A Novel Miniature and Selective CMOS Gas Sensor for Gas Mixture Analysis—Part 3: Extending the Chemical Modeling
Abstract
1. Introduction
2. Materials and Methods
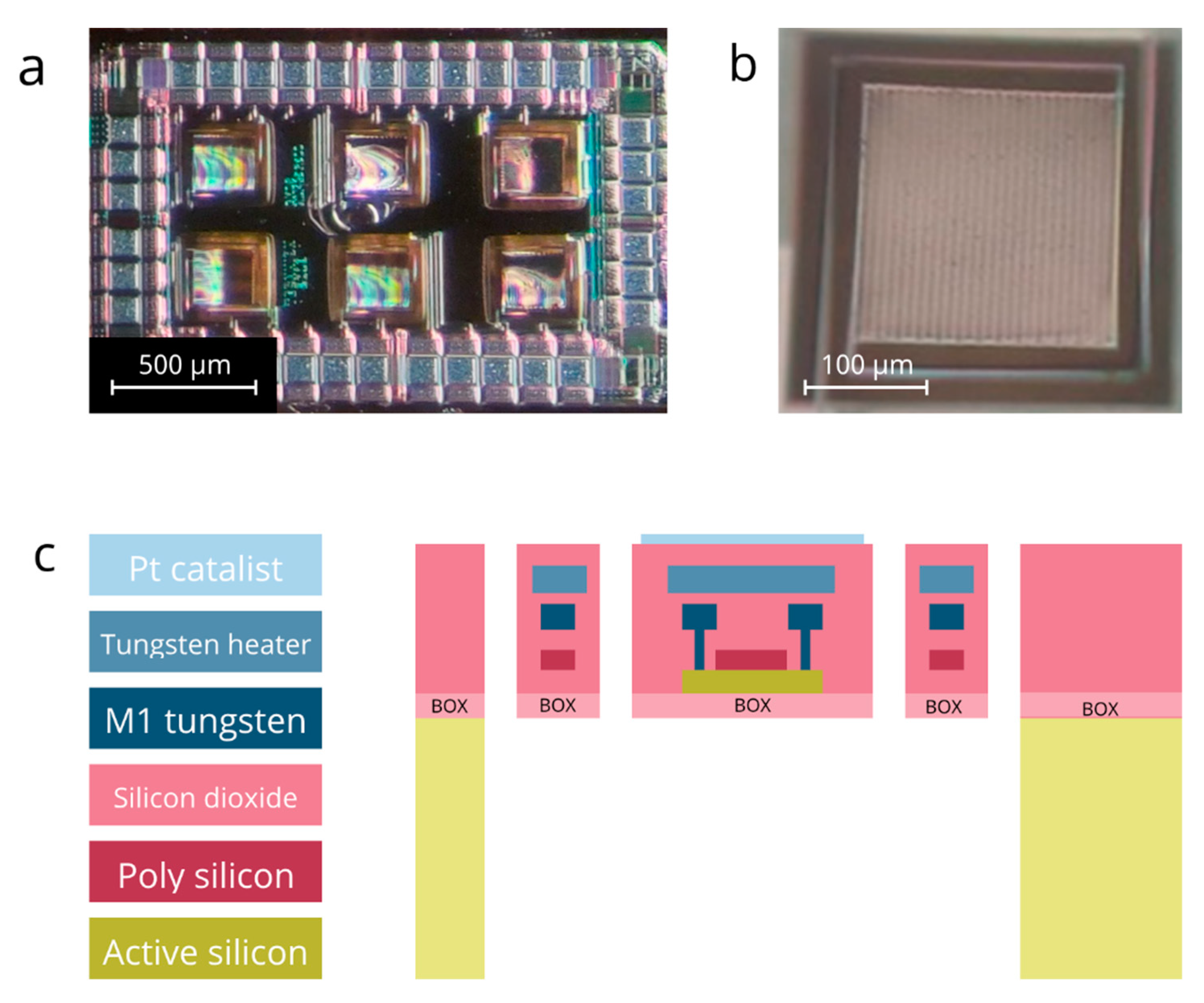
2.1. The GMOS Sensor
2.2. Simulation
3. Results
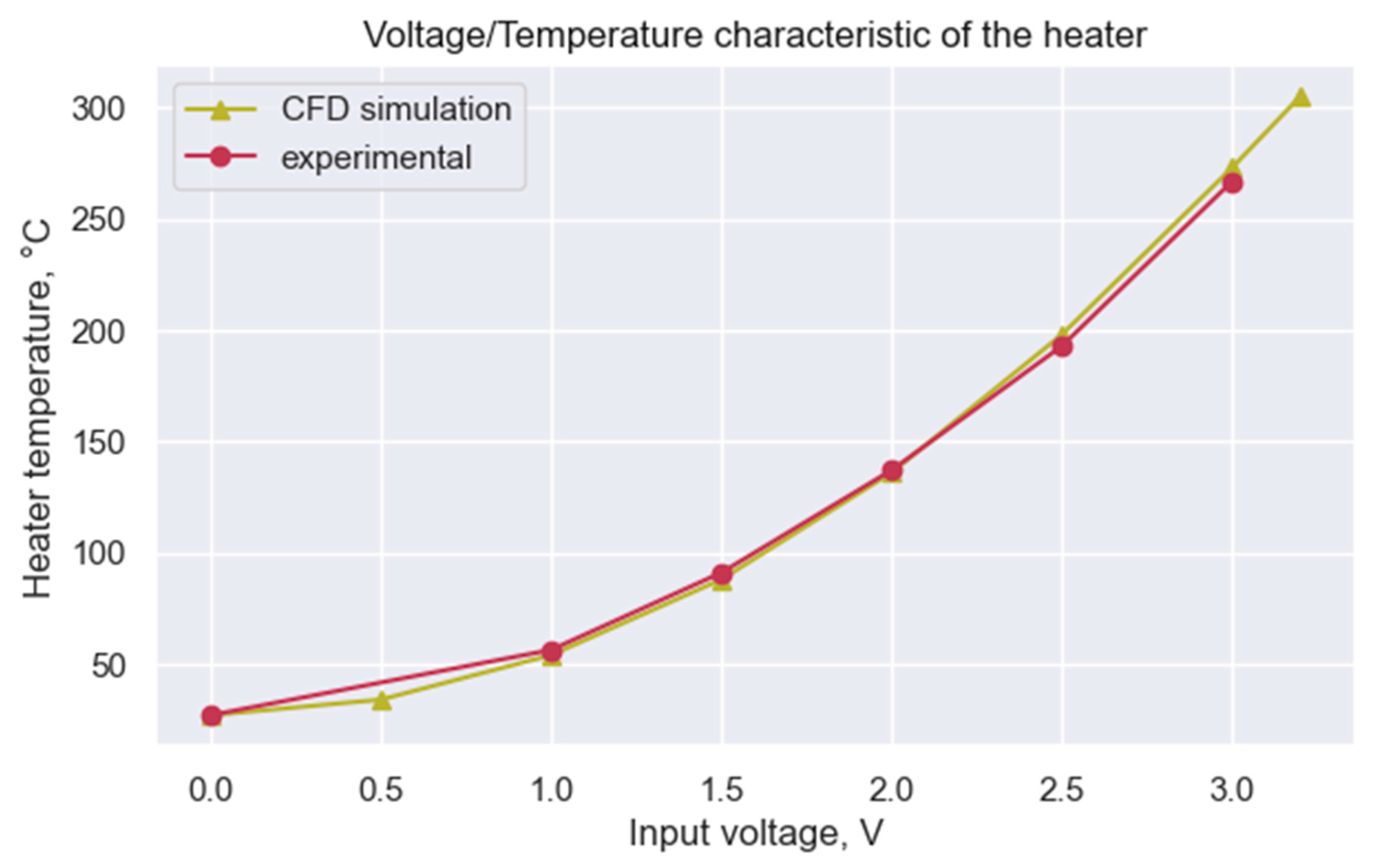
3.1. Voltage–Temperature Correlation
3.2. Ethanol Combustion under the Different Operating Temperatures
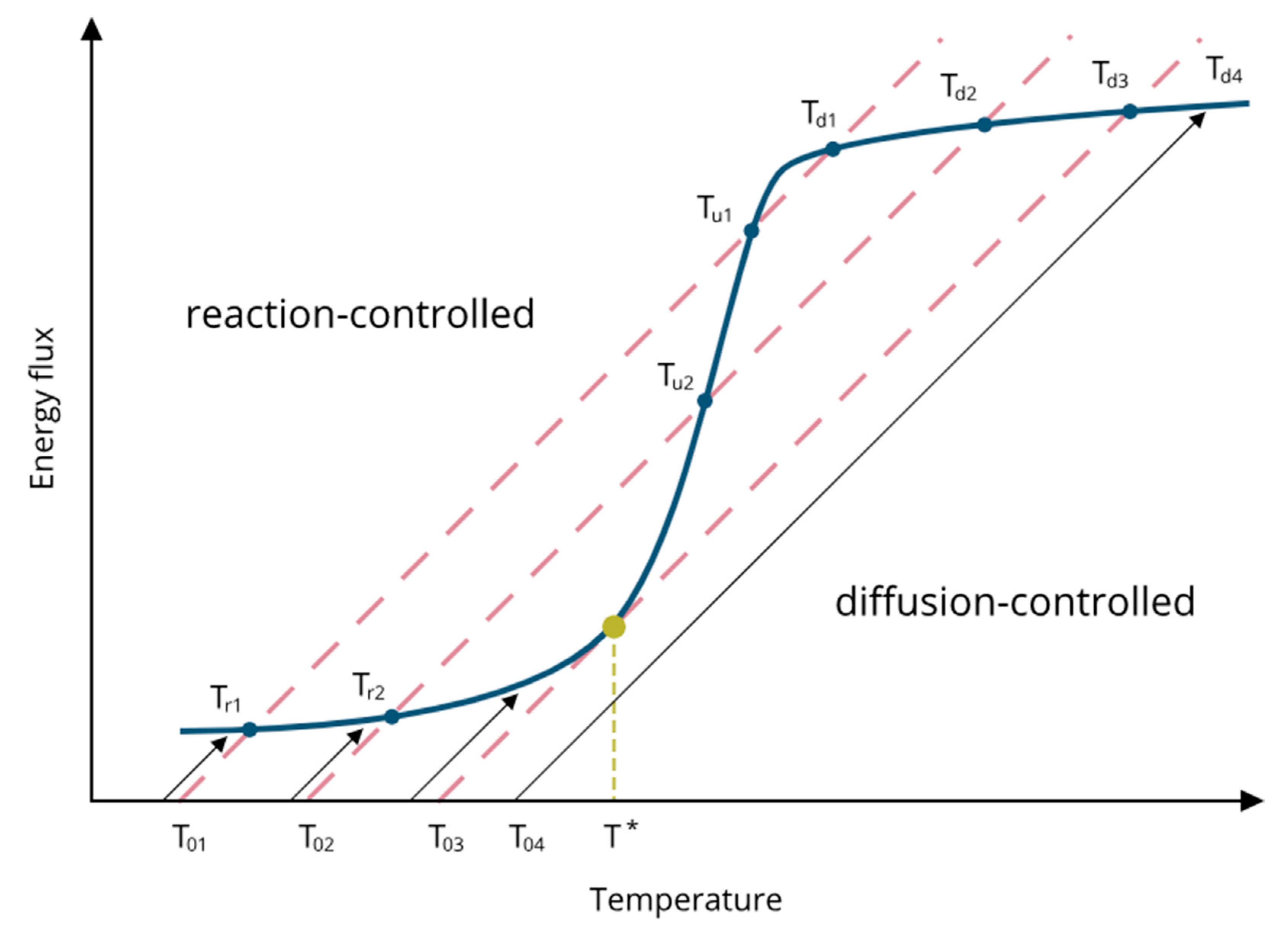
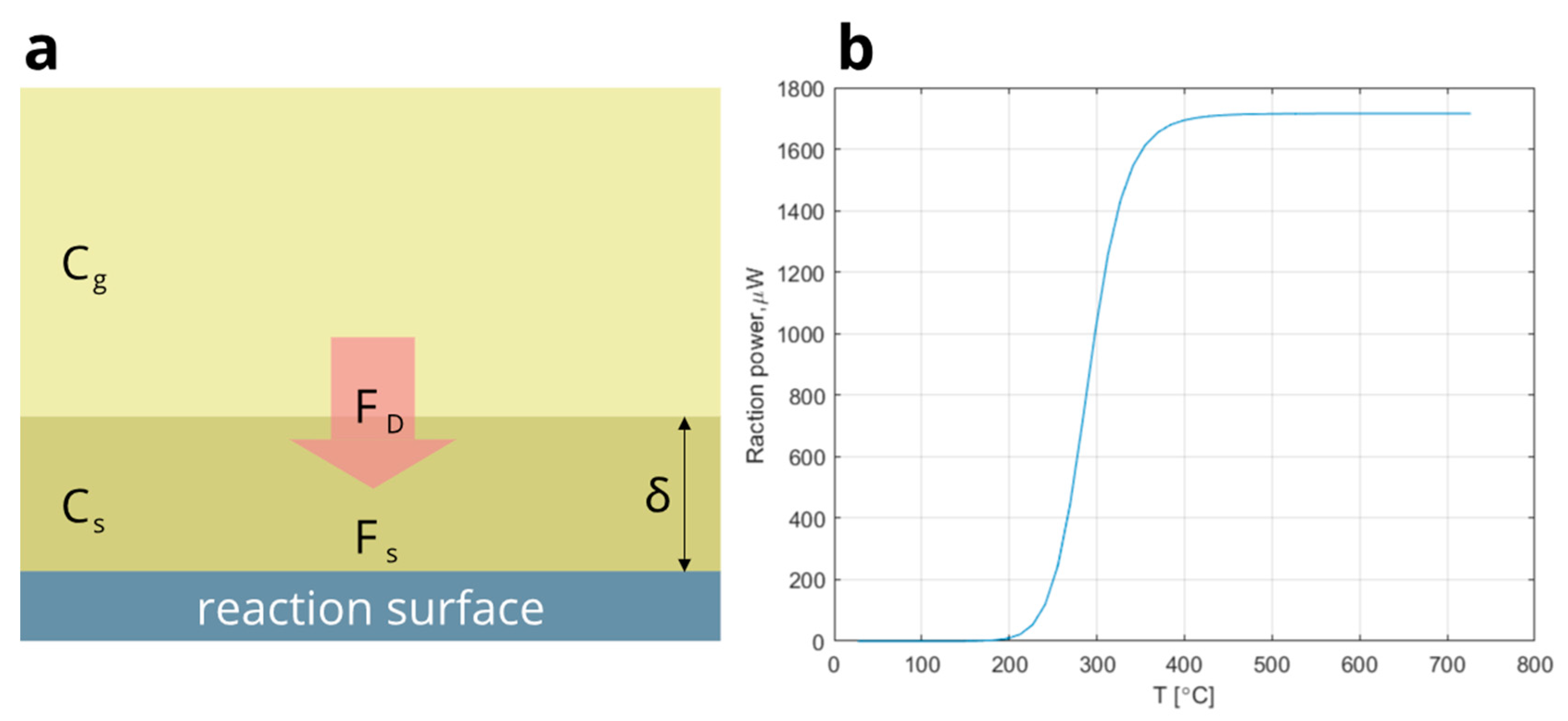
3.2.1. Theoretical
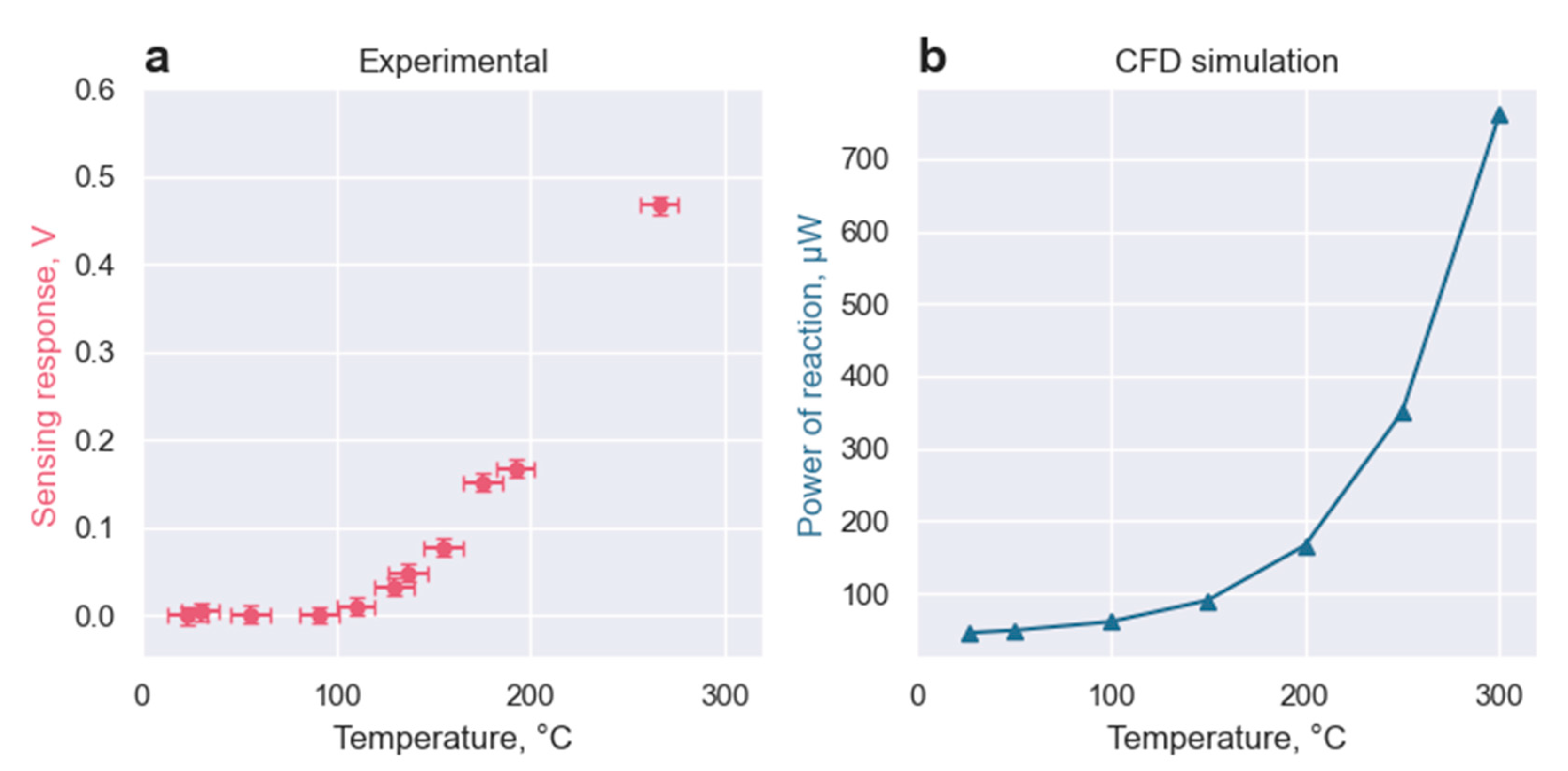
3.2.2. Experimental
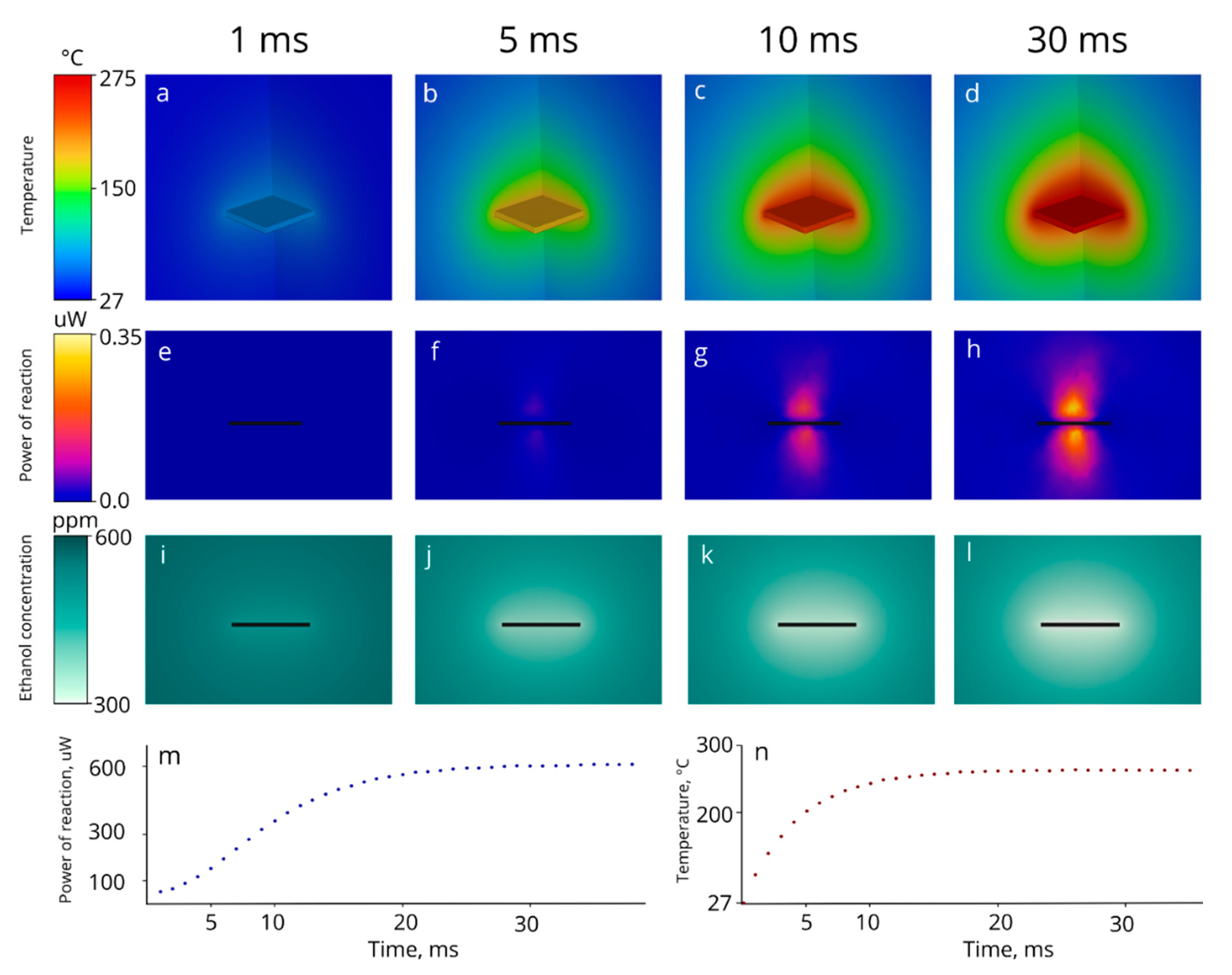
3.2.3. Matlab Simulation
3.3. Time Constant Investigation
4. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Love, C.; Nazemi, H.; El-Masri, E.; Ambrose, K.; Freund, M.; Emadi, A. A Review on Advanced Sensing Materials for Agricultural Gas Sensors. Sensors 2021, 21, 3423. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, M.; Dahlquist, E.; Lönnermark, A.; Säker, Ö. Gas sensors for early detection of fire hazards caused by vehicles in underground mines. In Proceedings of the 60th SIMS Conference on Simulation and Modelling SIMS 2019, Västerås, Sweden, 12–16 August 2019; Linköping University Electronic Press: Linköping, Sweden, 2020. [Google Scholar]
- Marder, D.; Tzanani, N.; Prihed, H.; Gura, S. Trace detection of explosives with a unique large volume injection gas chromatography-mass spectrometry (LVI-GC-MS) method. Anal. Methods 2018, 10, 2712–2721. [Google Scholar] [CrossRef]
- Placidi, P.; Gasperini, L.; Grassi, A.; Cecconi, M.; Scorzoni, A. Characterization of Low-Cost Capacitive Soil Moisture Sensors for IoT Networks. Sensors 2020, 20, 3585. [Google Scholar] [CrossRef] [PubMed]
- Shlenkevitch, D.; Stolyarova, S.; Blank, T.; Brouk, I.; Nemirovsky, Y. Novel Miniature and Selective Combustion-Type CMOS Gas Sensor for Gas-Mixture Analysis—Part 1: Emphasis on Chemical Aspects. Micromachines 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Avraham, M.; Stolyarova, S.; Blank, T.; Bar-Lev, S.; Golan, G.; Nemirovsky, Y. A Novel Miniature and Selective CMOS Gas Sensor for Gas Mixture Analysis—Part 2: Emphasis on Physical Aspects. Micromachines 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N., Jr.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Mikrochim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Zhang, C.; Shi, S.Q.; Zhang, H. High-Temperature Gas Sensors for Harsh Environment Applications: A Review. Clean Soil Air Water 2019, 47, 1800491. [Google Scholar] [CrossRef]
- Zito, C.; Perfecto, T.M.; Dippel, A.-C.; Volanti, D.P.; Koziej, D. Low-temperature carbon dioxide gas sensor based on yolk-shell Ceria nanospheres. ACS Appl. Mater. Interfaces 2020, 12, 17745–17751. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2016. [Google Scholar]
- Ansys Fluent. 2020. Available online: https://www.ansys.com/products/fluids/ansys-fluent (accessed on 1 December 2022).
- Zviagintsev, A.; Brouk, I.; Bloom, I.; Nemirovsky, Y. Self-heating effects in CMOS-SOI-NEMS transistors for uncooled passive IR sensors. In 2015 IEEE International Conference on Microwaves, Communications, Antennas and Electronic Systems (COMCAS); IEEE: Tel-Aviv, Israel, 2015. [Google Scholar]
- Svetlitza, A.; Blank, T.; Stolyarova, S.; Brouk, I.; Shefi, S.B.-L.; Nemirovsky, Y. CMOS-SOI-MEMS Thermal Antenna and Sensor for Uncooled THz Imaging. IEEE Trans. Electron Devices 2016, 63, 1260–1265. [Google Scholar] [CrossRef]
- Gitelman, L.; Stolyarova, S.; Bar-Lev, S.; Gutman, Z.; Ochana, Y.; Nemirovsky, Y. CMOS-SOI-MEMS Transistor for Uncooled IR Imaging. IEEE Trans. Electron Devices 2009, 56, 1935–1942. [Google Scholar] [CrossRef]
- Schwartz, A.; Holbrook, L.L.; Wise, H. Catalytic oxidation studies with platinum and palladium. J. Catal. 1971, 21, 199–207. [Google Scholar] [CrossRef]
- Sze, S.M.; Mattis, D.C. Physics of semiconductor devices. Phys. Today 1970, 23, 75. [Google Scholar] [CrossRef]
- X-fab. Available online: http://xfabulous.com (accessed on 10 December 2022).
- Vadasz, L.; Grove, A. Temperature dependence of MOS transistor characteristics below saturation. IEEE Trans. Electron Devices 1966, ED-13, 863–866. [Google Scholar] [CrossRef]
- AlZubaidi, I.; Govindan, S.M.; Enns, C.; Reiger, M.; Li, T. Diffusion coefficient of ethanol—Gasoline blends in air. In Proceedings of the 6th International Conference on Fluid Flow, Heat and Mass Transfer (FFHMT’19), Ottawa, ON, Canada, 18–19 June 2019. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goikhman, B.; Avraham, M.; Bar-Lev, S.; Stolyarova, S.; Blank, T.; Nemirovsky, Y. A Novel Miniature and Selective CMOS Gas Sensor for Gas Mixture Analysis—Part 3: Extending the Chemical Modeling. Micromachines 2023, 14, 270. https://doi.org/10.3390/mi14020270
Goikhman B, Avraham M, Bar-Lev S, Stolyarova S, Blank T, Nemirovsky Y. A Novel Miniature and Selective CMOS Gas Sensor for Gas Mixture Analysis—Part 3: Extending the Chemical Modeling. Micromachines. 2023; 14(2):270. https://doi.org/10.3390/mi14020270
Chicago/Turabian StyleGoikhman, Boris, Moshe Avraham, Sharon Bar-Lev, Sara Stolyarova, Tanya Blank, and Yael Nemirovsky. 2023. "A Novel Miniature and Selective CMOS Gas Sensor for Gas Mixture Analysis—Part 3: Extending the Chemical Modeling" Micromachines 14, no. 2: 270. https://doi.org/10.3390/mi14020270
APA StyleGoikhman, B., Avraham, M., Bar-Lev, S., Stolyarova, S., Blank, T., & Nemirovsky, Y. (2023). A Novel Miniature and Selective CMOS Gas Sensor for Gas Mixture Analysis—Part 3: Extending the Chemical Modeling. Micromachines, 14(2), 270. https://doi.org/10.3390/mi14020270





