Viscoelastic Particle Encapsulation Using a Hyaluronic Acid Solution in a T-Junction Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
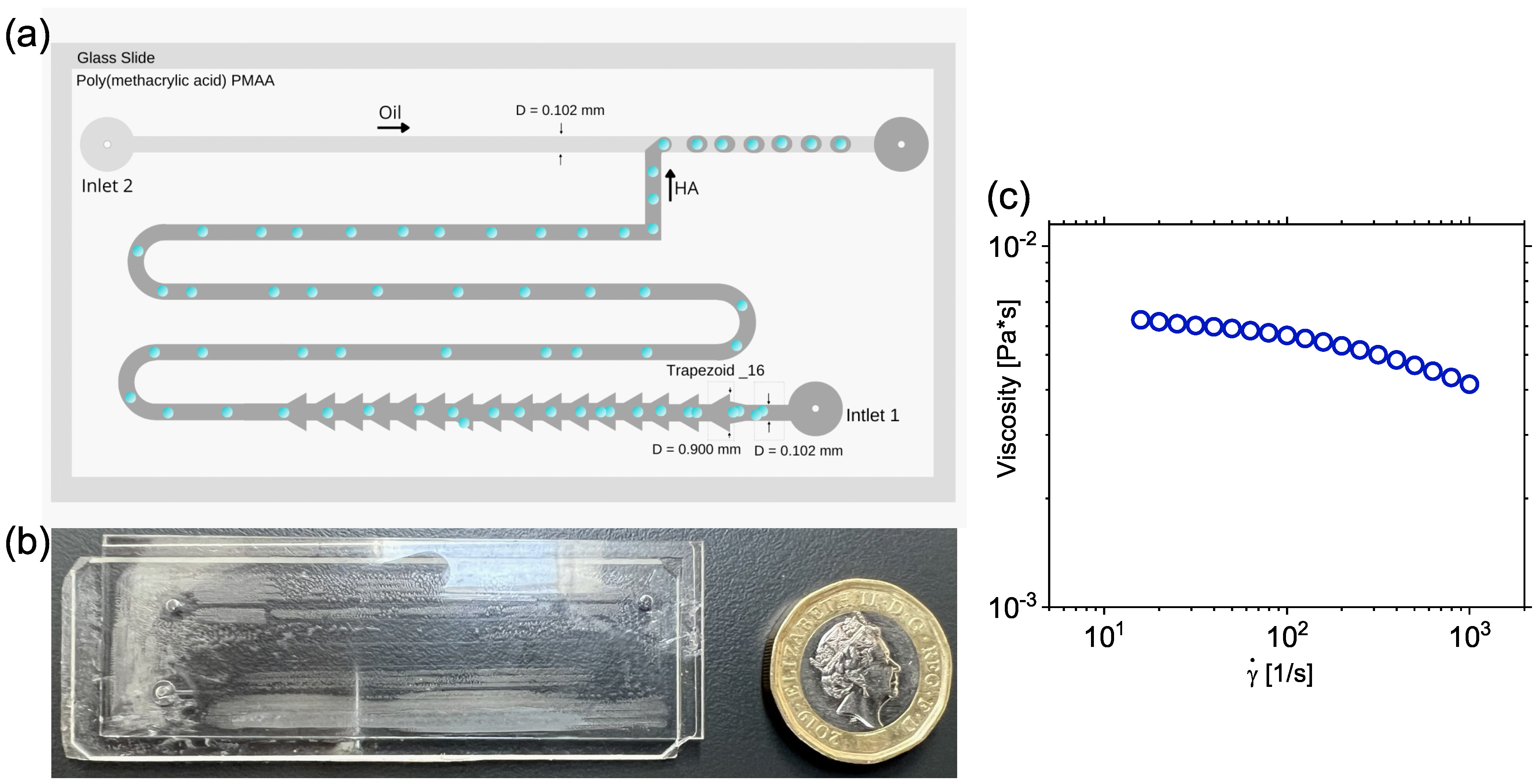
2.1. Microfluidic Device Design and Fabrication
2.2. Sample Preparation and Characterization
2.3. Experimental Apparatus and Particle Tracking
3. Results and Discussion
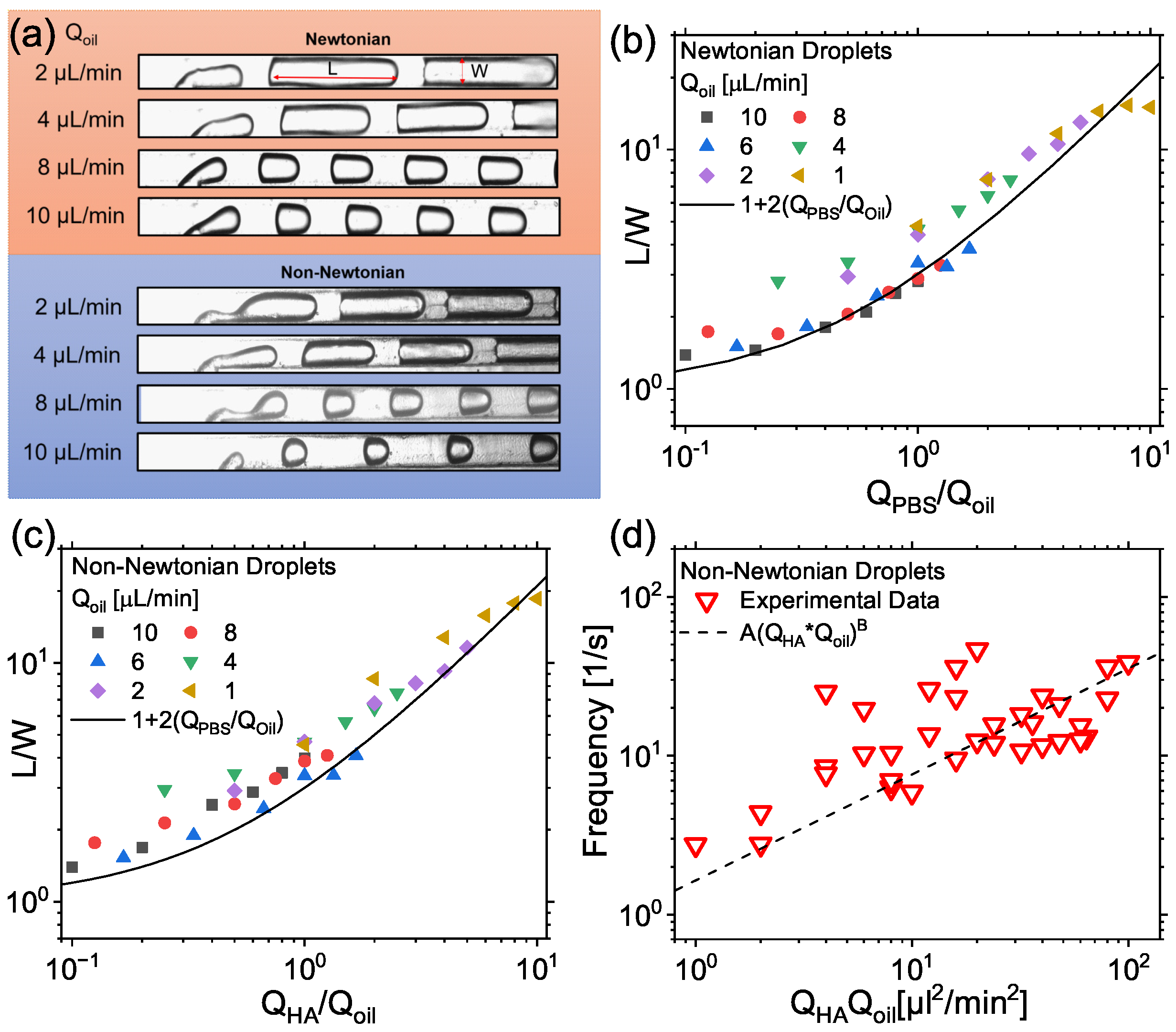
3.1. Droplet Formation
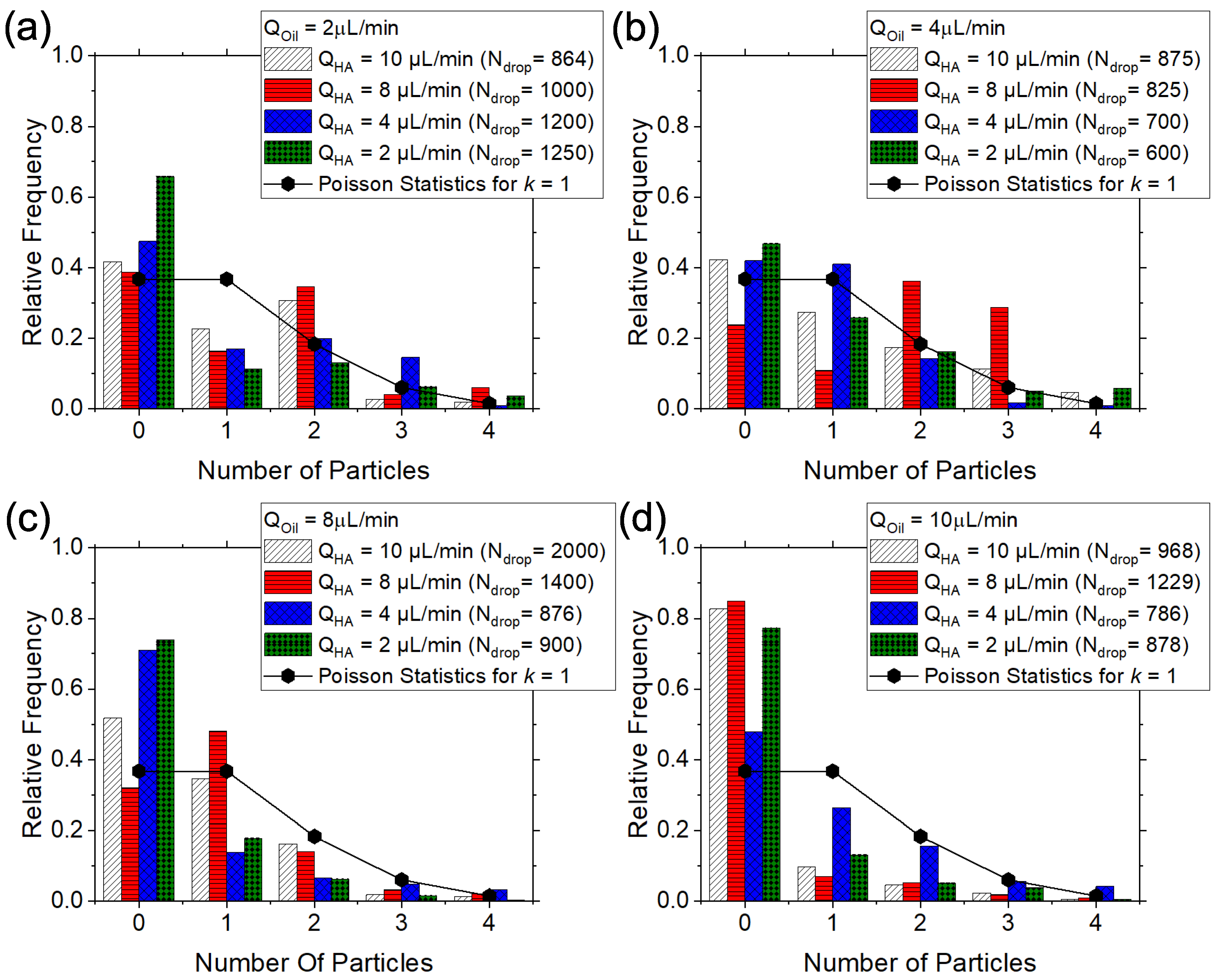
3.2. Viscoelastic Encapsulation of Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Del Giudice, F.; D’Avino, G.; Maffettone, P.L. Microfluidic formation of crystal-like structures. Lab Chip 2021, 21, 2069–2094. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Eskandari, M.; Ghazali, Z.S.; Ghazali, H.S. Cell encapsulation in alginate-based microgels using droplet microfluidics; a review on gelation methods and applications. Biomed. Phys. Eng. Express 2022, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yin, M.; Zhai, Q.; Chen, M. The encapsulation strategy to improve the survival of probiotics for food application: From rough multicellular to single-cell surface engineering and microbial mediation. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, Z.; Qiao, L.; Liu, B. Advances in microfluidic strategies for single-cell research. TrAC Trends Anal. Chem. 2022, 157, 116822. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Y.; Zhou, J. Interrogation of single-cell communications on microfluidic platforms. Cell Rep. Phys. Sci. 2022, 3, 101129. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- Sjostrom, S.L.; Bai, Y.; Huang, M.; Liu, Z.; Nielsen, J.; Joensson, H.N.; Svahn, H.A. High-throughput screening for industrial enzyme production hosts by droplet microfluidics. Lab Chip 2014, 14, 806–813. [Google Scholar] [CrossRef]
- Yanakieva, D.; Elter, A.; Bratsch, J.; Friedrich, K.; Becker, S.; Kolmar, H. FACS-based functional protein screening via microfluidic co-encapsulation of yeast secretor and mammalian reporter cells. Sci. Rep. 2020, 10, 10182. [Google Scholar] [CrossRef]
- Subedi, N.; Van Eyndhoven, L.C.; Hokke, A.M.; Houben, L.; Van Turnhout, M.C.; Bouten, C.V.; Eyer, K.; Tel, J. An automated real-time microfluidic platform to probe single NK cell heterogeneity and cytotoxicity on-chip. Sci. Rep. 2021, 11, 17084. [Google Scholar] [CrossRef]
- Santesson, S.; Degerman, E.; Rorsman, P.; Johansson, T.; Lemos, S.; Nilsson, S. Cell–cell communication between adipocytes and pancreatic β-cells in acoustically levitated droplets. Integr. Biol. 2009, 1, 595–601. [Google Scholar] [CrossRef]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. Nonlinear phenomena in microfluidics. Chem. Rev. 2022, 122, 6921–6937. [Google Scholar] [CrossRef]
- Nunes, J.; Tsai, S.; Wan, J.; Stone, H.A. Dripping and jetting in microfluidic multiphase flows applied to particle and fibre synthesis. J. Phys. D Appl. Phys. 2013, 46, 114002. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Edd, J.F.; Di Carlo, D.; Humphry, K.J.; Köster, S.; Irimia, D.; Weitz, D.A.; Toner, M. Controlled encapsulation of single-cells into monodisperse picolitre drops. Lab Chip 2008, 8, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Kemna, E.W.; Schoeman, R.M.; Wolbers, F.; Vermes, I.; Weitz, D.A.; Van Den Berg, A. High-yield cell ordering and deterministic cell-in-droplet encapsulation using Dean flow in a curved microchannel. Lab Chip 2012, 12, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Kahkeshani, S.; Haddadi, H.; Di Carlo, D. Preferred interparticle spacings in trains of particles in inertial microchannel flows. J. Fluid Mech. 2016, 786, R3. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Poitzsch, A.; Hwang, Y.; Lee, J.; Czerwinska, J.; Weitz, D. Impact of inlet channel geometry on microfluidic drop formation. Phys. Rev. E 2009, 80, 026310. [Google Scholar] [CrossRef]
- Chung, B.G.; Lee, K.H.; Khademhosseini, A.; Lee, S.H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 2012, 12, 45–59. [Google Scholar] [CrossRef]
- Yue, X.; Fang, X.; Sun, T.; Yi, J.; Kuang, X.; Guo, Q.; Wang, Y.; Gu, H.; Xu, H. Breaking through the Poisson Distribution: A compact high-efficiency droplet microfluidic system for single-bead encapsulation and digital immunoassay detection. Biosens. Bioelectron. 2022, 211, 114384. [Google Scholar] [CrossRef]
- Li, L.; Wu, P.; Luo, Z.; Wang, L.; Ding, W.; Wu, T.; Chen, J.; He, J.; He, Y.; Wang, H.; et al. Dean flow assisted single cell and bead encapsulation for high performance single cell expression profiling. ACS Sens. 2019, 4, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Lagus, T.P.; Edd, J.F. High-throughput co-encapsulation of self-ordered cell trains: Cell pair interactions in microdroplets. RSC Adv. 2013, 3, 20512–20522. [Google Scholar] [CrossRef]
- Moon, H.S.; Je, K.; Min, J.W.; Park, D.; Han, K.Y.; Shin, S.H.; Park, W.Y.; Yoo, C.E.; Kim, S.H. Inertial-ordering-assisted droplet microfluidics for high-throughput single-cell RNA-sequencing. Lab Chip 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Magaud, P.; Baldas, L.; Lafforgue, C.; Abbas, M.; Colin, S. Self-ordered particle trains in inertial microchannel flows. Microfluid. Nanofluidics 2017, 21, 154. [Google Scholar] [CrossRef]
- Dietsche, C.; Mutlu, B.R.; Edd, J.F.; Koumoutsakos, P.; Toner, M. Dynamic particle ordering in oscillatory inertial microfluidics. Microfluid. Nanofluidics 2019, 23, 83. [Google Scholar] [CrossRef]
- Hu, X.; Lin, J.; Chen, D.; Ku, X. Stability condition of self-organizing staggered particle trains in channel flow. Microfluid. Nanofluidics 2020, 24, 25. [Google Scholar] [CrossRef]
- Shahrivar, K.; Del Giudice, F. Controlled viscoelastic particle encapsulation in microfluidic devices. Soft Matter 2021, 17, 8068–8077. [Google Scholar] [CrossRef]
- Shahrivar, K.; Del Giudice, F. Beating Poisson stochastic particle encapsulation in flow-focusing microfluidic devices using viscoelastic liquids. Soft Matter 2022, 18, 5928–5933. [Google Scholar] [CrossRef]
- Del Giudice, F.; D’Avino, G.; Greco, F.; Maffettone, P.L.; Shen, A.Q. Fluid viscoelasticity drives self-assembly of particle trains in a straight microfluidic channel. Phys. Rev. Appl. 2018, 10, 64058. [Google Scholar] [CrossRef]
- Liu, L.; Xu, H.; Xiu, H.; Xiang, N.; Ni, Z. Microfluidic on-demand engineering of longitudinal dynamic self-assembly of particles. Analyst 2020, 145, 5128–5133. [Google Scholar] [CrossRef]
- D’Avino, G.; Maffettone, P.L. Numerical simulations on the dynamics of a particle pair in a viscoelastic fluid in a microchannel: Effect of rheology, particle shape, and confinement. Microfluid. Nanofluidics 2019, 23, 82. [Google Scholar] [CrossRef]
- D’Avino, G.; Maffettone, P.L. Numerical simulations on the dynamics of trains of particles in a viscoelastic fluid flowing in a microchannel. Meccanica 2020, 55, 317–330. [Google Scholar] [CrossRef]
- Hu, X.; Lin, J.; Chen, D.; Ku, X. Dynamics of self-organizing single-line particle trains in the channel flow of a power-law fluid. Chin. J. Chem. Eng. 2021, 34, 12–21. [Google Scholar] [CrossRef]
- Hu, X.; Lin, P.; Lin, J.; Zhu, Z.; Yu, Z. On the polydisperse particle migration and formation of chains in a square channel flow of non-Newtonian fluids. J. Fluid Mech. 2022, 936, A5. [Google Scholar] [CrossRef]
- Du, W.; Fu, T.; Zhang, Q.; Zhu, C.; Ma, Y.; Li, H.Z. Breakup dynamics for droplet formation in a flow-focusing device: Rupture position of viscoelastic thread from matrix. Chem. Eng. Sci. 2016, 153, 255–269. [Google Scholar] [CrossRef]
- Xue, C.D.; Chen, X.D.; Li, Y.J.; Hu, G.Q.; Cao, T.; Qin, K.R. Breakup dynamics of semi-dilute polymer solutions in a microfluidic flow-focusing device. Micromachines 2020, 11, 406. [Google Scholar] [CrossRef]
- Fatehifar, M.; Revell, A.; Jabbari, M. Non-Newtonian droplet generation in a cross-junction microfluidic channel. Polymers 2021, 13, 1915. [Google Scholar] [CrossRef]
- Fatehifar, M.; Revell, A.; Jabbari, M. Droplet Formation in a Cross-Junction Microfluidic Channel with Non-Newtonian Dispersed Phase. Eng. Proc. 2021, 4, 21. [Google Scholar]
- Del Giudice, F.; Calcagno, V.; Esposito Taliento, V.; Greco, F.; Netti, P.A.; Maffettone, P.L. Relaxation time of polyelectrolyte solutions: When μ-rheometry steps in charge. J. Rheol. 2017, 61, 13–21. [Google Scholar] [CrossRef]
- Cho, M.; Hong, S.O.; Lee, S.H.; Hyun, K.; Kim, J.M. Effects of ionic strength on lateral particle migration in shear-thinning xanthan gum solutions. Micromachines 2019, 10, 535. [Google Scholar] [CrossRef]
- Jeyasountharan, A.; Shahrivar, K.; D’Avino, G.; Del Giudice, F. Viscoelastic particle train formation in microfluidic flows using a xanthan gum aqueous solution. Anal. Chem. 2021, 93, 5503–5512. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, F.; Keays, M.; Hayes, S.; Albadarin, A.B.; Walker, G.M.; Kiely, P.A.; Collins, M.N. Enhanced cell viability in hyaluronic acid coated poly (lactic-co-glycolic acid) porous scaffolds within microfluidic channels. Int. J. Pharm. 2017, 532, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Jeyasountharan, A.; D’Avino, G.; Del Giudice, F. Confinement effect on the viscoelastic particle ordering in microfluidic flows: Numerical simulations and experiments. Phys. Fluids 2022, 34, 042015. [Google Scholar] [CrossRef]
- Ai, Y.; Xie, R.; Xiong, J.; Liang, Q. Microfluidics for biosynthesizing: From droplets and vesicles to artificial cells. Small 2020, 16, 1903940. [Google Scholar] [CrossRef]
- Zhao, C.X. Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1420–1446. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ambhorkar, P.; Samanipour, R.; Yang, A.; Ghafoor, A.; Kim, K. Microfluidics-based fabrication of cell-laden microgels. Biomicrofluidics 2020, 14, 021501. [Google Scholar] [CrossRef]
- Subramanian, B.; Kim, N.; Lee, W.; Spivak, D.A.; Nikitopoulos, D.E.; McCarley, R.L.; Soper, S.A. Surface modification of droplet polymeric microfluidic devices for the stable and continuous generation of aqueous droplets. Langmuir 2011, 27, 7949–7957. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Liu, X.; Chang, H. Ultra-monodisperse droplet formation using PMMA microchannels integrated with low-pulsation electrolysis micropumps. Sens. Actuators B Chem. 2016, 229, 466–475. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Lassemono, S.; Chollet, F.A. Optical detection for droplet size control in microfluidic droplet-based analysis systems. Sens. Actuators B Chem. 2006, 117, 431–436. [Google Scholar] [CrossRef]
- Hettiarachchi, S.; Melroy, G.; Mudugamuwa, A.; Sampath, P.; Premachandra, C.; Amarasinghe, R.; Dau, V. Design and development of a microfluidic droplet generator with vision sensing for lab-on-a-chip devices. Sens. Actuators A Phys. 2021, 332, 113047. [Google Scholar] [CrossRef]
- Del Giudice, F.; Haward, S.J.; Shen, A.Q. Relaxation time of dilute polymer solutions: A microfluidic approach. J. Rheol. 2017, 61, 327–337. [Google Scholar] [CrossRef]
- Kalmykova, T.; Kostina, Y.V.; Ilyin, S.; Bogdanova, Y.G.; Severin, A.; Ivanov, P.; Antonov, S. Effect of Synthesis Medium on the Structure and Physicochemical Properties of Biomineral Composites Based on Hydroxyapatite and Hyaluronic Acid. Polym. Sci. Ser. B 2020, 62, 61–71. [Google Scholar] [CrossRef]
- D’Avino, G.; Hulsen, M.A.; Maffettone, P.L. Dynamics of pairs and triplets of particles in a viscoelastic fluid flowing in a cylindrical channel. Comput. Fluids 2013, 86, 45–55. [Google Scholar] [CrossRef]
- Del Giudice, F. Simultaneous measurement of rheological properties in a microfluidic rheometer. Phys. Fluids 2020, 32, 052001. [Google Scholar] [CrossRef]
- Del Giudice, F.; Barnes, C. Rapid Temperature-Dependent Rheological Measurements of Non-Newtonian Solutions Using a Machine-Learning Aided Microfluidic Rheometer. Anal. Chem. 2022, 94, 3617–3628. [Google Scholar] [CrossRef]
- Rostami, B.; Morini, G.L. Generation of Newtonian and non-Newtonian droplets in silicone oil flow by means of a micro cross-junction. Int. J. Multiph. Flow 2018, 105, 202–216. [Google Scholar] [CrossRef]
- Wong, V.L.; Loizou, K.; Lau, P.L.; Graham, R.S.; Hewakandamby, B.N. Numerical studies of shear-thinning droplet formation in a microfluidic T-junction using two-phase level-SET method. Chem. Eng. Sci. 2017, 174, 157–173. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Z.; Shum, H.C. Breakup dynamics and dripping-to-jetting transition in a Newtonian/shear-thinning multiphase microsystem. Lab Chip 2015, 15, 121–134. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Song, Y.; Chen, B.; Christopher, D.M.; Li, X. Pressure-driven microfluidic droplet formation in Newtonian and shear-thinning fluids in glass flow-focusing microchannels. Int. J. Multiph. Flow 2021, 140, 103648. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—Scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.J.; Neild, A.; DeMello, A.; Liu, A.Q.; Ai, Y. The Poisson distribution and beyond: Methods for microfluidic droplet production and single cell encapsulation. Lab Chip 2015, 15, 3439–3459. [Google Scholar] [CrossRef] [PubMed]
- Colby, R.H. Structure and linear viscoelasticity of flexible polymer solutions: Comparison of polyelectrolyte and neutral polymer solutions. Rheol. Acta 2010, 49, 425–442. [Google Scholar] [CrossRef]
- Lashkaripour, A.; Rodriguez, C.; Mehdipour, N.; Mardian, R.; McIntyre, D.; Ortiz, L.; Campbell, J.; Densmore, D. Machine learning enables design automation of microfluidic flow-focusing droplet generation. Nat. Commun. 2021, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, D.; Lashkaripour, A.; Fordyce, P.; Densmore, D. Machine learning for microfluidic design and control. Lab Chip 2022, 22, 2925–2937. [Google Scholar] [CrossRef]
- Fukada, K.; Seyama, M. Microfluidic Devices Controlled by Machine Learning with Failure Experiments. Anal. Chem. 2022, 94, 7060–7065. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyasountharan, A.; Del Giudice, F. Viscoelastic Particle Encapsulation Using a Hyaluronic Acid Solution in a T-Junction Microfluidic Device. Micromachines 2023, 14, 563. https://doi.org/10.3390/mi14030563
Jeyasountharan A, Del Giudice F. Viscoelastic Particle Encapsulation Using a Hyaluronic Acid Solution in a T-Junction Microfluidic Device. Micromachines. 2023; 14(3):563. https://doi.org/10.3390/mi14030563
Chicago/Turabian StyleJeyasountharan, Anoshanth, and Francesco Del Giudice. 2023. "Viscoelastic Particle Encapsulation Using a Hyaluronic Acid Solution in a T-Junction Microfluidic Device" Micromachines 14, no. 3: 563. https://doi.org/10.3390/mi14030563
APA StyleJeyasountharan, A., & Del Giudice, F. (2023). Viscoelastic Particle Encapsulation Using a Hyaluronic Acid Solution in a T-Junction Microfluidic Device. Micromachines, 14(3), 563. https://doi.org/10.3390/mi14030563






