Gas-Sensitive Properties of ZnO/Ti2CTx Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Application
2.2. Instrumentation
3. Results and Discussion
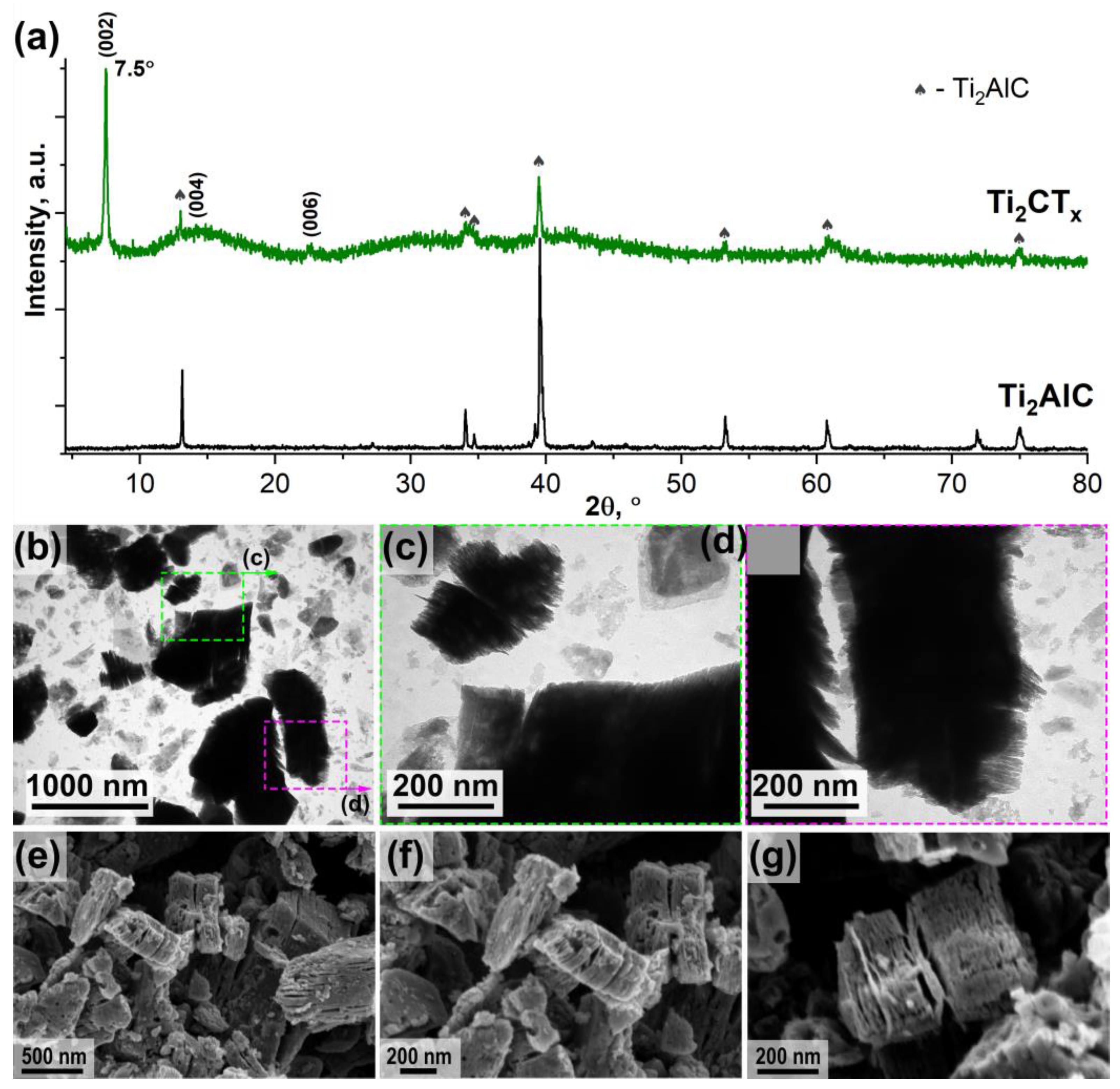
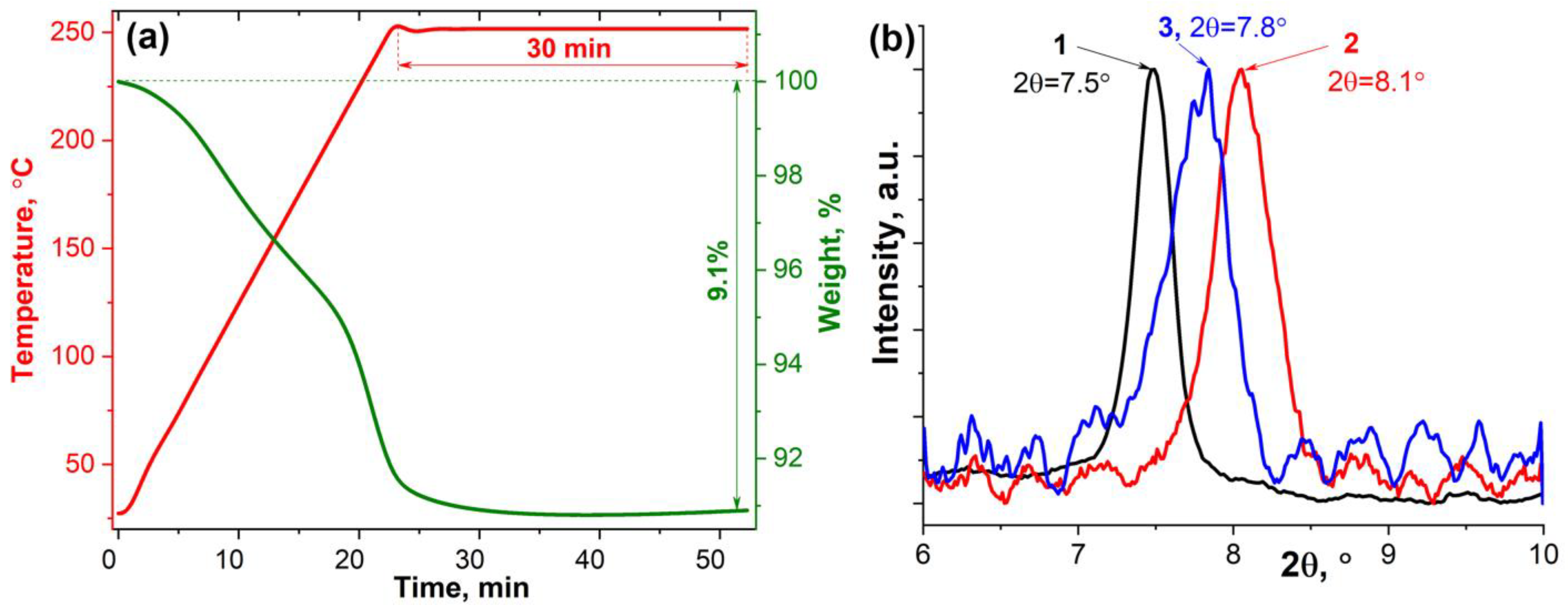
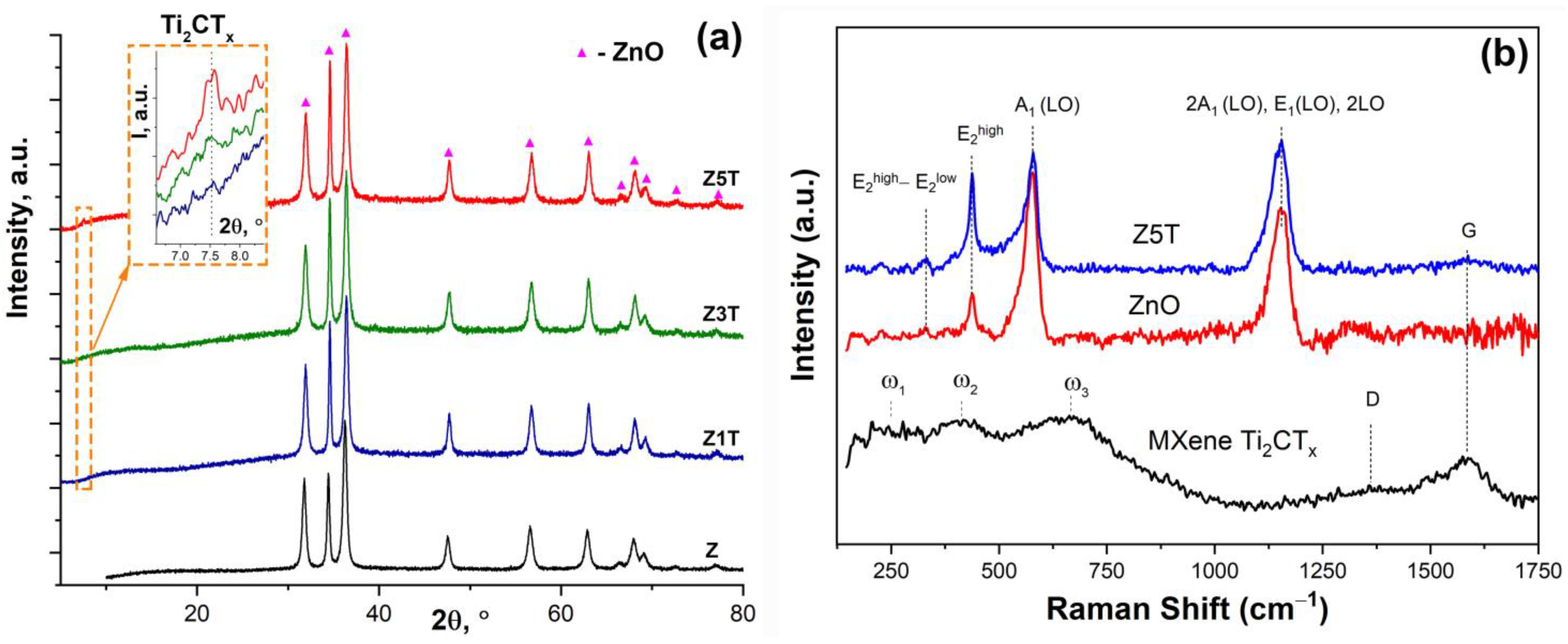
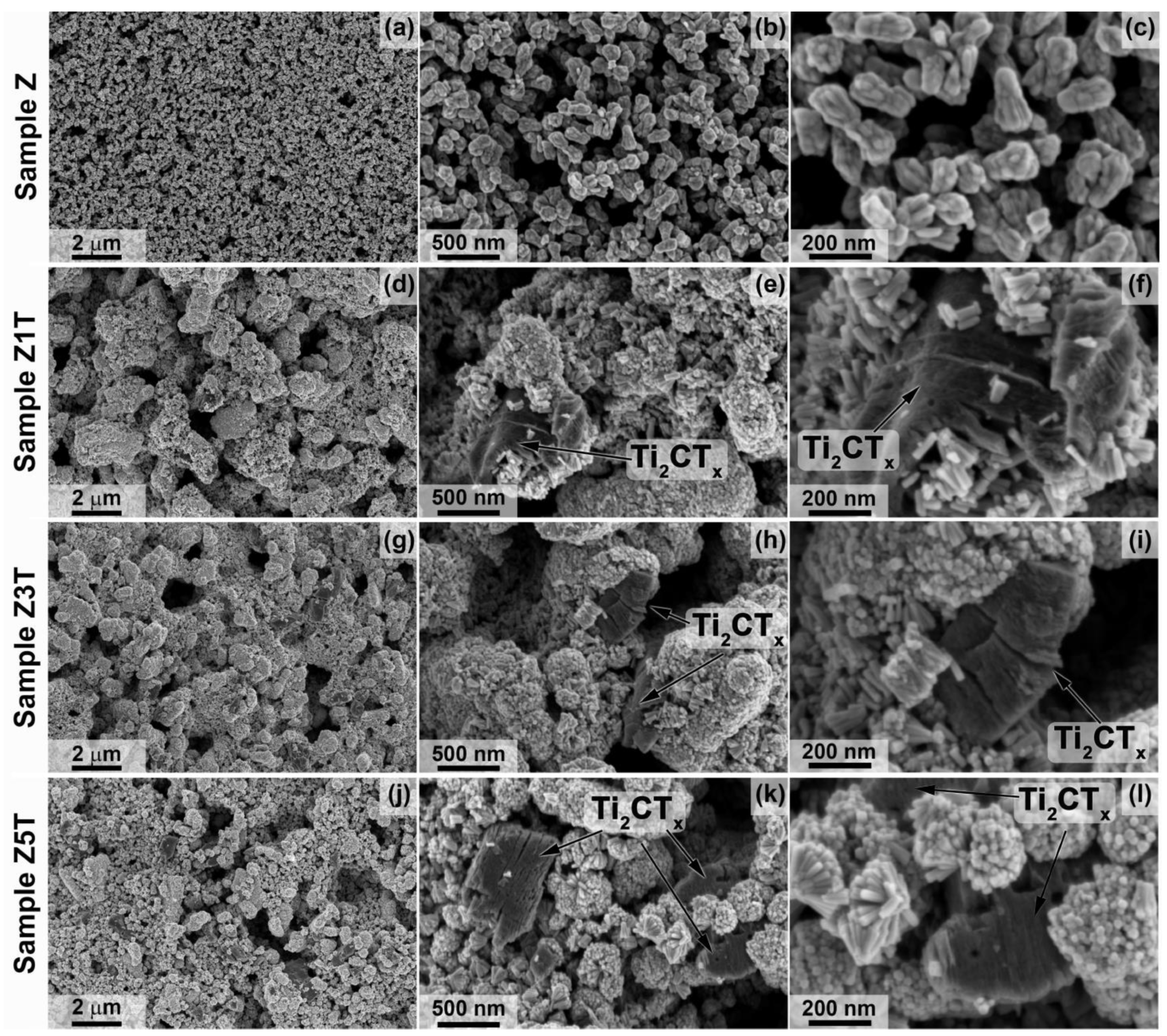
3.1. Synthesis and Investigation of Ti2CTx MXene Powder and ZnO/Ti2CTx Composite Powders
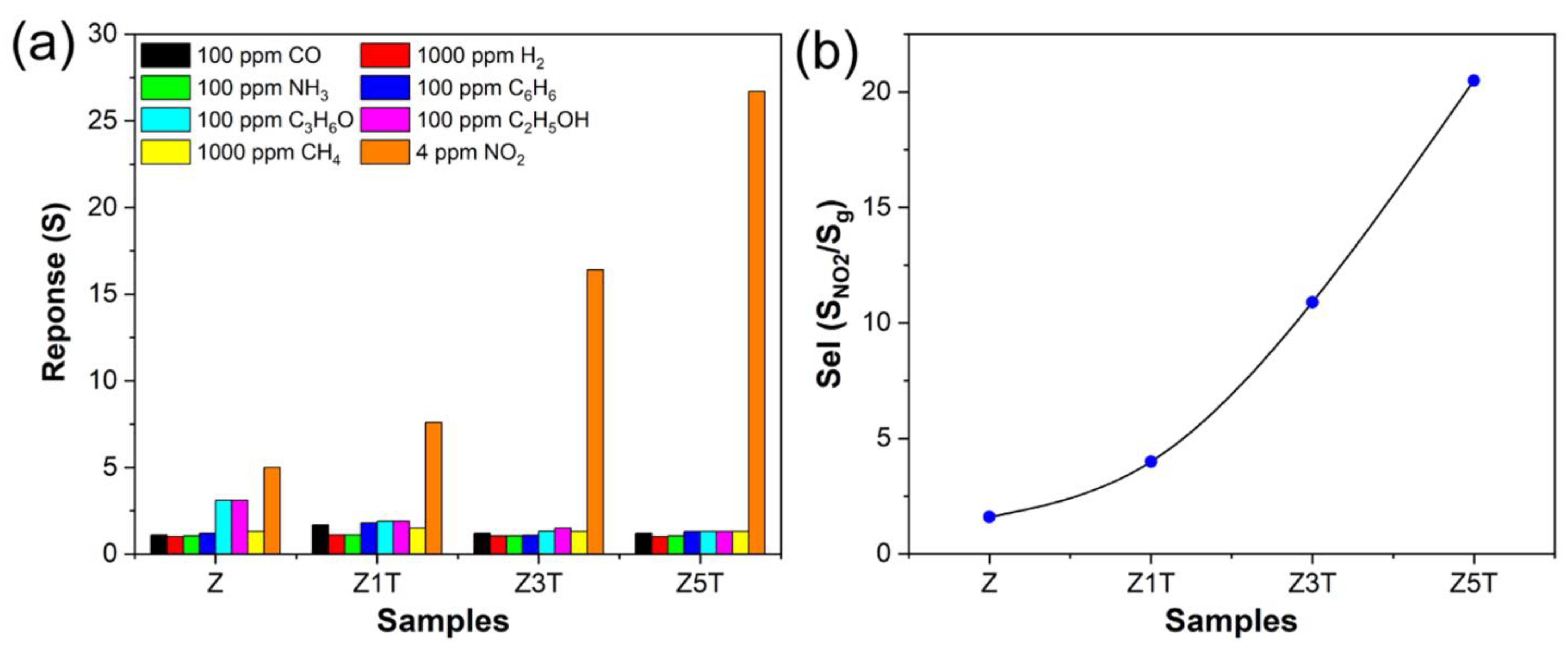
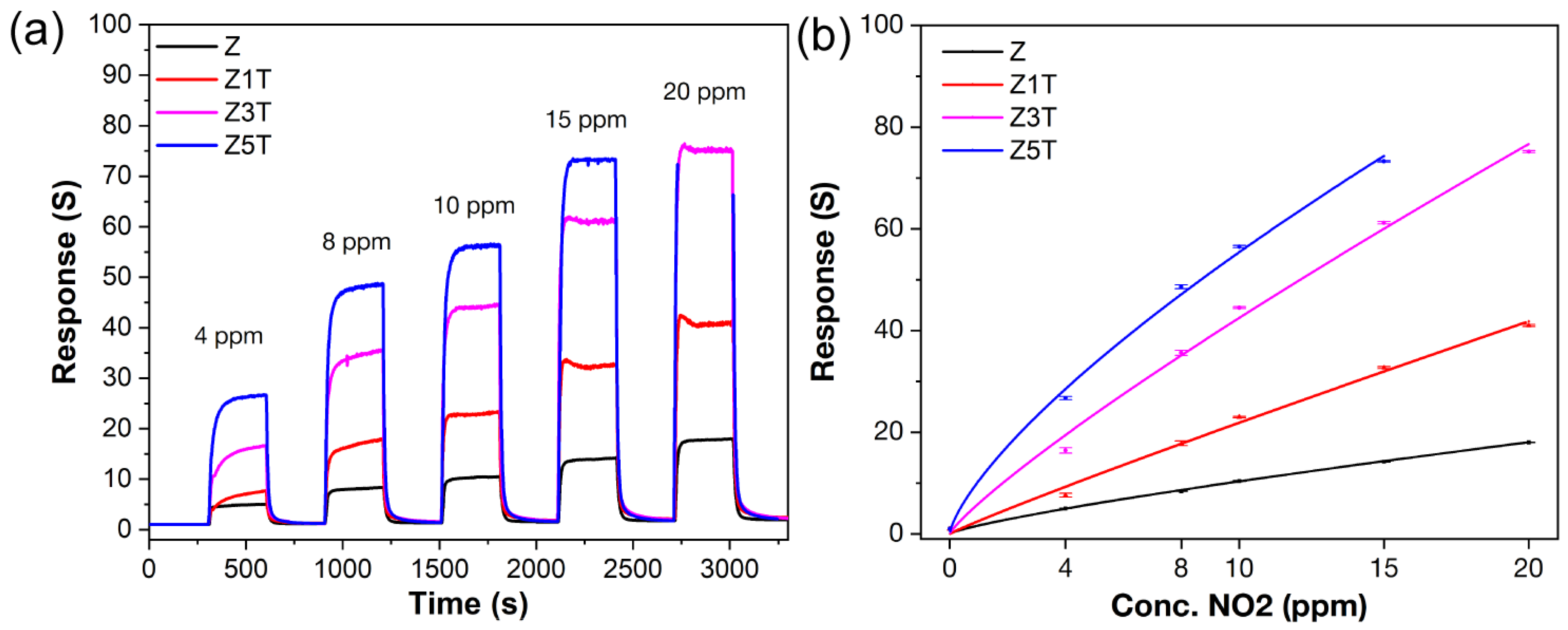
3.2. Chemoresistance Properties of ZnO/Ti2CTx Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhall, S.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Wilson, A. Advances in Electronic-Nose Technologies for the Detection of Volatile Biomarker Metabolites in the Human Breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Solovieva, S.; Karnaukh, M.; Panchuk, V.; Andreev, E.; Kartsova, L.; Bessonova, E.; Legin, A.; Wang, P.; Wan, H.; Jahatspanian, I.; et al. Potentiometric multisensor system as a possible simple tool for non-invasive prostate cancer diagnostics through urine analysis. Sens. Actuators B Chem. 2019, 289, 42–47. [Google Scholar] [CrossRef]
- Deisingh, A.K.; Stone, D.C.; Thompson, M. Applications of electronic noses and tongues in food analysis. Int. J. Food Sci. Technol. 2004, 39, 587–604. [Google Scholar] [CrossRef]
- Devabharathi, N.; Umarji, A.M.; Dasgupta, S. Fully Inkjet-Printed Mesoporous SnO2-Based Ultrasensitive Gas Sensors for Trace Amount NO2 Detection. ACS Appl. Mater. Interfaces 2020, 12, 57207–57217. [Google Scholar] [CrossRef]
- Thai, N.X.; Tonezzer, M.; Masera, L.; Nguyen, H.; Van Duy, N.; Hoa, N.D. Multi gas sensors using one nanomaterial, temperature gradient, and machine learning algorithms for discrimination of gases and their concentration. Anal. Chim. Acta 2020, 1124, 85–93. [Google Scholar] [CrossRef]
- Fedorov, F.S.; Simonenko, N.P.; Trouillet, V.; Volkov, I.A.; Plugin, I.A.; Rupasov, D.P.; Mokrushin, A.S.; Nagornov, I.A.; Simonenko, T.L.; Vlasov, I.S.; et al. Microplotter-Printed On-Chip Combinatorial Library of Ink-Derived Multiple Metal Oxides as an “Electronic Olfaction” Unit. ACS Appl. Mater. Interfaces 2020, 12, 56135–56150. [Google Scholar] [CrossRef]
- Goikhman, B.V.; Fedorov, F.S.; Simonenko, N.P.; Simonenko, T.L.; Fisenko, N.A.; Dubinina, T.S.; Ovchinnikov, G.; Lantsberg, A.V.; Lipatov, A.; Simonenko, E.P.; et al. Quantum of selectivity testing: Detection of isomers and close homologs using an AZO based e-nose without a prior training. J. Mater. Chem. A 2022, 10, 8413–8423. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, J.S.; Lee, J.H. Rational Design of Semiconductor-Based Chemiresistors and their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Arkhipushkin, I.A.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Gas-sensitive nanostructured ZnO films praseodymium and europium doped: Electrical conductivity, selectivity, influence of UV irradiation and humidity. Appl. Surf. Sci. 2022, 589, 152974. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Filatova, D.; Spiridonov, F.; Zaytsev, V.; Zaytseva, A.; Gaskov, A. Highly Sensitive ZnO(Ga, In) for Sub-ppm Level NO2 Detection: Effect of Indium Content. Chemosensors 2017, 5, 18. [Google Scholar] [CrossRef]
- Nagornov, I.A.; Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Gorobtsov, P.Y.; Sevastyanov, V.G.; Kuznetsov, N.T. Zinc oxide obtained by the solvothermal method with high sensitivity and selectivity to nitrogen dioxide. Ceram. Int. 2020, 46, 7756–7766. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Vladimirova, S.A.; Platonov, V.B.; Chizhov, A.S.; Batuk, M.; Hadermann, J.; Khmelevsky, N.O.; Gaskov, A.M. Sub-ppm H2S sensing by tubular ZnO-Co3O4 nanofibers. Sens. Actuators B Chem. 2020, 307, 127624. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; Yang, S.; Zhu, S.; Chen, X.; Dong, B.; Bai, X.; Wen, X.; Geyu, L.; Song, H. Highly dispersed Metal–Organic-Framework-Derived Pt nanoparticles on three-dimensional macroporous ZnO for trace-level H2S sensing. Sens. Actuators B Chem. 2020, 309, 127802. [Google Scholar] [CrossRef]
- Li, Z.; Yi, J. Drastically Enhanced Ammonia Sensing of Pt/ZnO Ordered Porous Ultra-Thin Films. Sens. Actuators B Chem. 2020, 317, 128217. [Google Scholar] [CrossRef]
- Shingange, K.; Tshabalala, Z.P.; Ntwaeaborwa, O.M.; Motaung, D.E.; Mhlongo, G.H. Highly selective NH3 gas sensor based on Au loaded ZnO nanostructures prepared using microwave-assisted method. J. Colloid Interface Sci. 2016, 479, 127–138. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Khamova, T.V.; Kopitsa, G.P.; Evzrezov, A.N.; Simonenko, E.P.; Sevastyanov, V.G.; et al. Chemoresistive gas-sensitive ZnO/Pt nanocomposites films applied by microplotter printing with increased sensitivity to benzene and hydrogen. Mater. Sci. Eng. B 2021, 271, 115233. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Gorban, Y.M.; Simonenko, T.L.; Simonenko, N.P.; Arkhipushkin, I.A.; Simonenko, E.P.; Kuznetsov, N.T. Effect of platinum nanoparticles on the chemoresistive gas sensitive properties of the ZnO/Pt composite. Ceram. Int. 2023. [Google Scholar] [CrossRef]
- Du, Y.; Gao, S.; Mao, Z.; Zhang, C.; Zhao, Q.; Zhang, S. Aerobic and anaerobic H2 sensing sensors fabricated by diffusion membranes depositing on Pt-ZnO film. Sens. Actuators B Chem. 2017, 252, 239–250. [Google Scholar] [CrossRef]
- Hassan, K.; Uddin, A.S.M.I.; Ullah, F.; Kim, Y.S.; Chung, G.-S. Platinum/palladium bimetallic ultra-thin film decorated on a one-dimensional ZnO nanorods array for use as fast response flexible hydrogen sensor. Mater. Lett. 2016, 176, 232–236. [Google Scholar] [CrossRef]
- Li, W.; Ma, S.; Li, Y.; Yang, G.; Mao, Y.; Luo, J.; Gengzang, D.; Xu, X.; Yan, S. Enhanced ethanol sensing performance of hollow ZnO–SnO2 core–shell nanofibers. Sens. Actuators B Chem. 2015, 211, 392–402. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Shao, C.; Li, X.; Ma, J.; Zhang, X.; Liu, Y. Composition-controllable p-CuO/n-ZnO hollow nanofibers for high-performance H2S detection. Sens. Actuators B Chem. 2019, 285, 495–503. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Fang, Y.; Wang, J.; Che, Q.; Wang, G.; Yang, P. Novel Construction of Morphology-Tunable C–N/SnO2/ZnO/Au Microspheres with Ultrasensitivity and High Selectivity for Triethylamine under Various Temperature Detections. ACS Appl. Mater. Interfaces 2019, 11, 8601–8611. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Co3O4-loaded ZnO nanofibers for excellent hydrogen sensing. Int. J. Hydrog. Energy 2019, 44, 27499–27510. [Google Scholar] [CrossRef]
- Zhou, Q.; Hong, C.; Li, Z.; Peng, S.; Wu, G.; Wang, Q.; Zhang, Q.; Xu, L. Facile Hydrothermal Synthesis and Enhanced Methane Sensing Properties of Pt-Decorated ZnO Nanosheets. J. Nanosci. Nanotechnol. 2018, 18, 3335–3340. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, X.; Chen, J.; Li, W.; Han, N.; Zhang, D.; Chen, Y. Enhanced gas-sensing performance of metal@ZnO core–shell nanoparticles towards ppb–ppm level benzene: The role of metal–ZnO hetero-interfaces. New J. Chem. 2019, 43, 2220–2230. [Google Scholar] [CrossRef]
- Wang, H.T.; Kang, B.S.; Ren, F.; Tien, L.C.; Sadik, P.W.; Norton, D.P.; Pearton, S.J.; Lin, J. Hydrogen-selective sensing at room temperature with ZnO nanorods. Appl. Phys. Lett. 2005, 86, 243503. [Google Scholar] [CrossRef]
- Van Tran, T.; Ahemad, M.J.; Kim, D.-S.; Le, T.D.; Dao, V.; Yu, Y.-T. Ultra high response for hydrogen gas on Pd–Augr-alloy@ZnO core-shell nanoparticles with Pd–Au gradient composition alloy core. Mater. Today Nano 2023, 21, 100292. [Google Scholar] [CrossRef]
- Anjitha, R.G.; Basu, P.K. Dual Activation and Photocatalyst-Induced Selective Detection in Defect Tuned Pd–ZnO Thin Films for Low ppm Detection of CH and CO. IEEE Sens. J. 2023, 23, 143–153. [Google Scholar] [CrossRef]
- Schütt, F.; Postica, V.; Adelung, R.; Lupan, O. Single and Networked ZnO–CNT Hybrid Tetrapods for Selective Room-Temperature High-Performance Ammonia Sensors. ACS Appl. Mater. Interfaces 2017, 9, 23107–23118. [Google Scholar] [CrossRef]
- Sinha, M.; Neogi, S.; Mahapatra, R.; Krishnamurthy, S.; Ghosh, R. Material dependent and temperature driven adsorption switching (p- to n- type) using CNT/ZnO composite-based chemiresistive methanol gas sensor. Sens. Actuators B Chem. 2021, 336, 129729. [Google Scholar] [CrossRef]
- Singh, G.; Choudhary, A.; Haranath, D.; Joshi, A.G.; Singh, N.; Singh, S.; Pasricha, R. ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon N. Y. 2012, 50, 385–394. [Google Scholar] [CrossRef]
- Anand, K.; Singh, O.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen sensor based on graphene/ZnO nanocomposite. Sens. Actuators B Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Hussein, H.T.; Kareem, M.H.; Abdul Hussein, A.M. Synthesis and characterization of carbon nanotube doped with zinc oxide nanoparticles CNTs-ZnO/PS as ethanol gas sensor. Optik 2021, 248, 168107. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Glukhova, O.E.; Plugin, I.A.; Kolosov, D.A.; Nagornov, I.A.; Simonenko, T.L.; Varezhnikov, A.S.; Simonenko, E.P.; Sysoev, V.V.; Kuznetsov, N.T. The Ti0.2V1.8C MXene Ink-Prepared Chemiresistor: From Theory to Tests with Humidity versus VOCs. Chemosensors 2022, 11, 7. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Nagornov, I.A.; Simonenko, T.L.; Gorobtsov, P.Y.; Mokrushin, A.S.; Kuznetsov, N.T. Synthesis and Chemoresistive Properties of Single-Layer MXene Ti2CTx. Russ. J. Inorg. Chem. 2022, 67, 1850–1859. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Averin, A.A.; Simonenko, T.L.; Simonenko, N.P.; Simonenko, E.P.; Kuznetsov, N.T. Chemoresistive Properties of V2CTx MXene and the V2CTx/V3O7 Nanocomposite Based on It. Chemosensors 2023, 11, 142. [Google Scholar] [CrossRef]
- Kedambaimoole, V.; Harsh, K.; Rajanna, K.; Sen, P.; Nayak, M.M.; Kumar, S. MXene wearables: Properties, fabrication strategies, sensing mechanism and applications. Mater. Adv. 2022, 3, 3784–3808. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Gorobtsov, P.Y.; Averin, A.A.; Simonenko, T.L.; Simonenko, N.P.; Simonenko, E.P.; Kuznetsov, N.T. Effect of Ti2CTx MXene Oxidation on Its Gas-Sensitive Properties. Chemosensors 2022, 11, 13. [Google Scholar] [CrossRef]
- Peng, B.; Huang, X. Research status of gas sensing performance of Ti3C2Tx-based gas sensors: A mini review. Front. Chem. 2022, 10, 1037732. [Google Scholar] [CrossRef] [PubMed]
- Pazniak, H.; Varezhnikov, A.S.; Kolosov, D.A.; Plugin, I.A.; Di Vito, A.; Glukhova, O.E.; Sheverdyaeva, P.M.; Spasova, M.; Kaikov, I.; Kolesnikov, E.A.; et al. 2D Molybdenum Carbide MXenes for Enhanced Selective Detection of Humidity in Air. Adv. Mater. 2021, 33, 2104878. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, M.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M. A review on MXene and its nanocomposites for the detection of toxic inorganic gases. Chemosphere 2022, 302, 134933. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T. MXenes as emerging two-dimensional analytical modalities for potential recognition of hazardous environmental contaminants. Mater. Today Chem. 2022, 24, 100859. [Google Scholar] [CrossRef]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.-Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef]
- Nahirniak, S.; Saruhan, B. MXene Heterostructures as Perspective Materials for Gas Sensing Applications. Sensors 2022, 22, 972. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Mokrushin, A.S.; Simonenko, T.L.; Gorobtsov, P.Y.; Nagornov, I.A.; Korotcenkov, G.; Sysoev, V.V.; Kuznetsov, N.T. Application of Titanium Carbide MXenes in Chemiresistive Gas Sensors. Nanomaterials 2023, 13, 850. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, J.; Wang, X.; Dai, J.; Wang, X.; Fan, H.; Wang, Z.; Li, H.; Huang, X.; Huang, W. Treatment-dependent surface chemistry and gas sensing behavior of the thinnest member of titanium carbide MXenes. Nanoscale 2020, 12, 16987–16994. [Google Scholar] [CrossRef]
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sens. 2019, 4, 2763–2770. [Google Scholar] [CrossRef]
- Li, X.; An, Z.; Lu, Y.; Shan, J.; Xing, H.; Liu, G.; Shi, Z.; He, Y.; Chen, Q.; Han, R.P.S.; et al. Room Temperature VOCs Sensing with Termination-Modified Ti3C2Tx MXene for Wearable Exhaled Breath Monitoring. Adv. Mater. Technol. 2022, 7, 2100872. [Google Scholar] [CrossRef]
- Majhi, S.M.; Ali, A.; Greish, Y.E.; El-Maghraby, H.F.; Qamhieh, N.N.; Hajamohideen, A.R.; Mahmoud, S.T. Accordion-like-Ti3C2 MXene-Based Gas Sensors with Sub-ppm Level Detection of Acetone at Room Temperature. ACS Appl. Electron. Mater. 2022, 4, 4094–4103. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Xia, Y.; Komarneni, S. Ti2CTx MXene: A novel p-type sensing material for visible light-enhanced room temperature methane detection. Ceram. Int. 2021, 47, 34437–34442. [Google Scholar] [CrossRef]
- Yao, L.; Tian, X.; Cui, X.; Zhao, R.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Two-dimensional Ti3C2Tx MXene/SnO nanocomposites: Towards enhanced response and selective ammonia vapor sensor at room temperature. Sens. Actuators B Chem. 2022, 358, 131501. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Lee, E.; VahidMohammadi, A.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.-J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Lee, J.; Kang, Y.C.; Koo, C.M.; Kim, S.J. Ti3C2Tx MXene Nanolaminates with Ionic Additives for Enhanced Gas-Sensing Performance. ACS Appl. Nano Mater. 2022, 5, 11997–12005. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Xia, Y. Mesoporous MXene/ZnO nanorod hybrids of high surface area for UV-activated NO2 gas sensing in ppb-level. Sens. Actuators B Chem. 2022, 353, 131087. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Song, Y.; Shen, T.; Sun, J. Facile solvothermal synthesis of ZnO/Ti3C2Tx MXene nanocomposites for NO2 detection at low working temperature. Sens. Actuators B Chem. 2022, 367, 132025. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Y.; Wu, D.; Jiang, G. Preparation and gas sensing properties of ZnO/MXene composite nanomaterials. Sens. Actuators A Phys. 2022, 344, 113740. [Google Scholar] [CrossRef]
- Bu, X.; Wu, Q.; Yuan, Y.; Wu, H.; Liu, W.; Li, X.; Han, C. The highly sensitive ethanol sensor based on nanocomposites of ZnO in situ grown on 2D Ti3C2Tx nanosheet. Nanotechnology 2023, 34, 105707. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, L.; Wang, J.; Liu, F.; He, J.; Liu, A.; Lv, S.; You, R.; Yan, X.; Sun, P.; et al. Flexible resistive NO2 gas sensor of three-dimensional crumpled MXene Ti3C2Tx/ZnO spheres for room temperature application. Sens. Actuators B Chem. 2021, 326, 128828. [Google Scholar] [CrossRef]
- Fan, C.; Shi, J.; Zhang, Y.; Quan, W.; Chen, X.; Yang, J.; Zeng, M.; Zhou, Z.; Su, Y.; Wei, H.; et al. Fast and recoverable NO2 detection achieved by assembling ZnO on Ti3C2Tx MXene nanosheets under UV illumination at room temperature. Nanoscale 2022, 14, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Simonenko, E.P.; Simonenko, N.P.; Nagornov, I.A.; Simonenko, T.L.; Mokrushin, A.S.; Sevastyanov, V.G.; Kuznetsov, N.T. Synthesis of MAX Phases in the Ti2AlC–V2AlC System as Precursors of Heterometallic MXenes Ti2–xVxC. Russ. J. Inorg. Chem. 2022, 67, 705–714. [Google Scholar] [CrossRef]
- Nagornov, I.A.; Mokrushin, A.S.; Simonenko, E.P.; Simonenko, N.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Liquid-Phase Growth of Nanocrystalline ZnO Thin Films and Their Gas-Sensitive Properties. Russ. J. Inorg. Chem. 2022, 67, 539–546. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Averin, A.A.; Simonenko, N.P.; Simonenko, T.L.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Effect of the Addition of Cerium Acetylacetonate on the Synthesis of ZnO Nanopowder. Russ. J. Inorg. Chem. 2021, 66, 638–644. [Google Scholar] [CrossRef]
- Simonenko, N.P.; Fisenko, N.A.; Fedorov, F.S.; Simonenko, T.L.; Mokrushin, A.S.; Simonenko, E.P.; Korotcenkov, G.; Sysoev, V.V.; Sevastyanov, V.G.; Kuznetsov, N.T. Printing Technologies as an Emerging Approach in Gas Sensors: Survey of Literature. Sensors 2022, 22, 3473. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Simonenko, E.P.; Vlasov, I.S.; Volkov, I.A.; Kuznetsov, N.T. Microplotter Printing of Hierarchically Organized Planar NiCo2O4 Nanostructures. Russ. J. Inorg. Chem. 2022, 67, 1848–1854. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Simonenko, E.P.; Kuznetsov, N.T. Microextrusion Printing of Multilayer Hierarchically Organized Planar Nanostructures Based on NiO, (CeO2)0.8(Sm2O3)0.2 and La0.6Sr0.4Co0.2Fe0.8O3−δ. Micromachines 2022, 14, 3. [Google Scholar] [CrossRef]
- Koh, H.J.; Kim, S.J.; Maleski, K.; Cho, S.Y.; Kim, Y.J.; Ahn, C.W.; Gogotsi, Y.; Jung, H.T. Enhanced Selectivity of MXene Gas Sensors through Metal Ion Intercalation: In Situ X-ray Diffraction Study. ACS Sens. 2019, 4, 1365–1372. [Google Scholar] [CrossRef]
- Güell, F.; Martínez-Alanis, P.R.; Khachadorian, S.; Rubio-García, J.; Franke, A.; Hoffmann, A.; Santana, G. Raman and photoluminescence properties of ZnO nanowires grown by a catalyst-free vapor-transport process using ZnO nanoparticle seeds. Phys. Status Solidi 2016, 253, 883–888. [Google Scholar] [CrossRef]
- Šćepanović, M.; Grujić-Brojčin, M.; Vojisavljević, K.; Bernik, S.; Srećković, T. Raman study of structural disorder in ZnO nanopowders. J. Raman Spectrosc. 2010, 41, 914–921. [Google Scholar] [CrossRef]
- Habib, I.; Ferrer, P.; Ray, S.C.; Ozoemena, K.I. Interrogating the impact of onion-like carbons on the supercapacitive properties of MXene (Ti2CTX). J. Appl. Phys. 2019, 126, 134301. [Google Scholar] [CrossRef]
- Lioi, D.B.; Neher, G.; Heckler, J.E.; Back, T.; Mehmood, F.; Nepal, D.; Pachter, R.; Vaia, R.; Kennedy, W.J. Electron-Withdrawing Effect of Native Terminal Groups on the Lattice Structure of Ti3C2Tx MXenes Studied by Resonance Raman Scattering: Implications for Embedding MXenes in Electronic Composites. ACS Appl. Nano Mater. 2019, 2, 6087–6091. [Google Scholar] [CrossRef]
- Melchior, S.A.; Raju, K.; Ike, I.S.; Erasmus, R.M.; Kabongo, G.; Sigalas, I.; Iyuke, S.E.; Ozoemena, K.I. High-Voltage Symmetric Supercapacitor Based on 2D Titanium Carbide (MXene, Ti2CTx)/Carbon Nanosphere Composites in a Neutral Aqueous Electrolyte. J. Electrochem. Soc. 2018, 165, A501–A511. [Google Scholar] [CrossRef]
- Nagornov, I.A.; Maeder, T.; Kuznetsov, N.T.; Mokrushin, A.S.; Simonenko, N.P.; Vasiliev, A.A.; Volkov, I.A.; Vlasov, I.S.; Gorobtsov, P.Y.; Simonenko, E.P.; et al. Nanocrystalline ZnO Obtained by the Thermal Decomposition of [Zn(H2O)(O2C5H7)2] in 1-Butanol: Synthesis and Testing as a Sensing Material. Russ. J. Inorg. Chem. 2018, 63, 1519–1528. [Google Scholar] [CrossRef]
- NOISH. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs). 1996. Available online: https://www.cdc.gov/niosh/idlh/pdfs/1994-IDLH-ValuesBackgroundDocs.pdf (accessed on 15 March 2023).
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The Key Role of Active Sites in the Development of Selective Metal Oxide Sensor Materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. Porous ZnO Polygonal Nanoflakes: Synthesis, Use in High-Sensitivity NO2 Gas Sensor, and Proposed Mechanism of Gas Sensing. J. Phys. Chem. C 2011, 115, 12763–12773. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Liu, G.; Wan, N.; Ge, C.; Hussain, S.; Meng, H.; Wang, M.; Qiao, G. Enhanced NO2 gas-sensing performance of 2D Ti3C2/TiO2 nanocomposites by in-situ formation of Schottky barrier. Appl. Surf. Sci. 2021, 567, 150747. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.; Cho, S.; Park, K.; Kang, H.; Kim, S.J.; Jung, H. In Situ Formation of Multiple Schottky Barriers in a Ti3C2 MXene Film and its Application in Highly Sensitive Gas Sensors. Adv. Funct. Mater. 2020, 30, 2003998. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hermawan, A.; Asakura, Y.; Hasegawa, T.; Kumagai, H.; Kato, H.; Kakihana, M.; Zhu, J.; Yin, S. SnO-SnO2 modified two-dimensional MXene Ti3C2T for acetone gas sensor working at room temperature. J. Mater. Sci. Technol. 2021, 73, 128–138. [Google Scholar] [CrossRef]



| Year | Composition | Gas | Conc., ppm | Temp, °C | Response | Response Time (s) | Sel 1 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2021 | ZnO—Ti3C2Tx | NO2 | 100 | RT | 41.39 2 | 34 | ~8 | [62] |
| 2022 | ZnO—Ti3C2Tx | NO2 | 20 | RT | 367.63 2 | 22 | ~6 | [63] |
| 2022 | ZnO—36.5% Ti3C2Tx | NO2 | 0.05 | RT, UV | 81 2 | 17 | ~4 | [58] |
| 2022 | ZnO—2% Ti3C2Tx | Acetone | 100 | 320 | 14.4 3 | 8 | ~1.03 | [60] |
| 2022 | ZnO—Ti3C2Tx | NO2 | 8 | 160 | 3.6 3 | 191 | ~3.6 | [59] |
| 2023 | ZnO—3.7% Ti3C2Tx | Ethanol | 50 | 300 | 59 3 | 22 | ~5 | [61] |
| 2023 | ZnO—(1–5%)Ti2CTx | NO2 | 20 | 200 | >73.3 3 | 22 | 20.5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonenko, E.P.; Nagornov, I.A.; Mokrushin, A.S.; Averin, A.A.; Gorban, Y.M.; Simonenko, T.L.; Simonenko, N.P.; Kuznetsov, N.T. Gas-Sensitive Properties of ZnO/Ti2CTx Nanocomposites. Micromachines 2023, 14, 725. https://doi.org/10.3390/mi14040725
Simonenko EP, Nagornov IA, Mokrushin AS, Averin AA, Gorban YM, Simonenko TL, Simonenko NP, Kuznetsov NT. Gas-Sensitive Properties of ZnO/Ti2CTx Nanocomposites. Micromachines. 2023; 14(4):725. https://doi.org/10.3390/mi14040725
Chicago/Turabian StyleSimonenko, Elizaveta P., Ilya A. Nagornov, Artem S. Mokrushin, Aleksey A. Averin, Yulia M. Gorban, Tatiana L. Simonenko, Nikolay P. Simonenko, and Nikolay T. Kuznetsov. 2023. "Gas-Sensitive Properties of ZnO/Ti2CTx Nanocomposites" Micromachines 14, no. 4: 725. https://doi.org/10.3390/mi14040725
APA StyleSimonenko, E. P., Nagornov, I. A., Mokrushin, A. S., Averin, A. A., Gorban, Y. M., Simonenko, T. L., Simonenko, N. P., & Kuznetsov, N. T. (2023). Gas-Sensitive Properties of ZnO/Ti2CTx Nanocomposites. Micromachines, 14(4), 725. https://doi.org/10.3390/mi14040725







