Synthesis and Characterization of Calcium Silicate Nanoparticles Stabilized with Amino Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
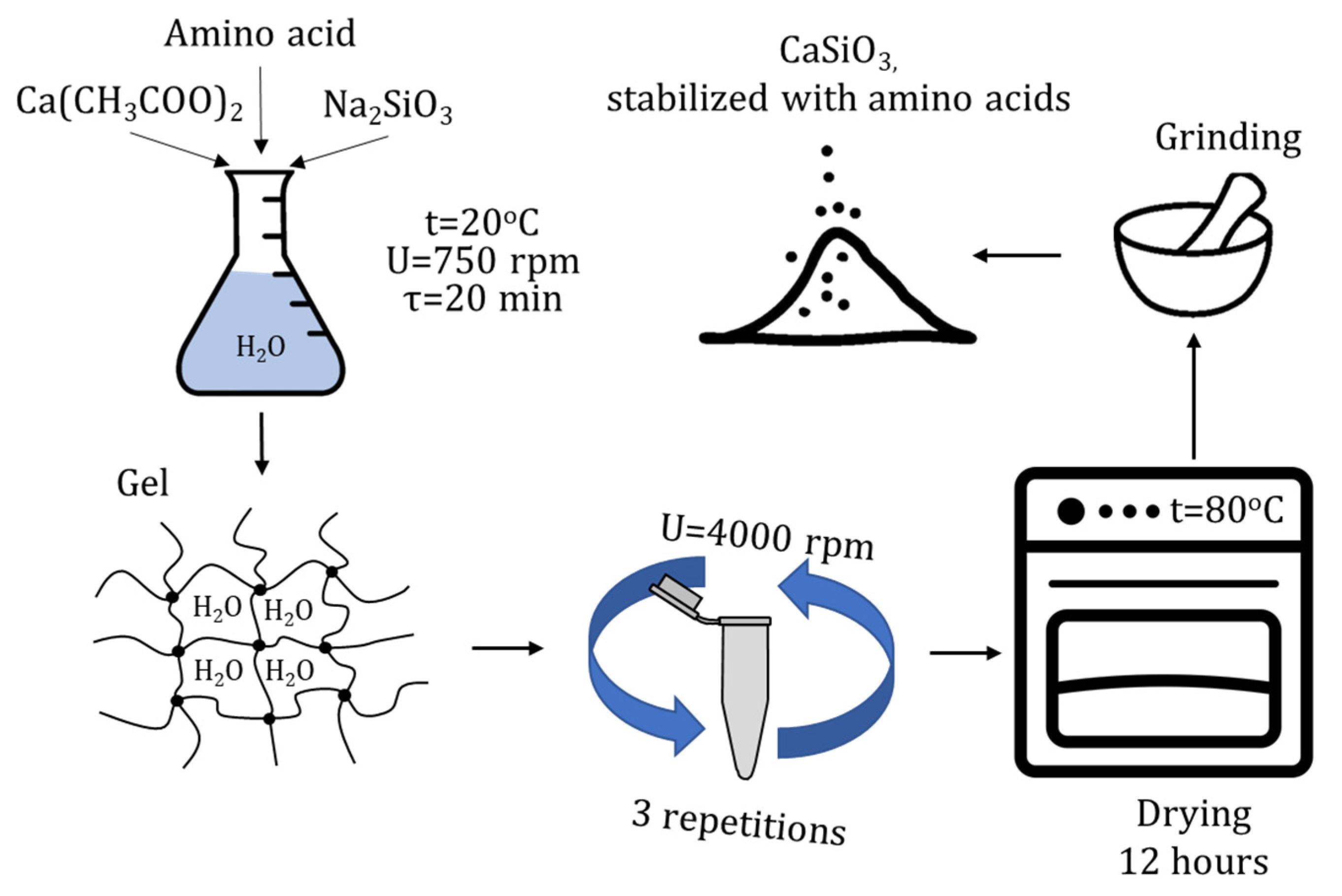
2.2. Method for the Synthesis of Calcium Silicate Nanoparticles
2.3. Method for the Synthesis of Calcium Silicate Nanoparticles Stabilized with Amino Acids
2.4. Methods for Studying Nanoparticles
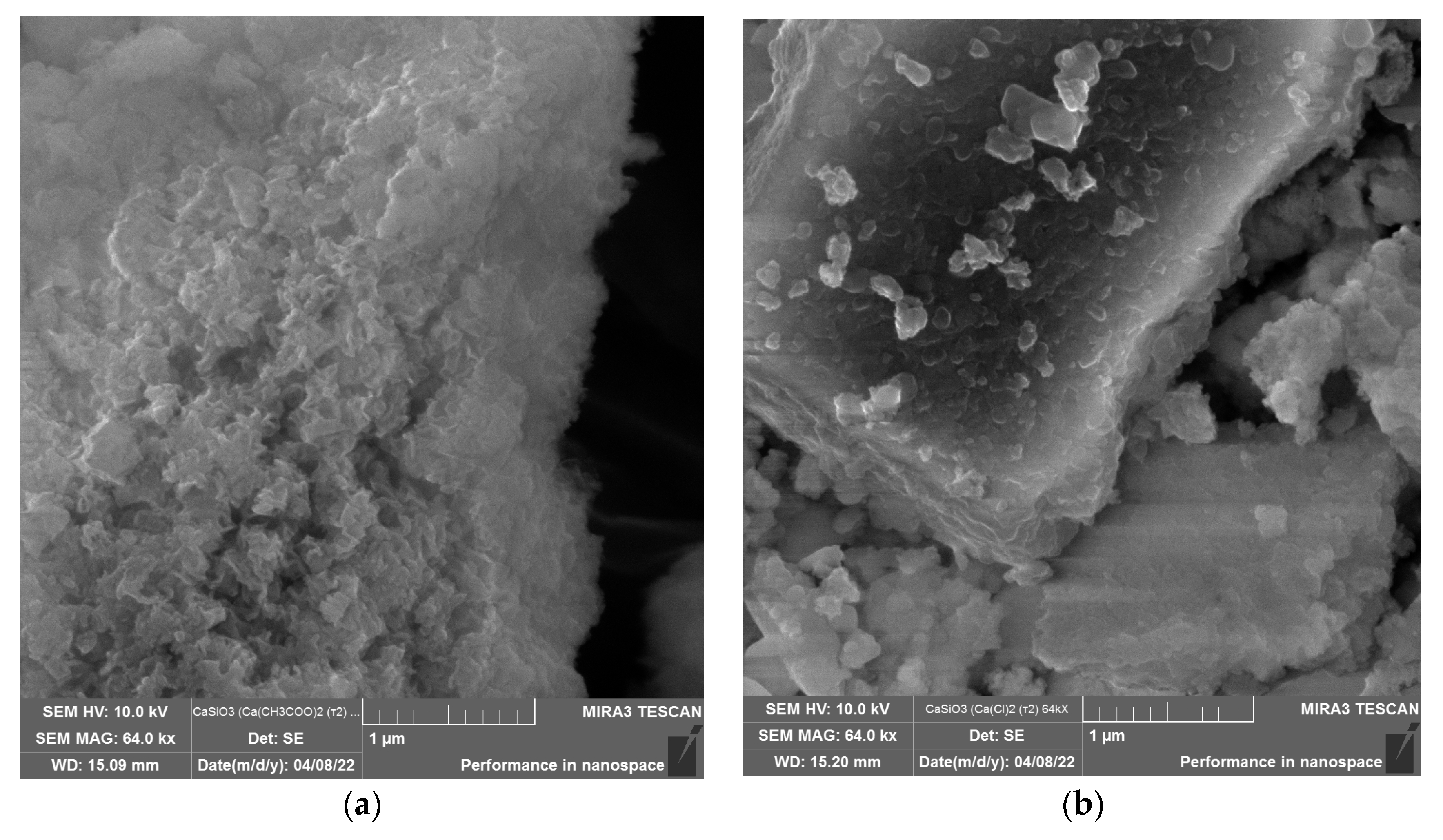
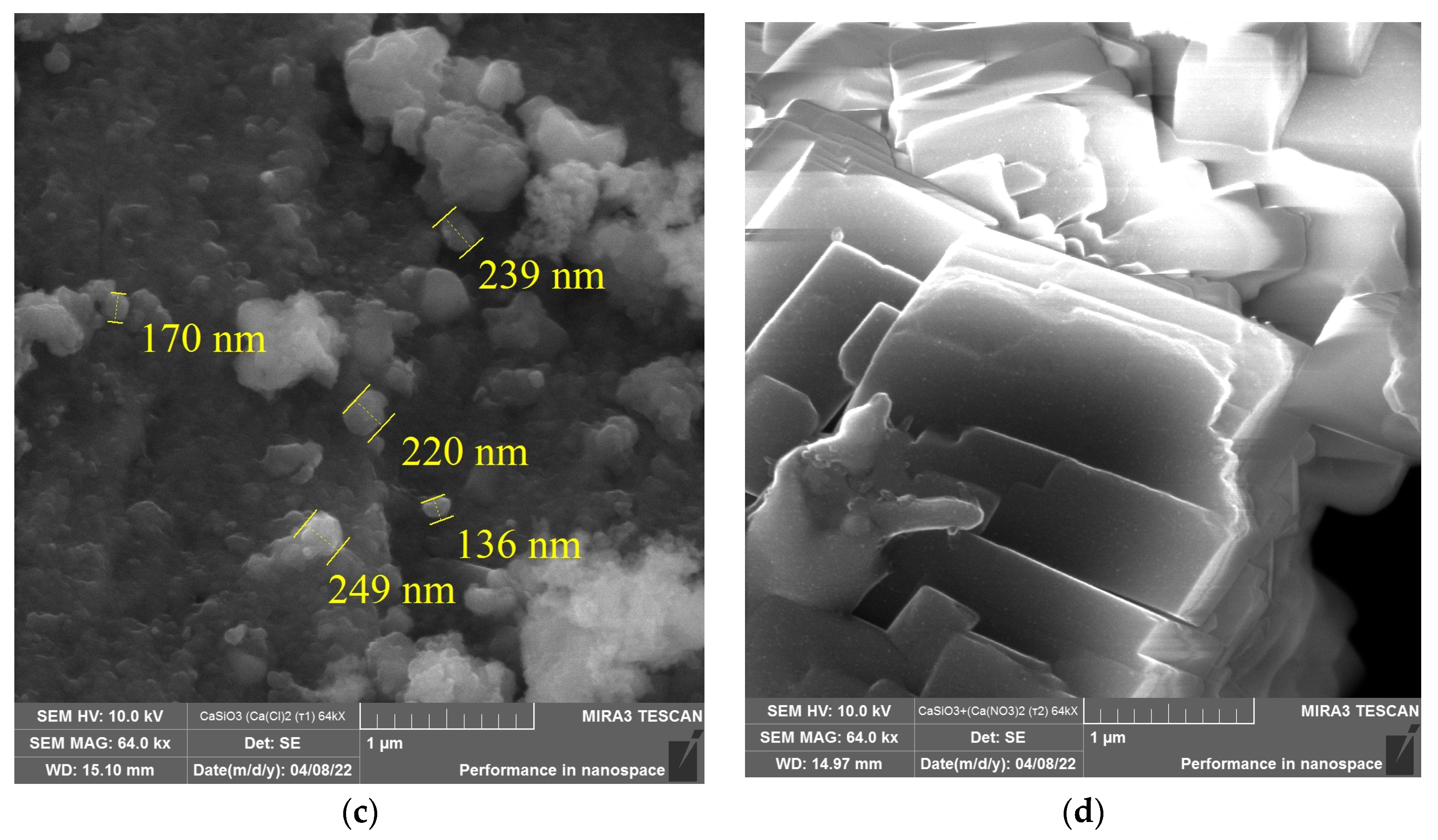
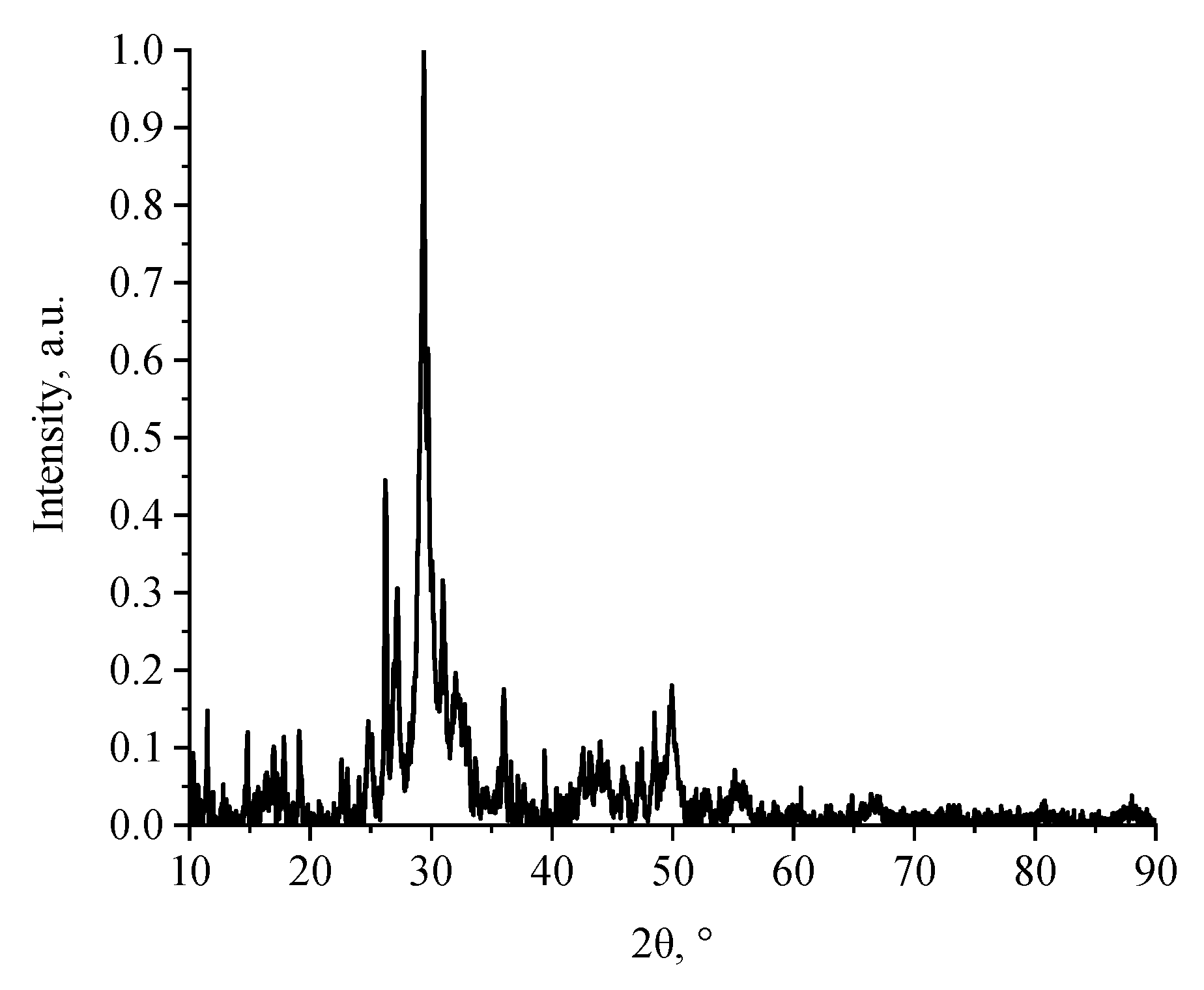
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.B. The Use of Hydrogels in Bone-Tissue Engineering. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e115–e118. [Google Scholar] [CrossRef]
- Sammarco, V.J.; Chang, L. Modern Issues in Bone Graft Substitutes and Advances in Bone Tissue Technology. Foot Ankle Clin. 2002, 7, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Einhorn, T.A.; Doll, B.A.; Sfeir, C. Bone Tissue Engineering. Woodhead Publishing Limited. 2011, 1, 1–21. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Nanotechnology for Bone Materials. Wiley Interdiscip. Rev. Nanomed. Nanobiotech. 2009, 1, 336–351. [Google Scholar] [CrossRef]
- Karlsson, K.H. Bone Implants—A Challenge to Materials Science. Ann. Chir. Gynaecol. 1999, 88, 226–235. [Google Scholar]
- Stevens, M.M. Biomaterials for Bone Tissue Engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef]
- Ducheyne, P.; Qiu, Q. Bioactive Ceramics: The Effect of Surface Reactivity on Bone Formation and Bone Cell Function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef]
- Oshida, Y.; Tuna, E.B.; Aktören, O.; Gençay, K. Dental Implant Systems. Int. J. Mol. Sci. 2010, 11, 1580–1678. [Google Scholar] [CrossRef]
- Sarda, S.; Ginebra, M.; Rodriguez, D.; Casals, J.; Navarro, M.; Aparicio, C.; Manero, J.; Gil, F.; Planell, J.; Nilsson, M.; et al. Materials in Dental Implantology. In Dental Biomechanics; CRC Press: Boca Raton, FL, USA, 2003; pp. 69–89. ISBN 9780080552941. [Google Scholar]
- Güngör, M.B.; Aydin, C.; Yilmaz, H.; Gül, E.B. An Overview of Zirconia Dental Implants: Basic Properties and Clinical Application of Three Cases. J. Oral Implantol. 2014, 40, 485–494. [Google Scholar] [CrossRef]
- Mathieu, V.; Vayron, R.; Richard, G.; Lambert, G.; Naili, S.; Meningaud, J.P.; Haiat, G. Biomechanical Determinants of the Stability of Dental Implants: Influence of the Bone-Implant Interface Properties. J. Biomech. 2014, 47, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, A.; Meijer, G.J.; Walboomers, X.F.; Jansen, J.A. Evaluation of Primary and Secondary Stability of Titanium Implants Using Different Surgical Techniques. Clin. Oral Implants Res. 2014, 25, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Buga, C.; Hunyadi, M.; Gácsi, Z.; Hegedűs, C.; Hakl, J.; Schmidt, U.; Ding, S.J.; Csík, A. Calcium Silicate Layer on Titanium Fabricated by Electrospray Deposition. Mater. Sci. Eng. C 2019, 98, 401–408. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive Coating as a Surface Modification Technique for Biocompatible Metallic Implants: A Review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef]
- Mihov, D.; Katerska, B. Some Biocompatible Materials Used in Medical Practice. Trakia J. Sci. 2010, 8, 119–125. [Google Scholar]
- Venkatraman, S.K.; Swamiappan, S. Review on Calcium- and Magnesium-Based Silicates for Bone Tissue Engineering Applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef]
- Cerruti, M.; Sahai, N. Silicate Biomaterials for Orthopaedic and Dental Implants. Rev. Miner. Geochem. 2006, 64, 283–313. [Google Scholar] [CrossRef]
- Hinata, G.; Yoshiba, K.; Han, L.; Edanami, N.; Yoshiba, N.; Okiji, T. Bioactivity and Biomineralization Ability of Calcium Silicate-Based Pulp-Capping Materials after Subcutaneous Implantation. Int. Endod. J. 2017, 50, e40–e51. [Google Scholar] [CrossRef]
- Buga, C.; Chen, C.-C.; Hunyadi, M.; Csík, A.; Hegedűs, C.; Ding, S.-J. Electrosprayed Calcium Silicate Nanoparticle-Coated Titanium Implant with Improved Antibacterial Activity and Osteogenesis. Colloids Surf. B Biointerfaces 2021, 202, 111699. [Google Scholar] [CrossRef]
- Knabikaite, I.; Eisinas, A.; Baltakys, K. Al3+ Influence on the Formation of Calcium Silicates by Using Two Step Synthesis. In Proceedings of the NBCM 2020: International Conference on Nanostructured Bioceramic Materials, Vilnius, Lithuania, 1–3 December 2020. [Google Scholar]
- Onay, E.; Yurtcu, E.; Terzi, Y.; Ungor, M.; Oguz, Y.; Sahin, F. Odontogenic Effects of Two Calcium Silicate-Based Biomaterials in Human Dental Pulp Cells. Adv. Clin. Exp. Med. 2018, 27, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Marques, J.A.; Santos, J.; Falacho, R.I.; Sequeira, D.; Diogo, P.; Caramelo, F.; Ramos, J.C.; Santos, J.M. Tooth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate-Based Cements—An Ex Vivo Study. Appl. Sci. 2020, 10, 5793. [Google Scholar] [CrossRef]
- Isabel, J.; Santos, D.P. Teeth Discoloration after Regenerative Endodontic Procedures with Calcium Silicate Cements; Dental Medicine from FUC; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Parfenova, L.V.; Galimshina, Z.R.; Gil’fanova, G.U.; Alibaeva, E.I.; Danilko, K.V.; Aubakirova, V.R.; Farrakhov, R.G.; Parfenov, E.V.; Valiev, R.Z. Modeling of Biological Activity of PEO-Coated Titanium Implants with Conjugates of Cyclic RGD Peptide with Amino Acid Bisphosphonates. Materials 2022, 15, 8120. [Google Scholar] [CrossRef]
- Byun, H.Y.; Choi, M.H.; Chung, I.J. Synthesis and Characterization of Resol Type Phenolic Resin/Layered Silicate Nanocomposites. Chem. Mater. 2001, 13, 4221–4226. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zadehnazari, A. Effect of Amino Acid-Functionalization on the Interfacial Adhesion and Behavior of Multi-Walled Carbon Nanotubes/Poly(Amide-Imide) Nanocomposites Containing Thiazole Side Unit. J. Polym. Res. 2013, 20, 192. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The Role of Amino Acids in Hydroxyapatite Mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef] [PubMed]
- MacDonell, R.; Hamrick, M.W.; Isales, C.M. Protein/Amino-Acid Modulation of Bone Cell Function. Bonekey Rep. 2016, 5, 827. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Fini, M.; Giavaresi, G.; Giardino, R. Human Osteopenic Bone-Derived Osteoblasts: Essential Amino Acids Treatment Effects. Artif. Cells Blood Substit. Biotechnol. 2003, 31, 35–46. [Google Scholar] [CrossRef]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Xia, L.; Zhao, C.; Chen, L.; Yi, D.; Chang, J.; Huang, L.; Zheng, X.; Zhu, H.; et al. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids Surf. B Biointerfaces 2015, 126, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Yu, Z.; Tang, S.; Pan, Y.; Wei, J.; Tang, T. Osseointegration of nanohydroxyapatite-or nano-calcium silicate-incorporated polyetheretherketone bioactive composites in vivo. Int. J. Nanomed. 2016, 11, 6023. [Google Scholar] [CrossRef]
- Ozaki, K.; Yamada, T.; Horie, T.; Ishizaki, A.; Hiraiwa, M.; Iezaki, T.; Park, G.; Fukasawa, K.; Kamada, H.; Tokumura, K.; et al. The L-type amino acid transporter LAT1 inhibits osteoclastogenesis and maintains bone homeostasis through the mTORC1 pathway. Sci. Signal. 2019, 12, eaaw3921. [Google Scholar] [CrossRef] [PubMed]
- Ratirotjanakul, W.; Suteewong, T.; Polpanich, D.; Tangboriboonrat, P. Amino acid as a biodegradation accelerator of mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2019, 282, 243–251. [Google Scholar] [CrossRef]
- Kamali, M.; Ghahremaninezhad, A. Effect of biomolecules on the nanostructure and nanomechanical property of calcium-silicate-hydrate. Sci. Rep. 2018, 8, 9491. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO nanoparticles stabilized with gelatin for potential use in food packaging applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef] [PubMed]
- Blinov, A.V.; Nagdalian, A.A.; Povetkin, S.N.; Gvozdenko, A.A.; Verevkina, M.N.; Rzhepakovsky, I.V.; Lopteva, M.S.; Maglakelidze, D.G.; Kataeva, T.S.; Blinova, A.A.; et al. Surface-Oxidized Polymer-Stabilized Silver Nanoparticles as a Covering Component of Suture Materials. Micromachines 2022, 13, 1105. [Google Scholar] [CrossRef]
- Richet, P.; Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Wollastonite, Pseudowollastonite and CaSiO3 Glass and Liquid. Eur. J. Mineral. 1991, 3, 475–484. [Google Scholar] [CrossRef]
- Maddalena, R.; Li, K.; Chater, P.A.; Michalik, S.; Hamilton, A. Direct synthesis of a solid calcium-silicate-hydrate (CSH). Constr. Build. Mater. 2019, 223, 554–565. [Google Scholar] [CrossRef]
- Rodriguez, E.T.; Hunnicutt, W.A.; Mondal, P.; Le Pape, Y. Examination of gamma-irradiated calcium silicate hydrates. Part I: Chemical-structural properties. J. Am. Ceram. Soc. 2019, 103, 558–568. [Google Scholar] [CrossRef]
- Yuan, M.; Shi, S.; Luo, Y.; Yu, Y.; Wang, S.; Chen, C. Fabrication of mesoporous SiO2@CaSiO3 hollow spheres as carriers for pH-sensitive drug delivery. Chem. Res. Chin. Univ. 2021, 38, 999–1004. [Google Scholar] [CrossRef]
- Karami, M.; Yousif, Q.A.; Ghanbari, M.; Mahdavi, K.; Salavati-Niasari, M. Green fabrication of graphene quantum dots from cotton with CaSiO3 nanostructure and enhanced photocatalytic performance for water treatment. Int. J. Hydrogen Energy 2022, 47, 7228–7241. [Google Scholar] [CrossRef]
- Steiner, S.; Lothenbach, B.; Proske, T.; Borgschulte, A.; Winnefeld, F. Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite. Cem. Concr. Res. 2020, 135, 106116. [Google Scholar] [CrossRef]
- Peng, X.Y.; Hu, M.; Liao, F.; Yang, F.; Ke, Q.F.; Guo, Y.P.; Zhu, Z.H. La-Doped mesoporous calcium silicate/chitosan scaffolds for bone tissue engineering. Biomater. Sci. 2019, 7, 1565–1573. [Google Scholar] [CrossRef] [PubMed]

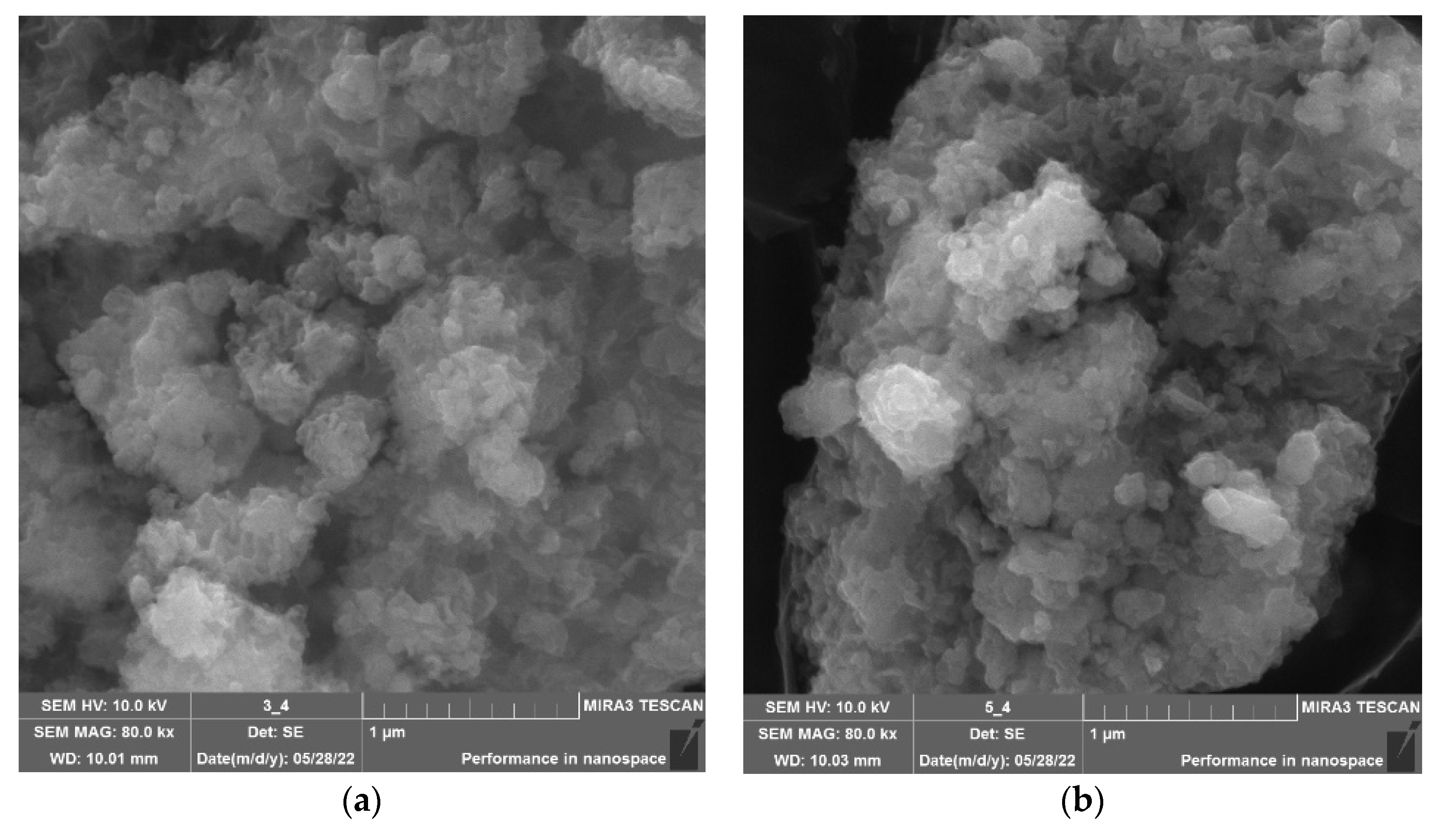
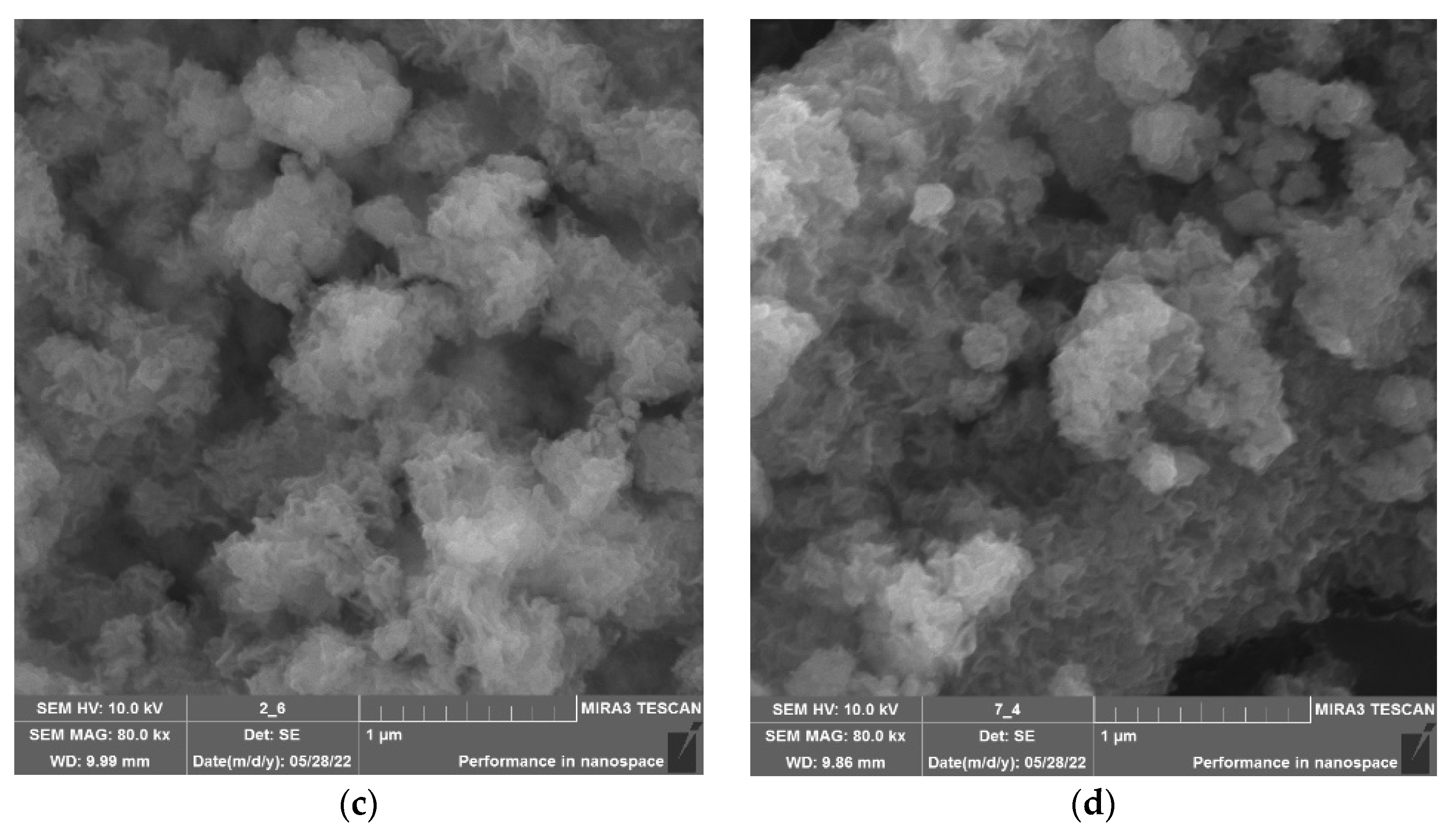

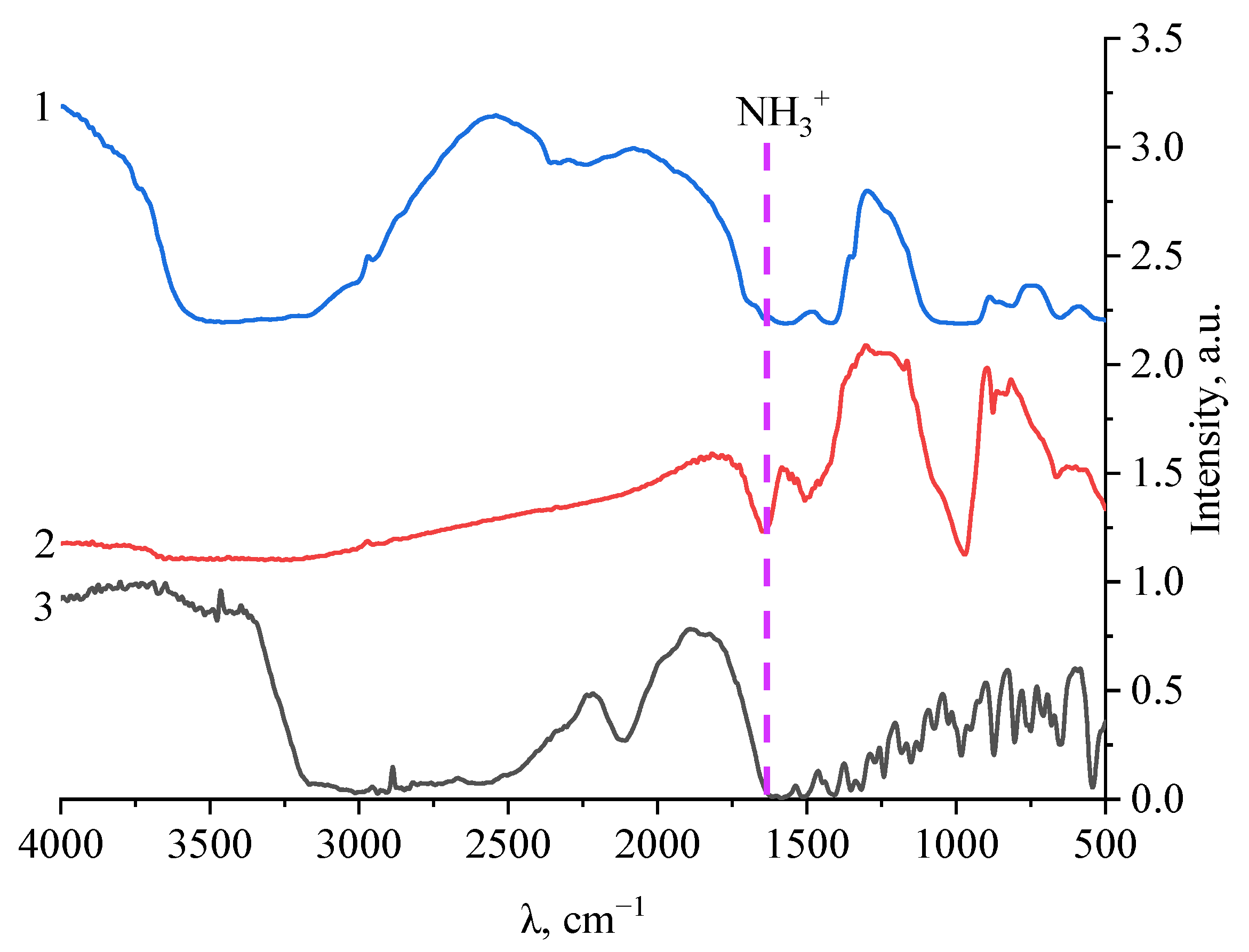
| Molecular System | Type of Interaction | E, kcal/mol | ∆E, kcal/mol | EHOMO, eV | ELUMO, eV | η, eV |
|---|---|---|---|---|---|---|
| CaSiO3 | - | −1185.674 | - | −0.268 | 0.033 | 0.151 |
| CaSiO3–L-valine | Amino acid | −402.112 | - | −0.249 | 0.016 | 0.133 |
| Through the amino group attached to the C2 atom of valine | −1661.171 | 1259.059 | −0.171 | 0.045 | 0.108 | |
| CaSiO3–L-leucine | Amino acid | −441.397 | - | −0.260 | 0.006 | 0.133 |
| Via an amino group attached to the C2 atom of leucine | −1700.028 | 1258.631 | −0.191 | 0.029 | 0.110 | |
| CaSiO3–L-isoleucine | Amino acid | −441.394 | - | −0.247 | 0.018 | 0.133 |
| Via an amino group attached to the C2 atom of isoleucine | −1700.108 | 1258.714 | −0.165 | 0.029 | 0.097 | |
| CaSiO3–L-methionine | Amino acid | −800.251 | - | −0.232 | 0.006 | 0.119 |
| Via an amino group attached to the C2 atom of methionine | −2058.497 | 1258.246 | −0.189 | 0.033 | 0.111 | |
| CaSiO3–L-threonine | Amino acid | −438.015 | - | −0.248 | 0.006 | 0.127 |
| Through an amino group attached to the C2 atom of threonine | −1696.802 | 1258.787 | −0.168 | 0.029 | 0.099 | |
| CaSiO3–L-lysine | Amino acid | −496.481 | - | −0.177 | −0.024 | 0.077 |
| Via an amino group attached to the C2 atom of lysine | −1754.871 | 1258.39 | −0.140 | 0.043 | 0.092 | |
| Via an amino group attached to the C6 atom of lysine | −1755.223 | 1258.742 | −0.138 | 0.042 | 0.090 | |
| CaSiO3–L-phenylalanine | Amino acid | −554.424 | - | −0.240 | 0.002 | 0.121 |
| Through an amino group attached to the C2 atom of phenylalanine | −1812.504 | 1258.08 | −0.156 | 0.037 | 0.097 | |
| CaSiO3–L-tryptophan | Amino acid | −685.684 | - | −0.195 | −0.035 | 0.080 |
| Through an amino group attached to the C2 atom of tryptophan | −1943.146 | 1257.462 | −0.162 | 0.035 | 0.099 | |
| Through the secondary amino group of indole in tryptophan | −1942.821 | 1257.137 | −0.168 | 0.021 | 0.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinova, A.A.; Karamirzoev, A.A.; Guseynova, A.R.; Maglakelidze, D.G.; Ilyaeva, T.A.; Gusov, B.A.; Meliksetyants, A.P.; Pirumian, M.M.; Taravanov, M.A.; Pirogov, M.A.; et al. Synthesis and Characterization of Calcium Silicate Nanoparticles Stabilized with Amino Acids. Micromachines 2023, 14, 245. https://doi.org/10.3390/mi14020245
Blinova AA, Karamirzoev AA, Guseynova AR, Maglakelidze DG, Ilyaeva TA, Gusov BA, Meliksetyants AP, Pirumian MM, Taravanov MA, Pirogov MA, et al. Synthesis and Characterization of Calcium Silicate Nanoparticles Stabilized with Amino Acids. Micromachines. 2023; 14(2):245. https://doi.org/10.3390/mi14020245
Chicago/Turabian StyleBlinova, Anastasiya A., Abdurasul A. Karamirzoev, Asiyat R. Guseynova, David G. Maglakelidze, Tatiana A. Ilyaeva, Batradz A. Gusov, Avetis P. Meliksetyants, Mari M. Pirumian, Maxim A. Taravanov, Maxim A. Pirogov, and et al. 2023. "Synthesis and Characterization of Calcium Silicate Nanoparticles Stabilized with Amino Acids" Micromachines 14, no. 2: 245. https://doi.org/10.3390/mi14020245
APA StyleBlinova, A. A., Karamirzoev, A. A., Guseynova, A. R., Maglakelidze, D. G., Ilyaeva, T. A., Gusov, B. A., Meliksetyants, A. P., Pirumian, M. M., Taravanov, M. A., Pirogov, M. A., Vakalov, D. S., Bernyukevich, T. V., Gvozdenko, A. A., Nagdalian, A. A., & Blinov, A. V. (2023). Synthesis and Characterization of Calcium Silicate Nanoparticles Stabilized with Amino Acids. Micromachines, 14(2), 245. https://doi.org/10.3390/mi14020245









