J.; Guo, F.; Tian, Z.; Ren, L.; Mao, Z.; Huang, P.H.; Li, P.; et al. Digital acoustofluidics enables contactless and programmable liquid handling. Nat. Commun. 2018, 9, 2928. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.; Quan, A.; Qiu, C.; Cao, W.; Fu, C.; Fu, Y.Q. Highly selective and label-free Love-mode surface acoustic wave biosensor for carcinoembryonic antigen detection using a self-assembled monolayer bioreceptor. Appl. Surf. Sci. 2020, 518, 146061. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.L.; Fan, Z.H. Sessile droplets for chemical and biological assays. Lab Chip 2017, 17, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, C.; Niu, D.; Jiang, W.; Shi, Y.; Yin, L.; Chen, B.; Liu, H.; Ding, Y. Exploitation of surface acoustic waves to drive nanoparticle concentration within an electrification-dependent droplet. RSC Adv. 2014, 4, 46502–46507. [Google Scholar]
- Wiklund, M.; Green, R.; Ohlin, M. Acoustofluidics 14: Applications of acoustic streaming in microfluidic devices. Lab Chip 2012, 12, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, S.; Matsui, Y.; Ueda, T. Liquid streaming and droplet formation caused by leaky Rayleigh waves. In Proceedings of the IEEE Ultrasonics Symposium, Montreal, QC, Canada, 3–6 October 1989; pp. 643–646. [Google Scholar]
- Liu, Y.; Ji, M.; Yu, N.; Zhao, C.; Xue, G.; Fu, W.; Qiao, X.; Zhang, Y.; Chou, X.; Geng, W. Enhanced Detection in Droplet Microfluidics by Acoustic Vortex Modulation of Particle Rings and Particle Clusters via Asymmetric Propagation of Surface Acoustic Waves. Biosensors 2022, 12, 399. [Google Scholar] [CrossRef]
- Habibi, R.; Neild, A. Sound wave activated nano-sieve (SWANS) for enrichment of nanoparticles. Lab Chip 2019, 19, 3032–3044. [Google Scholar] [CrossRef] [PubMed]
- Akther, A.; Marqus, S.; Rezk, A.R.; Yeo, L.Y. Submicron Particle and Cell Concentration in a Closed Chamber Surface Acoustic Wave Microcentrifuge. Anal. Chem. 2020, 92, 10024–10032. [Google Scholar] [CrossRef]
- Rogers, P.R.; Friend, J.R.; Yeo, L.Y. Exploitation of surface acoustic waves to drive size-dependent microparticle concentration within a droplet. Lab Chip 2010, 10, 2979–2985. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, C.; Rufo, J.; Shen, C.; Huang, T.J. Acoustofluidic Holography for Micro- to Nanoscale Particle Manipulation. ACS Nano 2020, 14, 14635–14645. [Google Scholar] [CrossRef]
- Reboud, J.; Bourquin, Y.; Wilson, R.; Pall, G.S.; Jiwaji, M.; Pitt, A.R.; Graham, A.; Waters, A.P.; Cooper, J.M. Shaping acoustic fields as a toolset for microfluidic manipulations in diagnostic technologies. Proc. Natl. Acad. Sci. USA 2012, 109, 15162–15167. [Google Scholar] [CrossRef]
- Shilton, R.J.; Travagliati, M.; Beltram, F.; Cecchini, M. Nanoliter-droplet acoustic streaming via ultra high frequency surface acoustic waves. Adv. Mater. 2014, 26, 4941–4946. [Google Scholar] [CrossRef]
- Maramizonouz, S.; Tao, X.; Rahmati, M.; Jia, C.; Tao, R.; Torun, H.; Zheng, T.; Jin, H.; Dong, S.; Luo, J.; et al. Flexible and bendable acoustofluidics for particle and cell patterning. Int. J. Mech. Sci. 2021, 202–203, 106536. [Google Scholar] [CrossRef]
- Han, J.L.; Hu, H.; Huang, Q.Y.; Lei, Y.L. Particle separation by standing surface acoustic waves inside a sessile droplet. Sens. Actuat. A-Physl. 2021, 326, 112731. [Google Scholar] [CrossRef]
- Alghane, M.; Chen, B.X.; Fu, Y.Q.; Li, Y.; Luo, J.K.; Walton, A.J. Experimental and numerical investigation of acoustic streaming excited by using a surface acoustic wave device on a 128° YX-LiNbO3 substrate. J. Micromech. Microeng. 2011, 21, 015005. [Google Scholar] [CrossRef]
- Wilson, R.; Reboud, J.; Bourquin, Y.; Neale, S.L.; Zhang, Y.; Cooper, J.M. Phononic crystal structures for acoustically driven microfluidic manipulations. Lab Chip 2011, 11, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.C.; Lin, Y.D. Microparticle concentration and separation inside a droplet using phononic-crystal scattered standing surface acoustic waves. Sens. Actuat. A-Physl. 2019, 300, 111651. [Google Scholar] [CrossRef]
- Destgeer, G.; Cho, H.; Ha, B.H.; Jung, J.H.; Park, J.; Sung, H.J. Acousticfluidic particle manipulation inside a sessile droplet: Four distinct regimes of particle concentration. Lab Chip 2016, 16, 660–667. [Google Scholar] [CrossRef]
- Bourquin, Y.; Reboud, J.; Wilson, R.; Cooper, J.M. Tuneable surface acoustic waves for fluid and particle manipulations on disposable chips. Lab Chip 2010, 10, 1898–1901. [Google Scholar] [CrossRef]
- Bourquin, Y.; Reboud, J.; Wilson, R.; Zhang, Y.; Cooper, J.M. Integrated immunoassay using tuneable surface acoustic waves and lensfree detection. Lab Chip 2011, 11, 2725–2730. [Google Scholar] [CrossRef]
- Shiokawa, S.; Matsui, Y.; Ueda, T. Study on SAW streaming and its application to fluid devices. Jpn. J. Appl. Phys. 1990, 29, 137–139. [Google Scholar] [CrossRef]
- Riaud, A.; Baudoin, M.; Bou Matar, O.; Thomas, J.L.; Brunet, P. On the influence of viscosity and caustics on acoustic streaming in sessile droplets: An experimental and a numerical study with a cost-effective method. J. Fluid Mech. 2017, 821, 384–420. [Google Scholar] [CrossRef]
- Arifin, D.R.; Yeo, L.Y.; Friend, J.R. Microfluidic blood plasma separation via bulk electrohydrodynamic flows. Biomicrofluidics 2007, 1, 014103. [Google Scholar] [CrossRef] [PubMed]
- García-Merino, J.A.; Torres-Torres, D.; Carrillo-Delgado, C.; Trejo-Valdez, M.; Torres-Torres, C. Optofluidic and strain measurements induced by polarization-resolved nanosecond pulses in gold-based nanofluids. Optik 2019, 182, 443–451. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, H.; Qian, J.; Liu, Y.; Lu, S.; Zhang, B.; Huang, L.; Hu, X.; Zhang, W. Swirl-like Acoustofluidic Stirring Facilitates Microscale Reactions in Sessile Droplets. Micromachines 2023, 14, 837. https://doi.org/10.3390/mi14040837
Lan H, Qian J, Liu Y, Lu S, Zhang B, Huang L, Hu X, Zhang W. Swirl-like Acoustofluidic Stirring Facilitates Microscale Reactions in Sessile Droplets. Micromachines. 2023; 14(4):837. https://doi.org/10.3390/mi14040837
Chicago/Turabian StyleLan, Huaize, Jingui Qian, Yansong Liu, Shanshan Lu, Bowei Zhang, Liang Huang, Xuefeng Hu, and Wei Zhang. 2023. "Swirl-like Acoustofluidic Stirring Facilitates Microscale Reactions in Sessile Droplets" Micromachines 14, no. 4: 837. https://doi.org/10.3390/mi14040837
APA StyleLan, H., Qian, J., Liu, Y., Lu, S., Zhang, B., Huang, L., Hu, X., & Zhang, W. (2023). Swirl-like Acoustofluidic Stirring Facilitates Microscale Reactions in Sessile Droplets. Micromachines, 14(4), 837. https://doi.org/10.3390/mi14040837





