Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore
Abstract
1. Introduction
- (i)
- To investigate the microstructure and chemical composition of as-received galena using scanning electron microscopy in conjugation with its energy dispersive X-ray spectrometer, as the properties of each batch of galena ore depend on its mining place.
- (ii)
- To determine, as a function of temperature, some physical properties for the used galena, such as density, specific heat capacity, and hardness.
- (iii)
- To produce ultra-fine and nanoscale particles of lead sulfide (PbS) starting from a commercial galena ore by employing high-vibration mechanical milling.
2. Experimental
2.1. Materials and Methods
- The following materials and instruments were used for conducting the practical work:
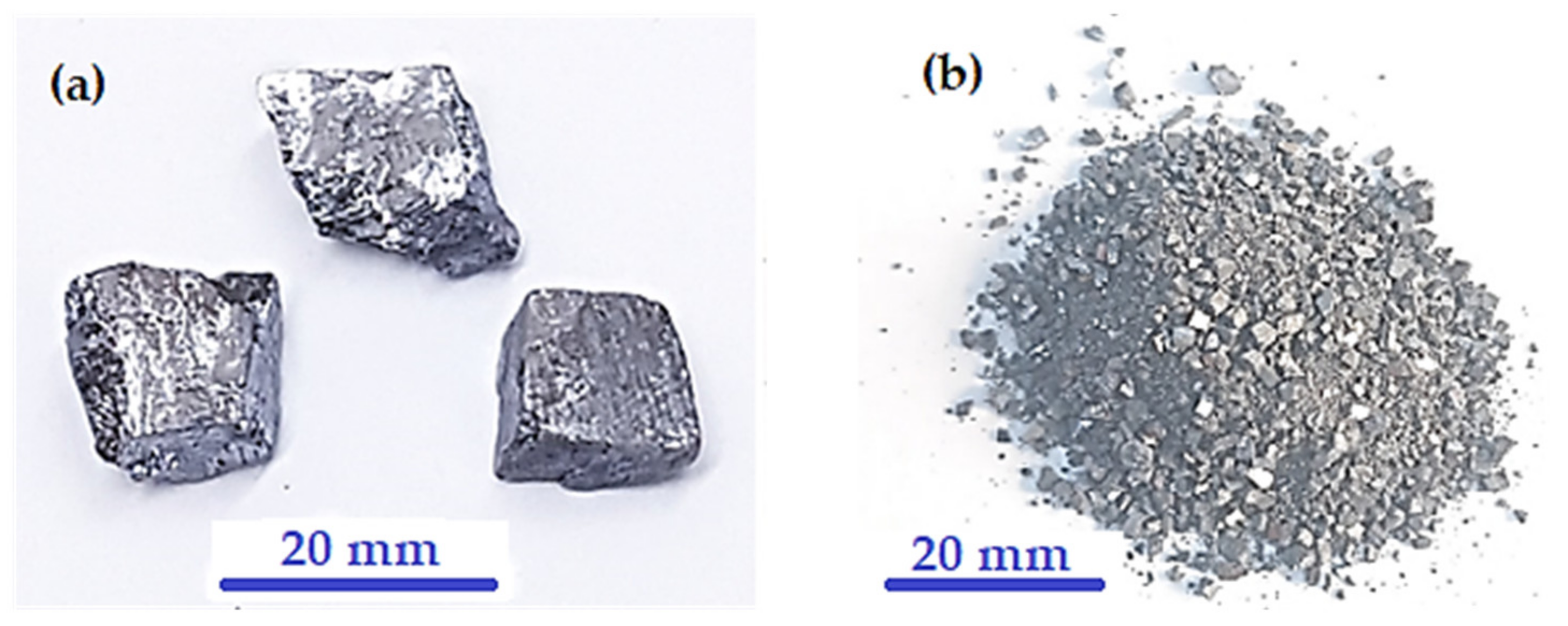
- Lumps of galena ore were purchased from a local market in Amman, Jordan.
- Digital micro-balance (Model SEJ-205, Taipei, Taiwan).
- Digital caliper with minimum reading 0.01 mm (Total, TMT 322001, Guangzhou, China).
- Scanning Electron Microscope (SEM), with integrated Energy Dispersive X-ray Spectrometer (EDS) (thermoscientific, JU-24112022, Waltham, USA).
- SEM (Inspect F50-FEI Company, Eindhoven, Netherlands).
- Differential Scanning Calorimeter (DSC) (NETZSCH DSC 204 F1).
- X-ray diffraction system (XRD) (Philips PW-1710), 40 kV and 30 mA with a Cu tube operating.
- Vibrating milling machine (TEMA, Woodford Halse UK) including tungsten carbide multi-rings with solid tungsten core.
- Micro Vickers Hardness Tester (MVHS), Mod/HTMV 2000M, SN/20190000187, Italian made, echo LAB.
- Different grades of emery papers for grinding and three types of diamond pastes for surface polishing.
- Compaction press, type Carver Lab. Press, model C-31000-564, UK.
- Homemade stainless-steel punch-die compaction system with a diameter of 1.21 cm.
- Vacuum oven (JEIO TECH, MODEL OV-11, AAH13115K, Korean made), temperature range from room temperature to 300 °C.
2.2. Investigation of the Physical Properties of Used Galena Ore
2.2.1. Density Determination
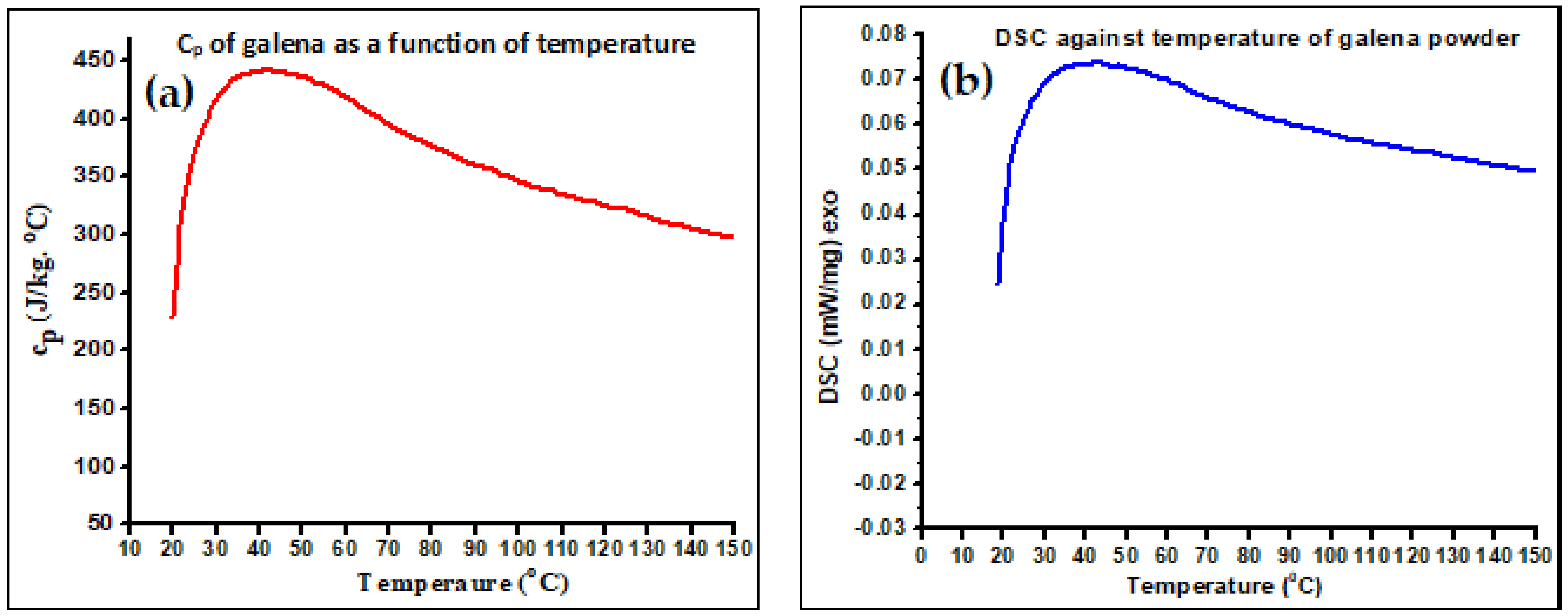
2.2.2. Specific Heat Capacity Determination Using Calorimetry and Differential Scanning Calorimeter (DSC) Techniques
2.2.3. Vickers Micro-Hardness Measurement
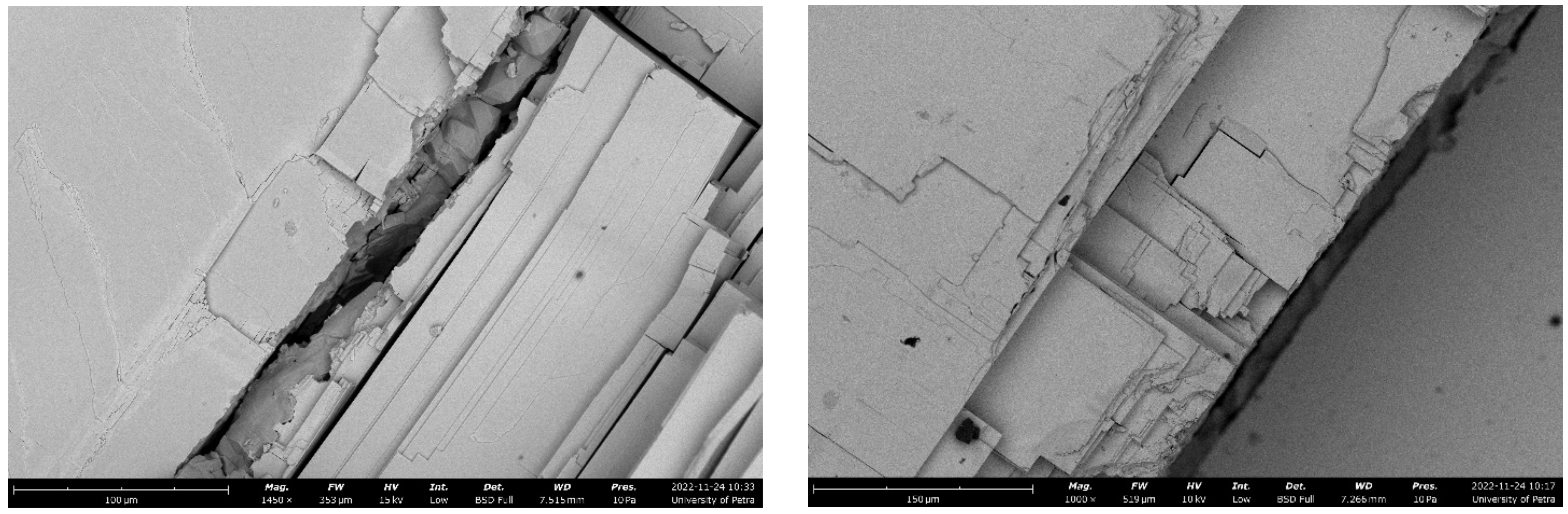
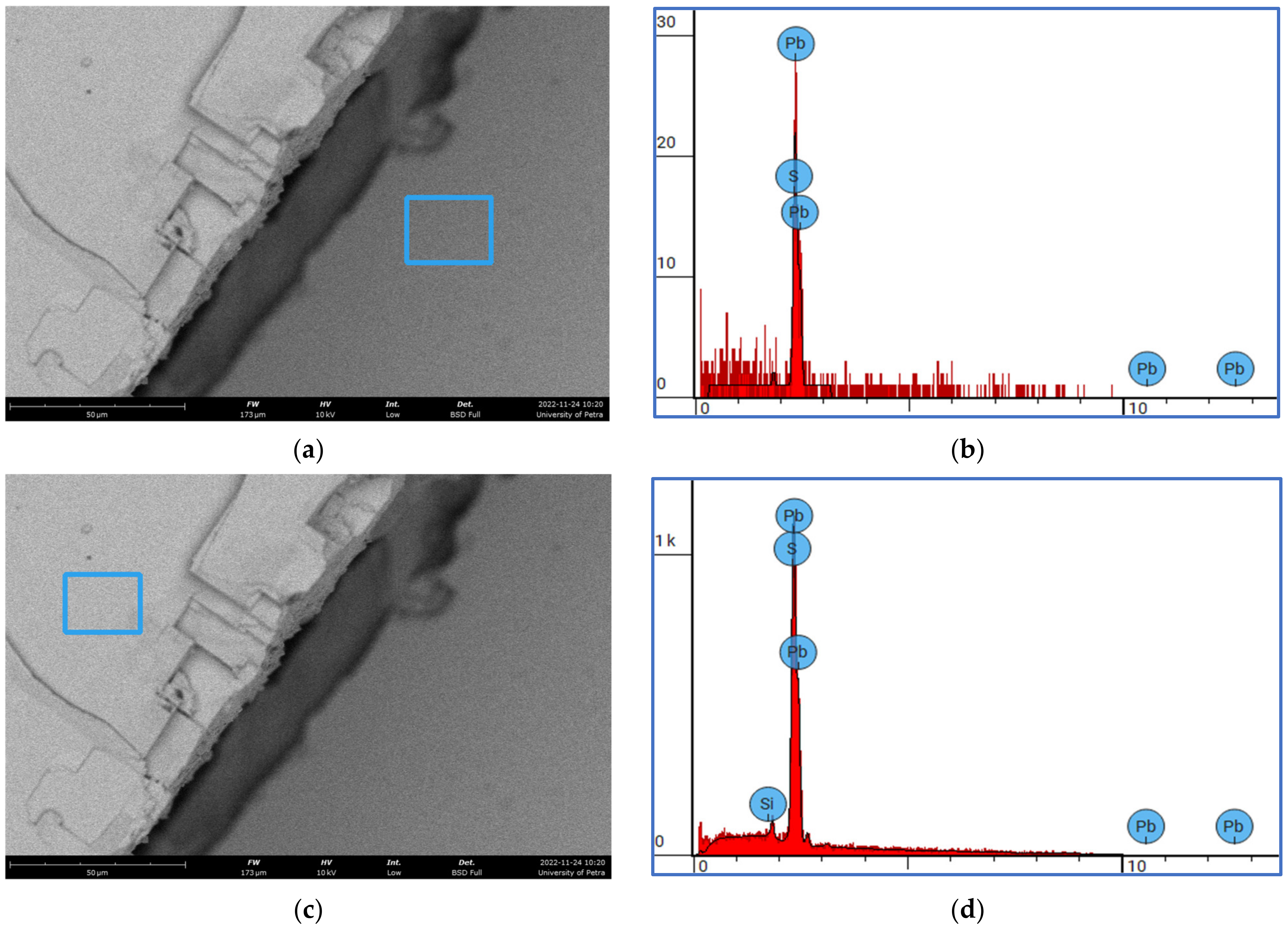
2.3. Scanning Electron Microscopy (SEM) Characterization of the As-Received Galena Ore
2.4. Milling Process of Galena Ore
2.5. X-ray Diffraction (XRD) Test
2.6. Compaction and Sintering of Galena Powder
3. Results and Discussion
3.1. Density Determination
3.2. Specific Heat Capacity Measurement Using Calorimetri Cand DSC Data
3.3. Micro-Hardness Measurement
3.4. Surface Characterization of the As-Received Fractured Galena Ore
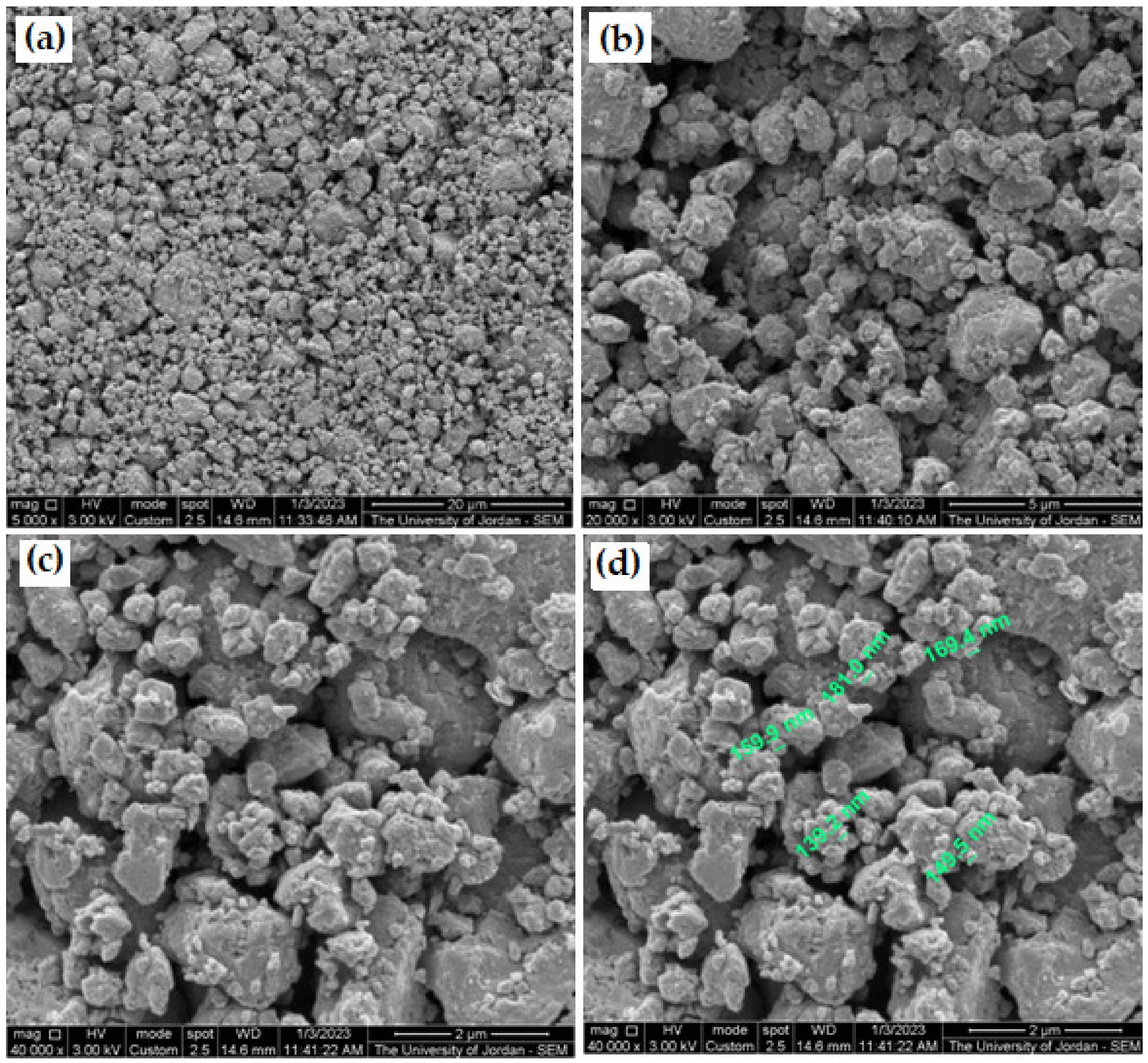
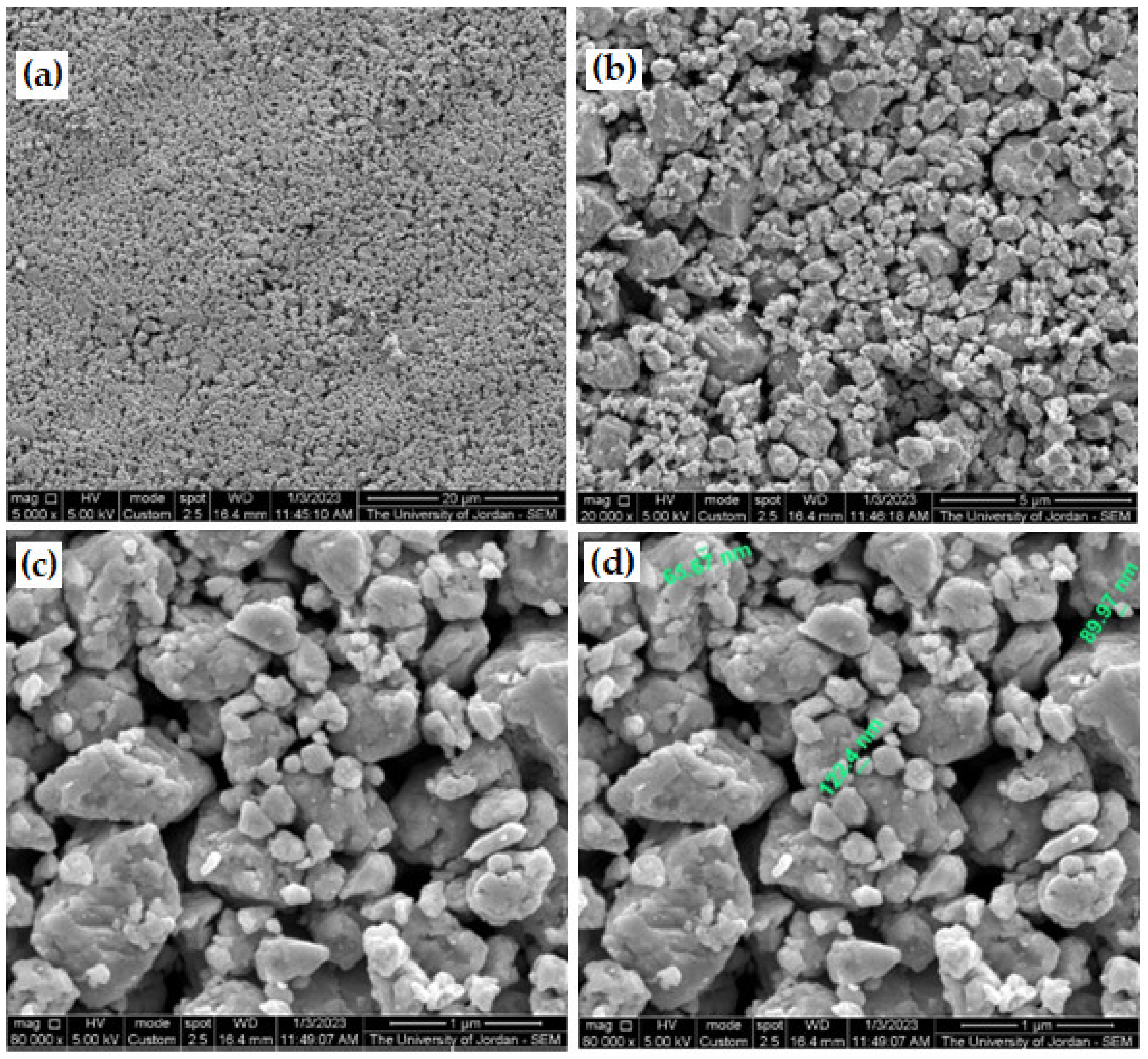
3.5. Milling of Galena Ore
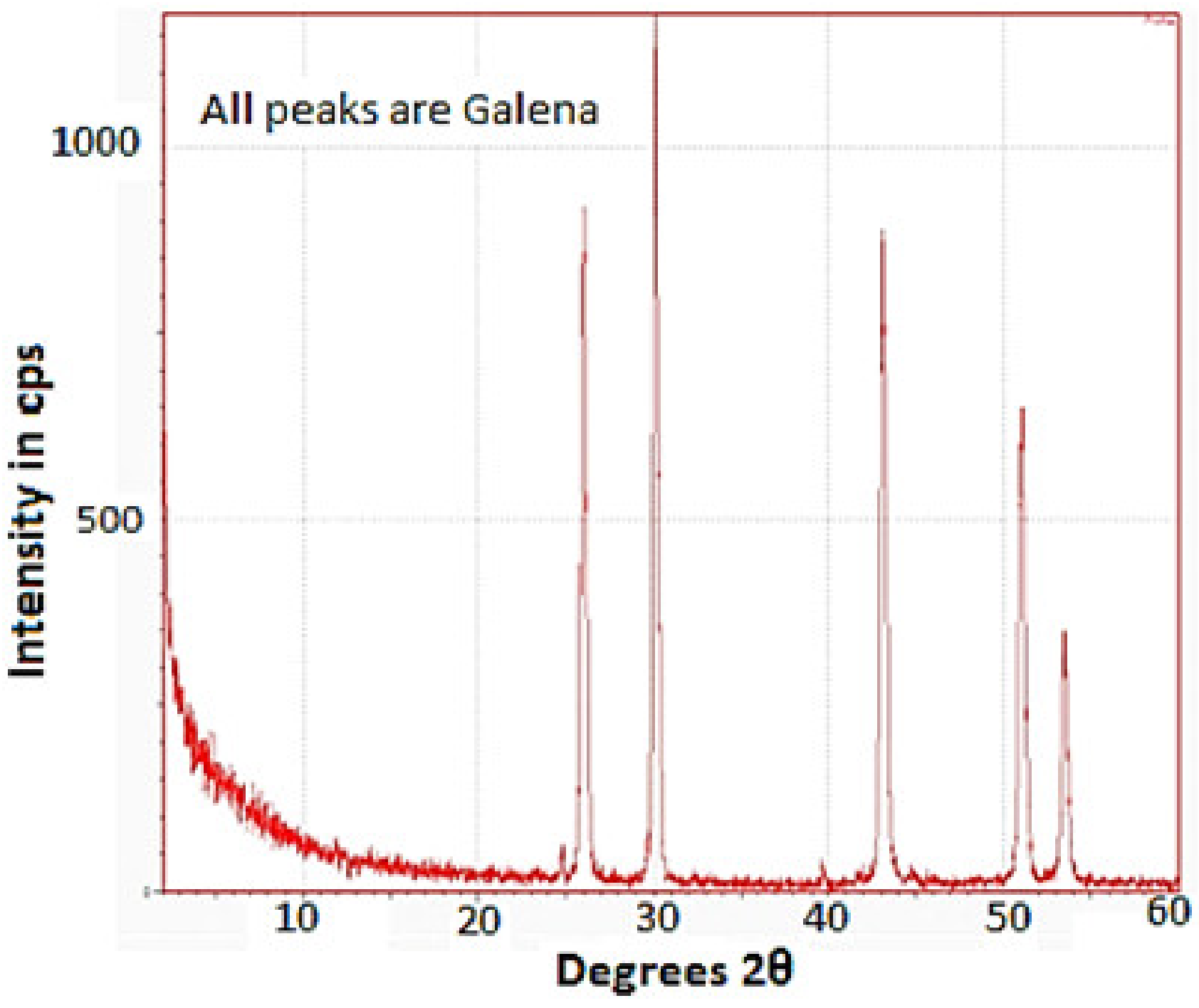
3.6. X-ray Diffraction Test
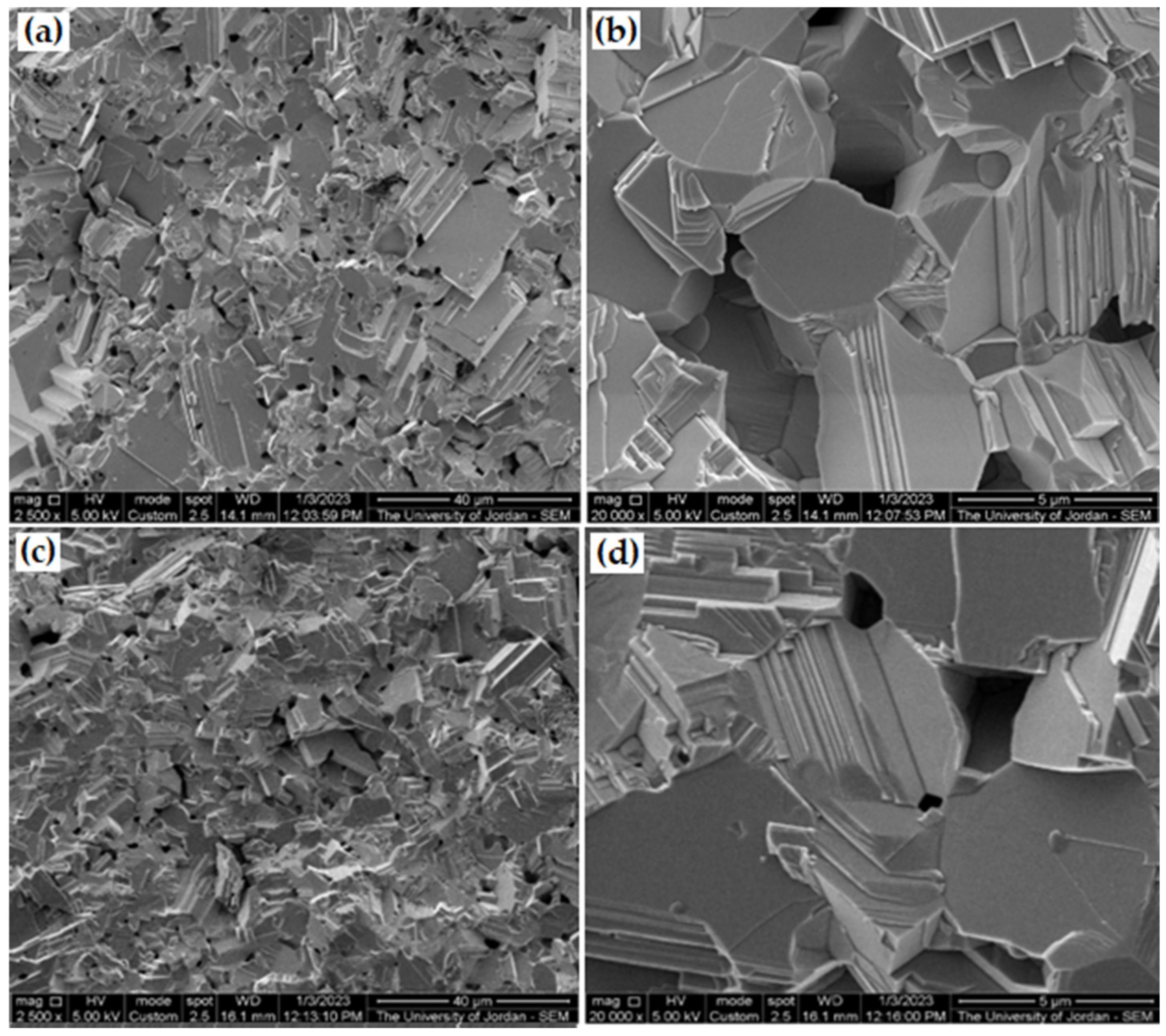
3.7. Yield of Compaction and Sintering of Galena Powder
4. Conclusions
- Some physical properties of the as-received galena lumps have been determined, such as density, specific heat capacity, and micro-hardness, as they depend on the mining site.
- The microstructures, chemical analysis of the as-received galena, the produced powder, and sintered samples were investigated using the SEM microscopy. The result confirmed that the main phase of the used galena was pure PbS.
- We have succeeded in producing nanoparticles of approximately 90 nm and ultra-fine powder of PbS, using a simple high-vibration tungsten carbide ring milling machine in a very short time of approximately 15 min under atmospheric conditions.
- The sintering process of galena powder must be carried out in vacuum or under inert gas to avoid oxidation.
- A relative density of approximately 97% of sintered galena produced powder was achieved at the sintering temperature of 700 °C for 2 h, which is a very encouraging result for practical applications.
- The milling and sintering of high PbS phase percentage galena ore can be employed to produce the bulk of PbS for different scientific applications as required.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amalia, D.; Ramanda, Y.; Maryono, M. Extraction of lead from galena concentrates using fluosilicic acid and peroxide. Indones. Min. J. 2017, 20, 69–80. [Google Scholar] [CrossRef]
- Raza, M.; Bhatti, M.; Nasir, S.; Bashir, F.; Mahmood, Z.; Kazmi, K.; Hafeez, I. Study on low-grade galena-barite ore beneficiation in Khuzdar, Balochistan, Pakistan. Min. Miner. Deposits 2019, 13, 1–8. [Google Scholar] [CrossRef]
- Nnanwube, I.A.; Onukwuli, O.D. Hydrometallurgical Processing of a Nigerian Galena Ore in Nitric Acid: Characterization and Dissolution Kinetics. J. Miner. Mater. Charact. Eng. 2018, 6, 271–293. [Google Scholar] [CrossRef]
- Chatterjee, S. Low-cost solar selective absorbers from Indian galena. Opt. Eng. 1993, 32, 2923. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhu, H.; Li, H.; Su, T.; Li, S.; Hu, M.; Fan, H. Hydrothermal synthesis and thermoelectric properties of PbS. Mater. Sci.-Pol. 2016, 34, 754–759. [Google Scholar] [CrossRef]
- Moosa, I.S.; Maqableh, B.B. Temperature Difference with Respect to Exposure Time for Black Paint and Galena Powder-Black Paint Composite Selective Surfaces. In Transition Towards 100% Renewable Energy: Selected Papers from the World Renewable Energy Congress WREC; Springer: Cham, Switzerland, 2018; Volume 13, pp. 139–147. [Google Scholar] [CrossRef]
- Barrios-Salgado, E.; Rodríguez-Lazcano, Y.; Pérez-Orozco, J.P.; Colin, J.; Altuzar, P.; Campos, J.; Quesada, D. Effect of Deposition Time on the Optoelectronics Properties of PbS Thin Films Obtained by Microwave-Assisted Chemical Bath Deposition. Adv. Condens. Matter Phys. 2019, 2019, 5960587. [Google Scholar] [CrossRef]
- Göde, F.; Yavuz, F.; Kariper, A. Preparation and Characterization of Nanocrystalline PbS Thin Films Produced by Chemical Bath Deposition. Acta Phys. Pol. A 2015, 128, B-215. [Google Scholar] [CrossRef]
- Ezekoye, B.; Emeakaroha, T.; Ezekoye, V.; Ighodalo, K.; Offor, P. Optical and structural properties of lead sulphide (PbS) thin films synthesized by chemical method. Int. J. Phys. Sci. 2015, 10, 385–390. [Google Scholar] [CrossRef]
- Akbarbeiglou, F. Effect of temperature and time on the structural properties of PbS nanofilms. Sci. Iran. 2018, 26, 2007–2012. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C. PbS Nanoparticles Prepared Using 1, 10-Phenanthroline Adduct of Lead(II) Bis(N-alkyl-N-phenyl dithiocarbamate) as Single Source Precursors. Molecules 2020, 25, 2097. [Google Scholar] [CrossRef]
- Mlowe, S.; Revaprasadu, N. Preparation of spin coated PbS thin films using bis-tetrahydroquinolinedithiocarbamatolead(II) complex as a single source precursor. Inorg. Nano-Metal. Chem. 2022, 52, 1019–1023. [Google Scholar] [CrossRef]
- Moosa, I.S. Powder Metallurgy and its Application in the Production of Permanent Magnets. Int. J. Adv. Res. Eng. Technol. 2013, 4, 127–141. [Google Scholar]
- Marinca, T.; Neamțu, B.; Popa, F. Powder Metallurgy and Advanced Materials: RoPM&AM 2017 (8) (Mater. Res. Proc.); Materials Research Forum LLC: Millersville, PA, USA, 2018. [Google Scholar]
- Novák, P. Advanced Powder Metallurgy Technologies; MDPI: Basel, Switzerland, 2020. [Google Scholar] [CrossRef]
- Mohammad, F.; Suhaib, M.; Prveen, A. Introduction to Powder Metallurgy: A Review. J. Eng. Technol. Res. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Qu, X.; Qin, M.; Que, Z.; Wei, Z.; Guo, C.; Lu, X.; Dong, Y. Powder metallurgy route to ultrafine-grained refractory metals. Adv. Mater. 2022, 34, 2205807. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Alrbaihat, M.; Moosa, I.; Abu-Afifeh, Q.; Al-Fayyad, H.; Hamadneh, I.; Al-Rawajfeh, A. Mechanochemical Preparation of a Novel Slow-Release Fertilizer Based on K2SO4-kaolinite. Agronomy 2022, 12, 3016. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Altwaiq, A.M.; Esaifan, M.; Al-Fayyad, H.; Shraideh, Z.; Moosa, I.S.; Hamadneh, I. Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications. Metals 2022, 12, 1635. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Moosa, I.S.; Al-Fayyad, H.; Lahlouh, B.; Kazem, H.A.; Abu-Afifeh, Q.; Al-Saqarat, B.S.; Esaifan, M.; Hamadneh, I. Performance Comparison and Light Reflectance of Al, Cu, and Fe Metals in Direct Contact Flat Solar Heating Systems. Energies 2022, 15, 8888. [Google Scholar] [CrossRef]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent Developments on the Synthesis of Nanocomposite Materials via Ball Milling Approach for Energy Storage Applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Serway, R.A.; Jewett, J.W. Physics for Scientists and Engineers, 10th ed.; Cengage Learning: Stanford, CT, USA, 2018; Volume 2. [Google Scholar]
- Mettler-Toledo, A.G. Available online: https://www.mse.ucr.edu/media/416/download (accessed on 16 January 2023).
- Waples, D.W.; Waples, J.S. A Review and Evaluation of Specific Heat Capacities of Rocks, Minerals, and Subsurface Fluids. Part 2: Fluids and Porous Rocks. Nat. Resour. Res. 2004, 13, 123–130. [Google Scholar] [CrossRef]
- Osadchii, V.O.; Gurevich, V.M.; Polyakov, V.B.; Gavrichev, K.S.; Osadchii, E.G. Heat capacity and thermodynamic properties of PbS: Optimization based on calorimetric and electrochemical data. J. Alloys Compd. 2022, 909, 164695. [Google Scholar] [CrossRef]
- Mindat. Available online: https://www.mindat.org/min-1641.html (accessed on 16 January 2023).


| Parameters | Galena | Water |
|---|---|---|
| mg and mw (kg) | 0.06332 | 0.08044 |
| cg and cw (J/kg·°C) | x (unknown) 1 | 4186 |
| Tg and Tw (°C) | 87.1 | 17.8 |
| Tf | 20.6 | 20.6 |
| Sample No. | Micro HV | Used Load |
|---|---|---|
| 1 | 81.33 | 0.98 N |
| 2 | 80.28 | |
| 3 | 80.07 | |
| 4 | 78.43 | |
| 5 | 78.56 | |
| 6 | 69.75 | |
| 7 | 71.82 | |
| 8 | 72.53 | |
| 9 | 79.23 | |
| 10 | 80.54 | |
| Mean value | 77.25 |
| Property | Value in the SI Units |
|---|---|
| Density | 7.32 × 103 kg/m3 |
| Vickers Hardness | 77.25 HV |
| Specific heat capacity | 214.86 J/kg·K |
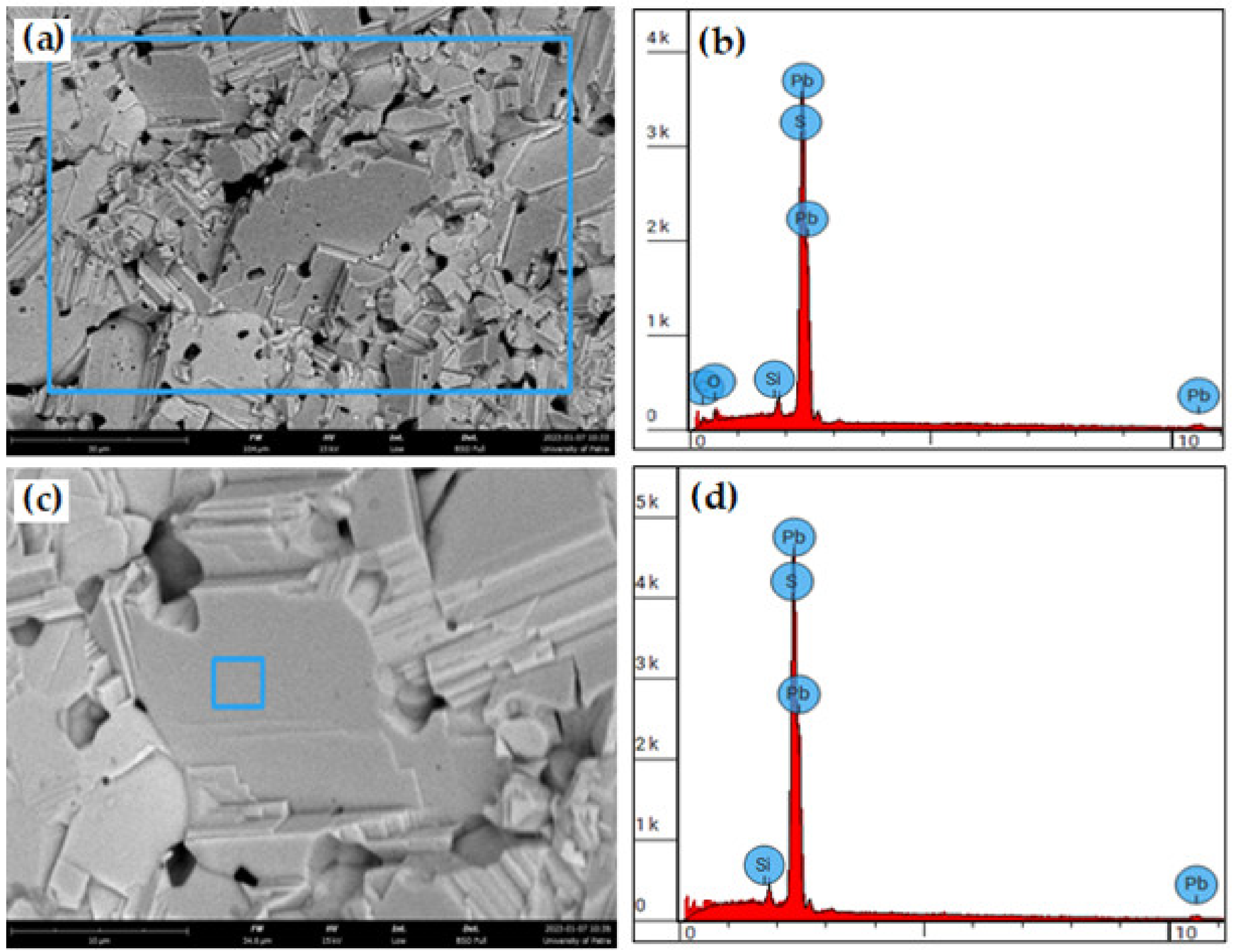
| Tested Area | Element Atomic Number | Element Symbol | Element Name | Atomic % | Mass % |
|---|---|---|---|---|---|
| Figure 4b | 16 | S | Sulfur | 55.17 | 16.00 |
| 82 | Pb | Lead | 44.83 | 84.00 | |
| Figure 4d | 14 | Si | Silicon | 0.83 | 0.20 |
| 16 | S | Sulfur | 50.95 | 14.03 | |
| 82 | Pb | Lead | 48.22 | 85.77 |
| Powder Description | Compact Density ×103 kg/m3 | Sintered Density ×103 kg/m3 | Compaction Pressure MPa | Sintering Condition |
|---|---|---|---|---|
| Powder after 15 min. milling time | 6.00 | 7.11 | 300 | 700 °C, 2 h, rate of heating 10 °C/min, under vacuum of about 10−3 torr, furnace cooling at the same rate of heating |
| Powder after suspension in water, and separated | 5.95 | 7.13 | ||
| Residual powder after removing the fine powder of water suspension | 6.08 | 7.04 |
| Analyzed Area | Element Name | Element Symbol | Atomic Conc. | Mass Conc. |
|---|---|---|---|---|
| Figure 10a Blue square | Carbon | C | 17.08 | 2.50 |
| Oxygen | O | 13.68 | 2.60 | |
| Silicon | Si | 0.87 | 0.21 | |
| Sulfur | S | 35.26 | 13.40 | |
| Lead | Pb | 33.10 | 81.28 | |
| Figure 10c | Sulfur | S | 50.95 | 14.03 |
| Small Blue square | Silicon | Si | 0.83 | 0.20 |
| Lead | Pb | 48.22 | 85.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saqarat, B.S.; Al-Mobydeen, A.; AL-Masri, A.N.; Esaifan, M.; Hamadneh, I.; Moosa, I.S.; AlShamaileh, E. Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore. Micromachines 2023, 14, 564. https://doi.org/10.3390/mi14030564
Al-Saqarat BS, Al-Mobydeen A, AL-Masri AN, Esaifan M, Hamadneh I, Moosa IS, AlShamaileh E. Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore. Micromachines. 2023; 14(3):564. https://doi.org/10.3390/mi14030564
Chicago/Turabian StyleAl-Saqarat, Bety S., Ahmed Al-Mobydeen, Ahmed N. AL-Masri, Muayad Esaifan, Imad Hamadneh, Iessa Sabbe Moosa, and Ehab AlShamaileh. 2023. "Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore" Micromachines 14, no. 3: 564. https://doi.org/10.3390/mi14030564
APA StyleAl-Saqarat, B. S., Al-Mobydeen, A., AL-Masri, A. N., Esaifan, M., Hamadneh, I., Moosa, I. S., & AlShamaileh, E. (2023). Facile Production Method of PbS Nanoparticles via Mechanical Milling of Galena Ore. Micromachines, 14(3), 564. https://doi.org/10.3390/mi14030564










