Biomechanical Assessment of Red Blood Cells in Pulsatile Blood Flows
Abstract
1. Introduction
2. Materials and Methods
2.1. Microfluidic Device and Experimental Setup
2.2. Acquisition of Microscopic Image Intensity and Blood Velocity
2.3. Analytical Formula of Time Constant of Blood Flow
2.4. Blood Preparation for Stimulating RBCs Sedimentation in the Driving Syringe
3. Results and Discussion
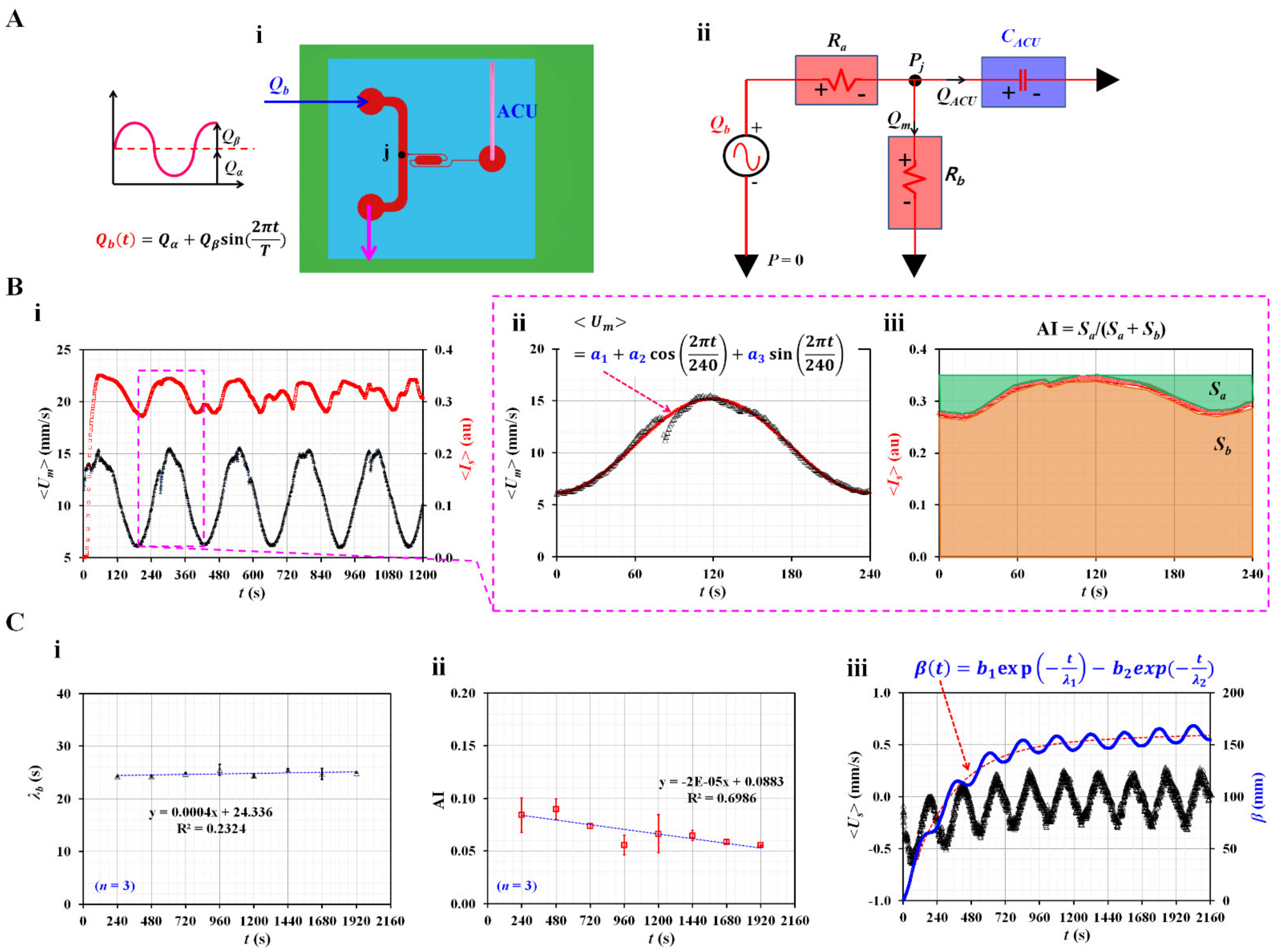
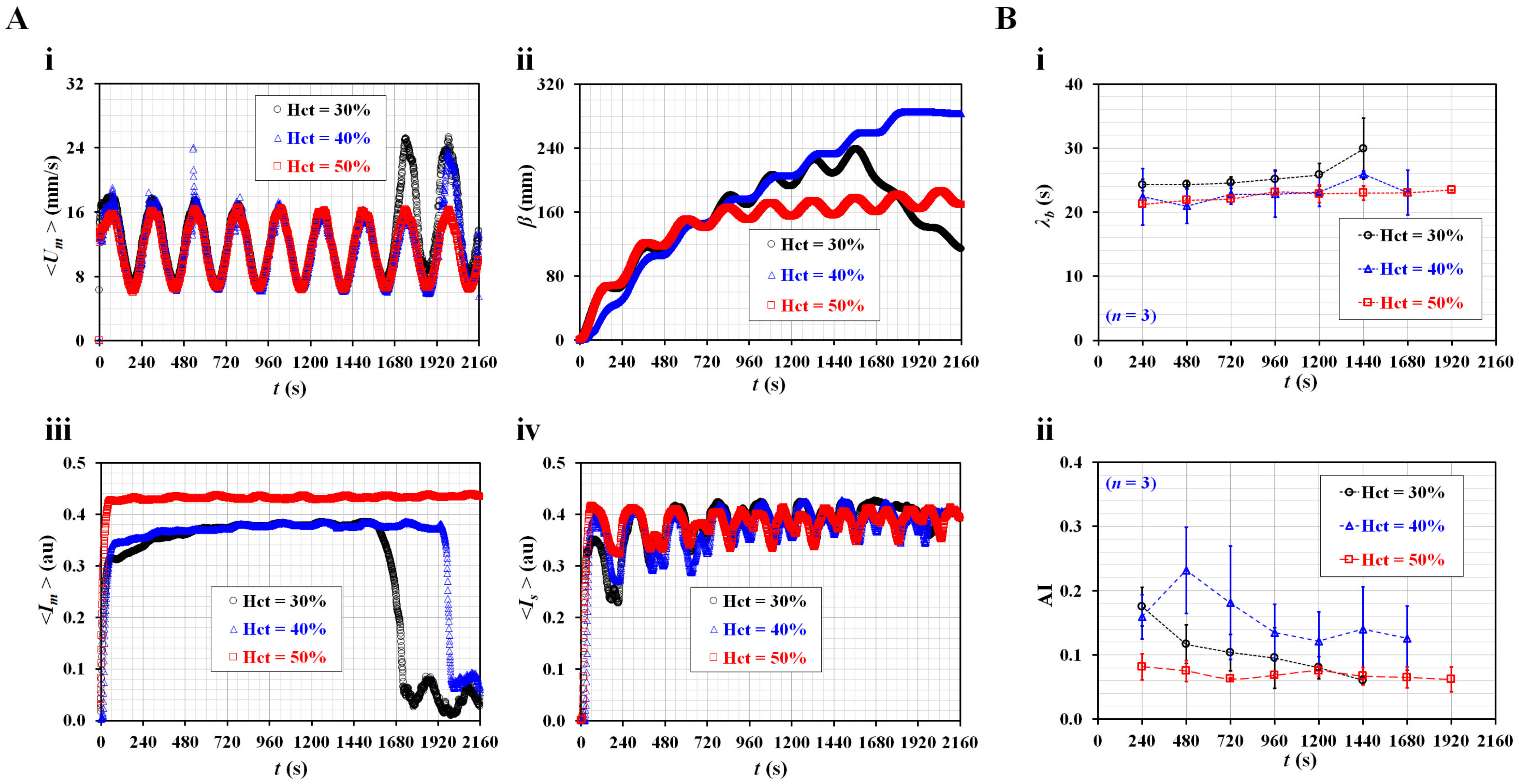
3.1. Quantification of Three Rheological Properties in Pulsatile Blood Flow
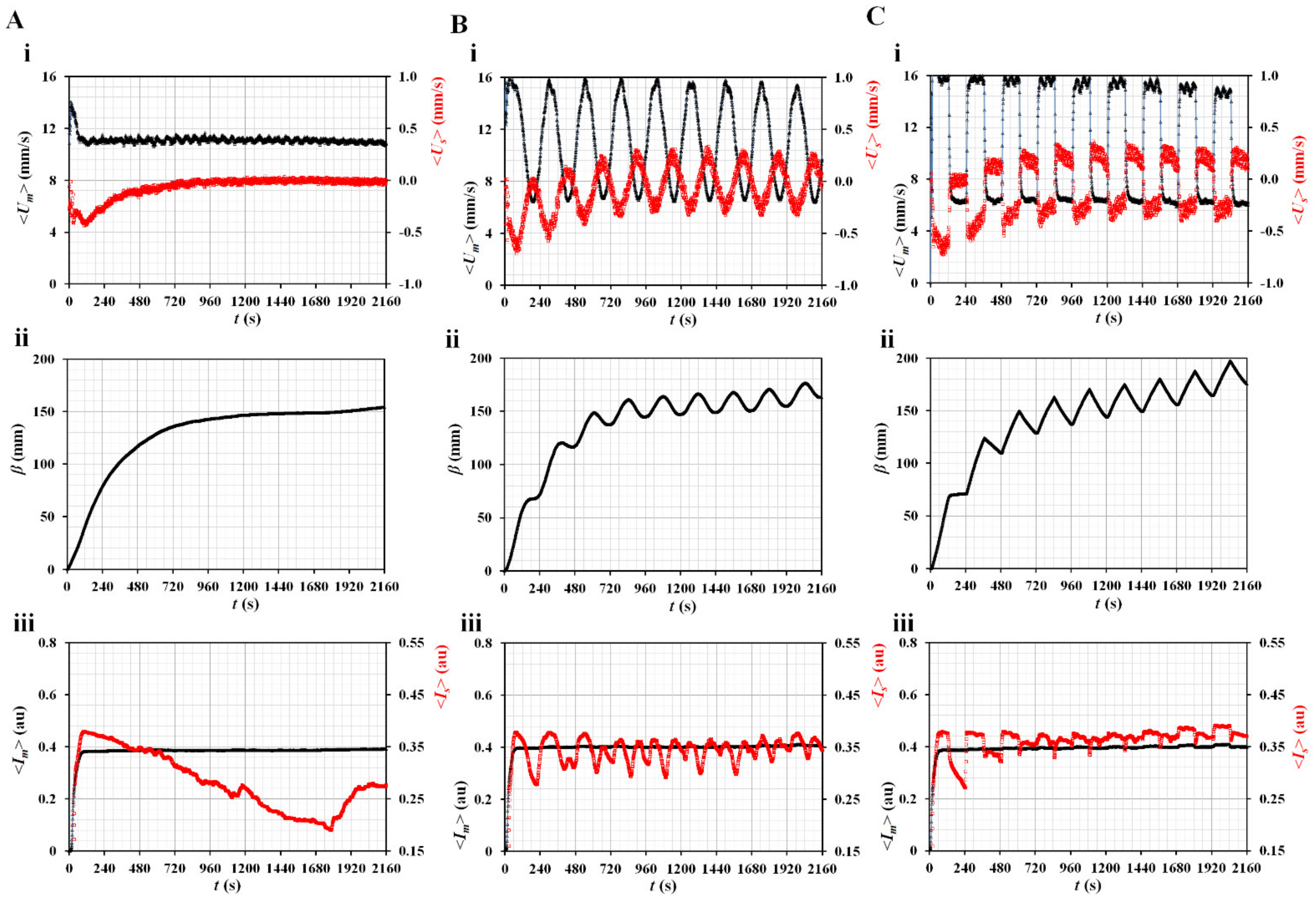
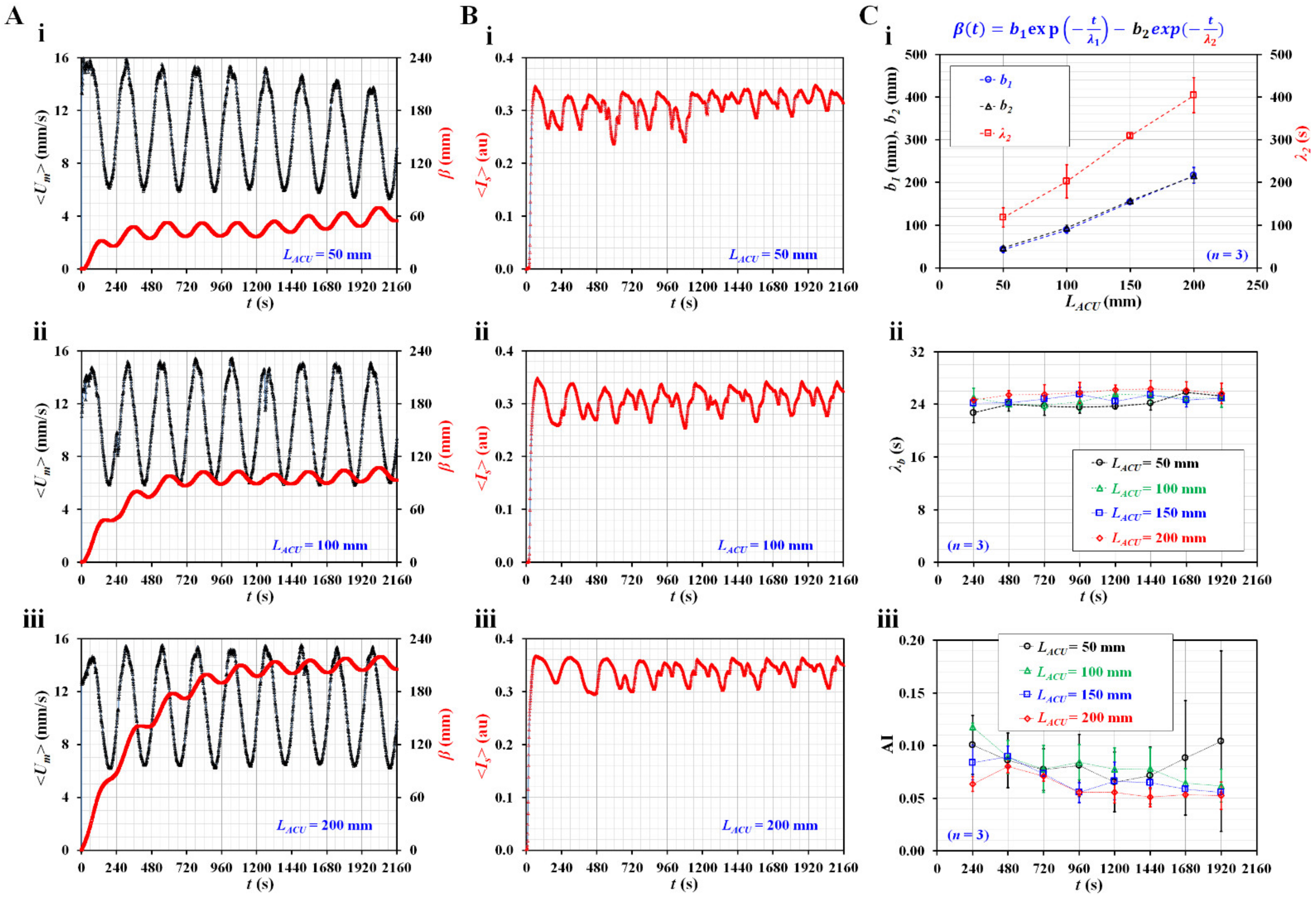
3.2. Contributions of Three Vital Factors (i.e., Flow Profile, Air Cavity, and Hematocrit) to Rheological Properties
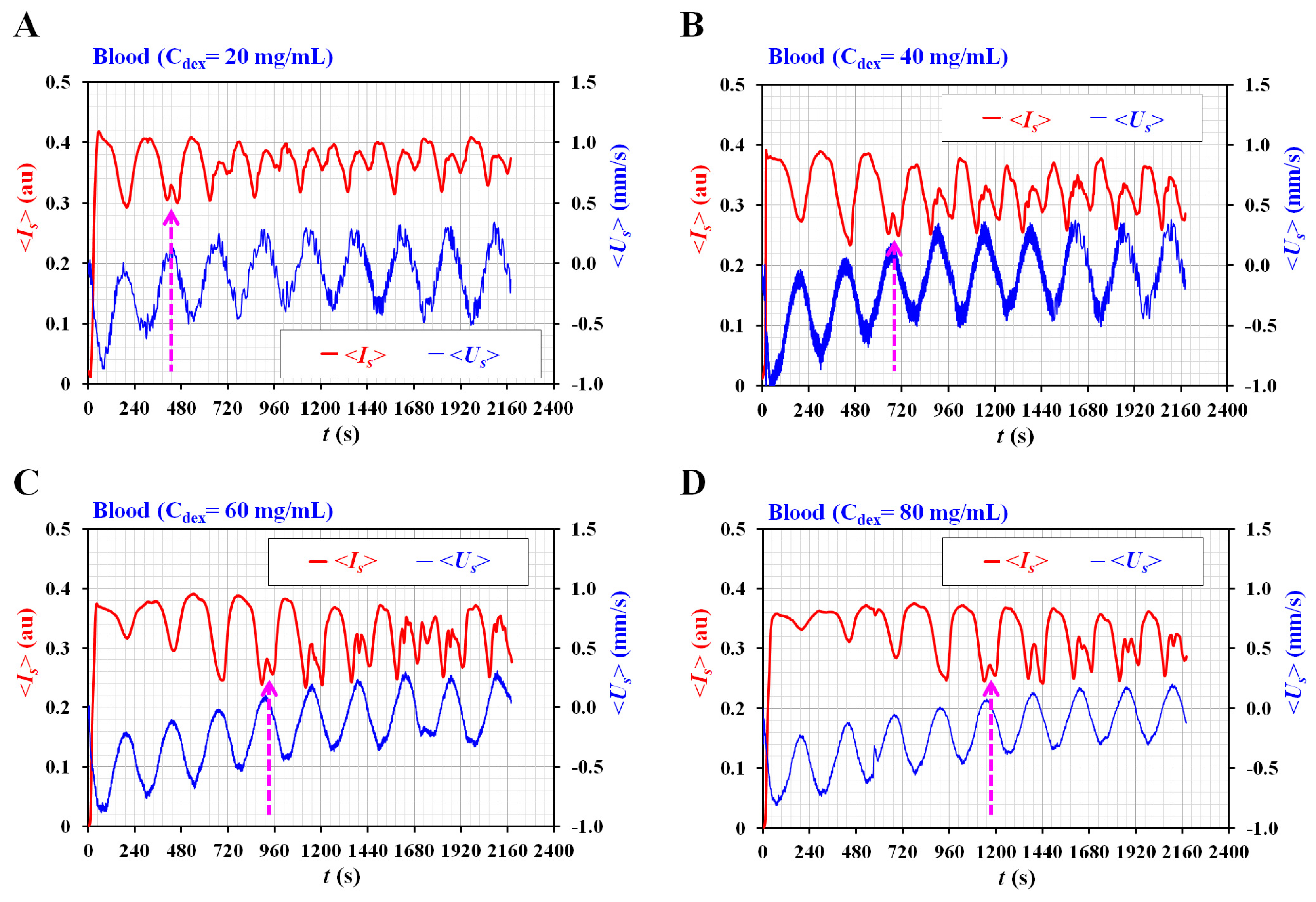
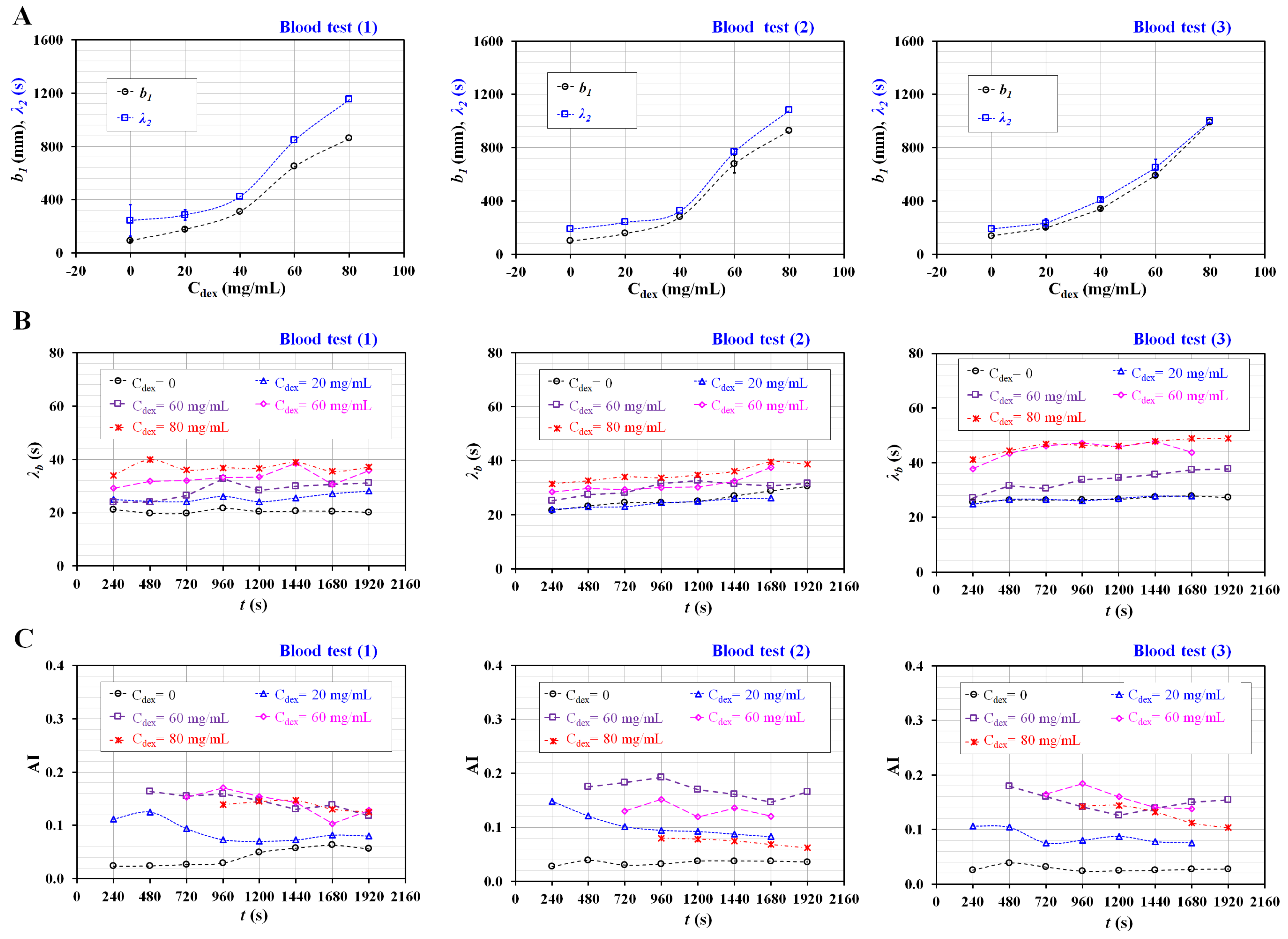
3.3. Quantification of Three Rheological Properties for Aggregated Blood and Hardened Blood
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar]
- Secomb, T.W. Red blood cell mechanics and capillary blood rheology. Cell Biophys. 1991, 18, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Dumanskiy, Y.V.; Stoliarova, O.Y.; Syniachenko, O.V.; Giulmamedova, M.F.; Potapov, Y.A. Adsorption-rheological properties of blood serum in lung cancer patients. Exp. Oncol. 2017, 39, 304–307. [Google Scholar] [CrossRef]
- Nader, E.; Romana, M.; Connes, P. The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease. Front Immunol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Ballas, S.K.; Connes, P. Rheological properties of sickle erythrocytes in patients with sickle-cell anemia: The effect of hydroxyurea, fetal hemoglobin, and α-thalassemia. Eur. J. Haematol. 2018, 101, 798–803. [Google Scholar] [CrossRef]
- Vent-Schmidt, J.; Waltz, X.; Romana, M.; Hardy-Dessources, M.-D.; Lemonne, N.; Billaud, M.; Etienne-Julan, M.; Connes, P. Blood Thixotropy in Patients with Sickle Cell Anaemia: Role of Haematocrit and Red Blood Cell Rheological Properties. PLoS ONE 2014, 9, e114412. [Google Scholar] [CrossRef]
- Marcinkowska-Gapińska, A.; Kowal, P. Influence of magnetostimulation therapy on rheological properties of blood in neurological patients. Electromagn. Biol. Med. 2016, 35, 260–264. [Google Scholar] [CrossRef]
- França, E.L.; Ribeiro, E.B.; Scherer, E.F.; Cantarini, D.G.; Pessôa, R.S.; França, F.L.; Honorio-França, A.C. Effects of Momordica charantia L. on the Blood Rheological Properties in Diabetic Patients. BioMed. Res. Int. 2014, 2014, 1–8. [Google Scholar]
- Vermes, I.; Steinmetz, E.T.; Zeyen, L.J.J.M.; Van Der Veen, E.A. Rheological properties of white blood cells are changed in diabetic patients with microvascular complications. Diabetologia 1987, 30, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Słoczyńska, K.; Kózka, M.; Marona, H. Rheological properties of young and aged erythrocytes in chronic venous disease patients with varicose veins. Clin. Hemorheol. Microcirc. 2015, 60, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kasperska-Zajac, A.; Brzoza, Z.; Koczy-Baron, E.; Jagodzinska, J.; Slowinska, L.; Rogala, B. Blood Rheological Properties in Patients with Atopic Dermatitis (AD). Inflammation 2009, 32, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.X.; Ji, M.; Han, L.Z.; Xu, C.C.; Pan, F.F.; Hu, T.J.; Zhong, Y. Erythrocyte rheological properties but not whole blood and plasma viscosity are associated with severity of hypertension in older people. Z Gerontol. Geriatr. 2017, 50, 233–238. [Google Scholar] [CrossRef]
- Fornal, M.; Korbut, R.A.; Lekka, M.; Pyka-Fościak, G.; Wizner, B.; Styczen, J.; Grodzicki, T. Rheological properties of erythrocytes in patients with high risk of cardiovascular disease. Clin. Hemorheol. Microcirc. 2008, 39, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.D.; Drummond, M.M.; Lorimer, A.R.; Hutton, I.; Forbes, C.D.; Prentice, C.R.; Barbenel, J.C. Relation between extent of coronary artery disease and blood viscosity. Br. Med. J. 1980, 280, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.F.; Shirure, V.S.; Chu, Y.E.; Soetikno, A.G.; George, S.C. Microfluidic device to attain high spatial and temporal control of oxygen. PLoS ONE 2018, 13, e0209574. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; Van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. BioMedical. Eng. Online 2020, 1, 1–19. [Google Scholar] [CrossRef]
- Bukowska, D.M.; Derzsi, L.; Tamborski, S.; Szkulmowski, M.; Garstecki, P.; Wojtkowski, M. Assessment of the flow velocity of blood cells in a microfluidic device using joint spectral and time domain optical coherence tomography. Opt. Express 2013, 21, 24025. [Google Scholar] [CrossRef] [PubMed]
- Cairone, F.; Ortiz, D.; Cabrales, P.J.; Intaglietta, M.; Bucolo, M. Emergent behaviors in RBCs flows in micro-channels using digital particle image velocimetry. Microvasc. Res. 2018, 116, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, D.; Liu, X.; Xie, Y.; Shan, J.; Huang, H.; Yu, X.; Chen, Y.; Zheng, W.; Li, Z. Point-of-Care Blood Coagulation Assay Based on Dynamic Monitoring of Blood Viscosity Using Droplet Microfluidics. ACS Sens. 2022, 7, 2170–2177. [Google Scholar] [CrossRef]
- Khnouf, R.; Karasneh, D.; Abdulhay, E.; Abdelhay, A.; Sheng, W.; Fan, Z.H. Microfluidics-based device for the measurement of blood viscosity and its modeling based on shear rate, temperature, and heparin concentration. Biomed. Microdevices 2019, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.E.; Li, Y.; McCormick, J.; McCracken, B.; Colmenero, C.; Ward, K.; Burns, M.A. A droplet-based microfluidic viscometer for the measurement of blood coagulation. Biomicrofluidics 2020, 14, 014109. [Google Scholar] [CrossRef]
- Agarwal, R.; Sarkar, A.; Paul, S.; Chakraborty, S. A portable rotating disc as blood rheometer. Biomicrofluidics 2019, 13, 064120. [Google Scholar] [CrossRef]
- Park, J.H.; Go, T.; Lee, S.J. Label-Free Sensing and Classification of Old Stored Blood. Ann. Biomed. Eng. 2017, 45, 2563–2573. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Zhbanov, A.; Yang, S. A physiometer for simultaneous measurement of whole blood viscosity and its determinants: Hematocrit and red blood cell deformability. Analyst 2019, 144, 3144–3157. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Franke, T. Acoustic erythrocytometer for mechanically probing cell viscoelasticity. Lab Chip 2020, 20, 1991–1998. [Google Scholar] [CrossRef]
- Toderi, M.A.; Castellini, H.V.; Riquelme, B.D. Descriptive parameters of the erythrocyte aggregation phenomenon using a laser transmission optical chip. J. Biomed. Opt. 2017, 22, 17003. [Google Scholar] [CrossRef]
- Kaliviotis, E.; Yianneskis, M. Blood viscosity modelling: Influence of aggregate network dynamics under transient conditions. Biorheology 2011, 48, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Serhrouchni, S.; Makhro, A.; Bogdanova, A.; Lee, S.S. Simple Assessment of Red Blood Cell Deformability Using Blood Pressure in Capillary Channels for Effective Detection of Subpopulations in Red Blood Cells. ACS Omega 2022, 7, 38576–38588. [Google Scholar] [CrossRef]
- Shin, D.A.; Lee, J.C.; Shin, H.; Cho, Y.-J.; Kim, H.C. Point-of-care testing of plasma free hemoglobin and hematocrit for mechanical circulatory support. Sci. Rep. 2021, 11, 3788. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, S.; Das, C.; Chandra, S.; Sharma, K.D.; Karmakar, A.; Chattoapadhyay, S. On-chip estimation of hematocrit level for diagnosing anemic conditions by Impedimetric techniques. Biomed. Microdevices 2020, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Zhbanov, A.; Yang, S. Microfluidic Systems for Blood and Blood Cell Characterization. Biosensors 2023, 13, 13. [Google Scholar] [CrossRef]
- Kang, Y.J.; Lee, S.-J. In vitro and ex vivo measurement of the biophysical properties of blood using microfluidic platforms and animal models. Analyst 2018, 143, 2723–2749. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, S.Y.; Jee, S.; Atajanov, A.; Yang, S. Micro-viscometer for measuring shear-varying blood viscosity over a wide-ranging shear rate. Sensors 2017, 17, 1442. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yoon, S.Y.; Lee, K.-H.; Yang, S. A highly accurate and consistent microfluidic viscometer for continuous blood viscosity measurement. Artif. Organs 2010, 34, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Ryu, J.; Lee, S.-J. Label-free viscosity measurement of complex fluids using reversal flow switching manipulation in a microfluidic channel. Biomicrofluidics 2013, 7, 044106. [Google Scholar]
- Srivastava, N.; Davenport, R.D.; Burns, M.A. Nanoliter Viscometer for Analyzing Blood Plasma and Other Liquid Samples. Anal. Chem. 2005, 77, 383–392. [Google Scholar] [CrossRef]
- Lee, J.; Chou, T.-C.; Kang, D.; Kang, H.; Chen, J.; Baek, K.I.; Wang, W.; Ding, Y.; Carlo, D.D.; Tai, Y.-C.; et al. A Rapid Capillary-Pressure Driven Micro-Channel to Demonstrate Newtonian Fluid Behavior of Zebrafish Blood at High Shear Rates. Sci. Rep. 2017, 7, 1980. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, D.; Lam, R. Microfluidic Viscometer Using a Suspending Micromembrane for Measurement of Biosamples. Micromachines 2020, 11, 934. [Google Scholar] [CrossRef]
- Mustafa, A.; Eser, A.; Aksu, A.C.; Kiraz, A.; Tanyeri, M.; Erten, A.; Yalcin, O. A micropillar-based microfluidic viscometer for Newtonian and non-Newtonian fluids. Anal. Chim. Acta 2020, 1135, 107–115. [Google Scholar] [CrossRef]
- Lee, T.A.; Liao, W.H.; Wu, Y.F.; Chen, Y.L.; Tung, Y.C. Electrofluidic Circuit-Based Microfluidic Viscometer for Analysis of Newtonian and Non-Newtonian Liquids under Different Temperatures. Anal. Chem. 2018, 90, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood rheology: Key parameters, impact on blood flow, role in sickle cell disease and effects of exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Sabuncu, A.C.; Muldur, S.; Cetin, B.; Usta, O.B.; Aubry, N. β-dispersion of blood during sedimentation. Sci. Rep. 2021, 11, 2642. [Google Scholar]
- Kang, Y.; Kim, B. Multiple and Periodic Measurement of RBC Aggregation and ESR in Parallel Microfluidic Channels under On-Off Blood Flow Control. Micromachines 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Namgung, B.; Lee, T.; Tan, J.K.S.; Poh, D.K.H.; Park, S.; Chng, K.Z.; Agrawal, R.; Park, S.Y.; Leo, H.L.; Kim, S. Vibration motor-integrated low-cost, miniaturized system for rapid quantification of red blood cell aggregation. Lab Chip 2020, 20, 3930–3937. [Google Scholar] [CrossRef]
- Isiksacan, Z.; Erel, O.; Elbuken, C. A portable microfluidic system for rapid measurement of the erythrocyte sedimentation rate. Lab Chip 2016, 16, 4682–4690. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.; Lee, B.K.; Shin, S. Influence of shear stress on erythrocyte aggregation. Clin. Hemorheol. Microcirc. 2016, 62, 165–171. [Google Scholar] [CrossRef]
- Lee, K.; Kinnunen, M.; Danilina, A.V.; Ustinov, V.D.; Shin, S.; Meglinski, I.; Priezzhev, A.V. Characterization at the individual cell level and in whole blood samples of shear stress preventing red blood cells aggregation. J. Biomech. 2016, 49, 1021–1026. [Google Scholar] [CrossRef]
- Kang, Y.J. Red Blood Cell Sedimentation Index Using Shear Stress of Blood Flow in Microfluidic Channel. Biosensors 2022, 12, 547. [Google Scholar] [CrossRef]
- Kang, Y.J. Assessment of blood biophysical properties using pressure sensing with micropump and microfluidic comparator. Micromachines 2022, 13, 483. [Google Scholar] [CrossRef]
- Rajeeva Pandian, N.K.; Jain, A. In silico analyses of blood flow and oxygen transport in human micro-veins and valves. Clin. Hemorheol. Microcirc. 2022, 81, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Kim, M.G.; Son, K.H.; Lim, C.H.; Son, H.S.; Yoon, S.Y.; Kwon, H.S.; Yang, S. Experimental investigation of pulsatility effect on the deformability and hemolysis of blood cells. Artif. Organs 2010, 34, E103–E109. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Kang, Y.J.; Kim, M.G.; Yang, S.; Lim, C.H.; Son, H.S.; Kim, J.S.; Lee, S.Y.; Son, K.H.; Sun, K. The Effect of Pulsatile Versus Nonpulsatile Blood Flow on Viscoelasticity and Red Blood Cell Aggregation in Extracorporeal Circulation. Korean J. Thorac. Cardiovasc. Surg. 2016, 49, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J. Contributions of red blood cell sedimentation in a driving syringe to blood flow in capillary channels. Micromachines 2022, 13, 909. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jeong, S.; Kang, Y.J. Ultrasound standing wave-based cell-to-liquid separation for measuring viscosity and aggregation of blood sample. Sensors 2020, 20, 2284. [Google Scholar] [CrossRef] [PubMed]
- Thielicke, W.; Stamhuis, E.J. PIVlab–Towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. J. Open Res. Softw. 2014, 2, e30. [Google Scholar] [CrossRef]
- Bourdon, C.J.; Olsen, M.G.; Gorby, A.D. The depth of correction in miciro-PIV for high numerical aperaure and immersion objectives. J. Fluid Eng. T. ASME 2006, 128, 883–886. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Xiang, N.; Ni, Z. A microfluidic gas damper for stabilizing gas pressure in portable microfluidic systems. Biomicrofluidics 2016, 10, 054123. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Yan, J.; Zhu, T.; Guo, S.; Li, S.; Li, T. Standing Air Bubble-Based Micro-Hydraulic Capacitors for Flow Stabilization in Syringe Pump-Driven Systems. Micromachines 2020, 11, 396. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, K.; Ahn, B.; Furlani, E.P. Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 2012, 12, 515–545. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Lee, S.-J. Blood viscoelasticity measurement using steady and transient flow controls of blood in a microfluidic analogue of Wheastone-bridge channel. Biomicrofluidics 2013, 7, 054122. [Google Scholar]
- Kang, Y.J.; Yang, S. Fluidic low pass filter for hydrodynamic flow stabilization in microfluidic environments. Lab Chip 2012, 12, 1881–1889. [Google Scholar] [CrossRef]
- Yang, B.; Lin, Q. A Compliance-Based Microflow Stabilizer. J. Microelectromech. Syst. 2009, 18, 539–546. [Google Scholar] [CrossRef]
- Veenstra, T.T.; Sharma, N.R.; Forster, F.K.; Gardeniers, J.G.E.; Elwenspoek, M.C.; Berg, A.v.d. The design of an in-plane compliance structure for microfluidical systems. Sens. Actuator B Chem. 2002, 81, 377–383. [Google Scholar] [CrossRef]
- Kang, Y.J. Sequential quantification of blood and diluent using red cell sedimentation-based separation and pressure-induced work in a microfluidic channel. Anal. Methods 2022, 14, 1194–1207. [Google Scholar] [CrossRef]
- Kang, Y.J. Microfluidic-based biosensor for blood viscosity and erythrocyte sedimentation rate using disposable fluid delivery system. Micromachines 2020, 11, 215. [Google Scholar] [CrossRef]
- Kang, Y.J. Microfluidic quantification of blood pressure and compliance properties using velocity fields under periodic on-off blood flows. App. Sci. Basel. 2020, 10, 5273. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.J. Biomechanical Assessment of Red Blood Cells in Pulsatile Blood Flows. Micromachines 2023, 14, 317. https://doi.org/10.3390/mi14020317
Kang YJ. Biomechanical Assessment of Red Blood Cells in Pulsatile Blood Flows. Micromachines. 2023; 14(2):317. https://doi.org/10.3390/mi14020317
Chicago/Turabian StyleKang, Yang Jun. 2023. "Biomechanical Assessment of Red Blood Cells in Pulsatile Blood Flows" Micromachines 14, no. 2: 317. https://doi.org/10.3390/mi14020317
APA StyleKang, Y. J. (2023). Biomechanical Assessment of Red Blood Cells in Pulsatile Blood Flows. Micromachines, 14(2), 317. https://doi.org/10.3390/mi14020317






