Enzymes Immobilized into Starch- and Gelatin-Based Hydrogels: Properties and Application in Inhibition Assay
Abstract
:1. Introduction
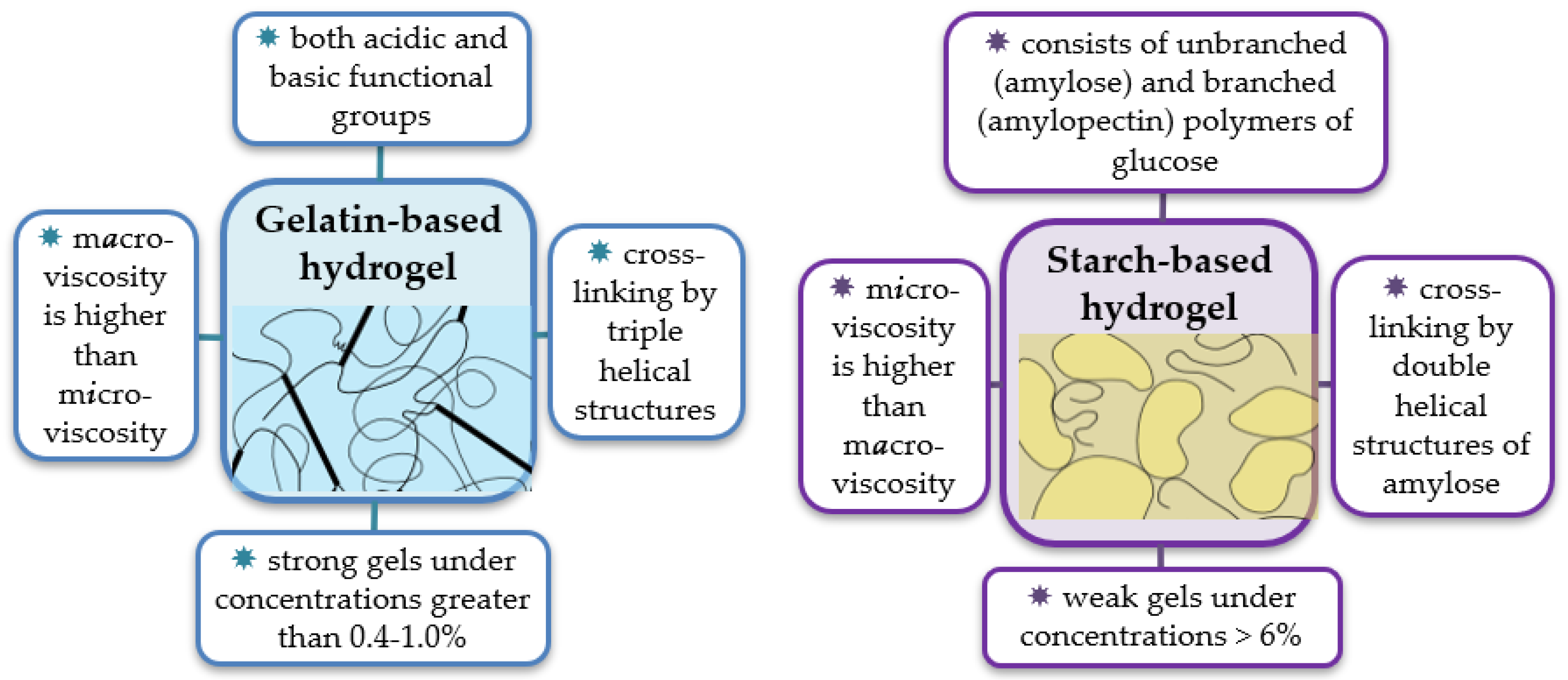
2. Molecular Structure of the Starch and Gelatin Biopolymers and Architecture of Their Hydrogels
2.1. Starch—A Storage Polysaccharide of Plants
2.2. Gelatin—A Structural Polypeptide of Mammals
2.3. Properties of the Starch and Gelatin Hydrogels
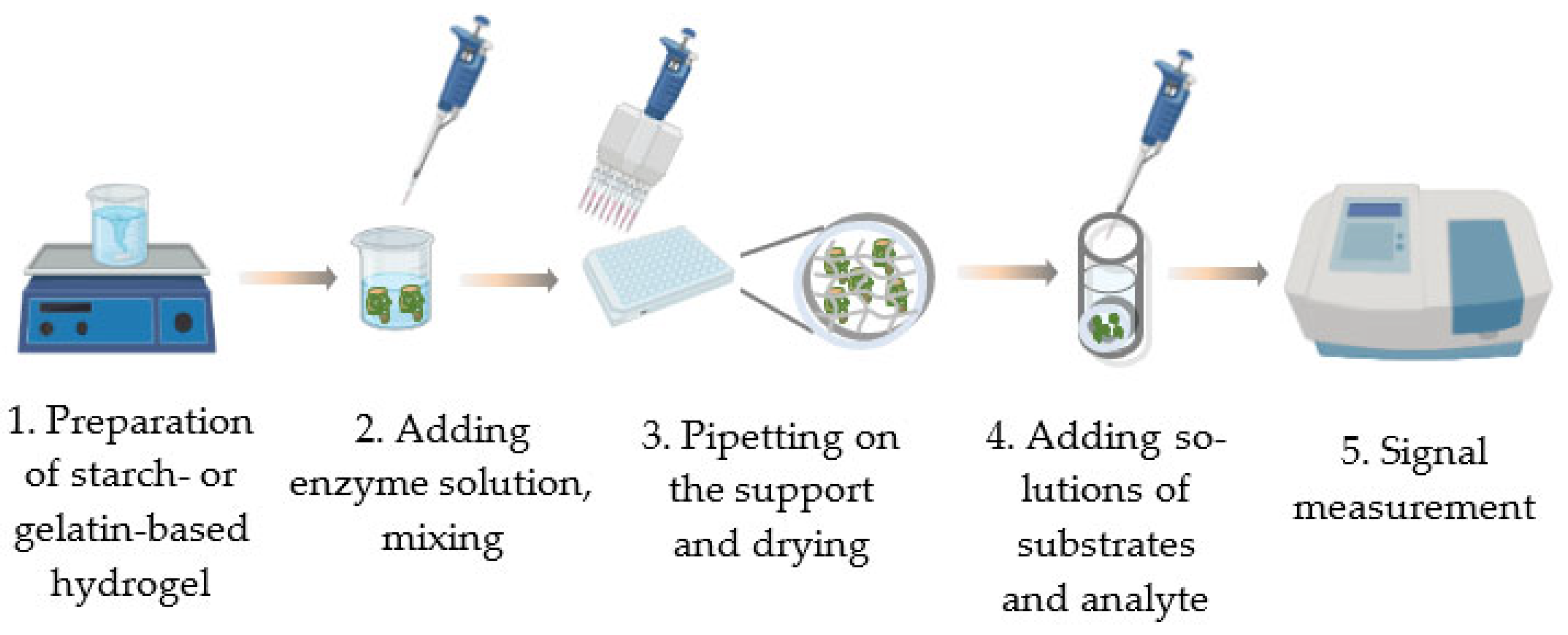
3. Entrapment of the Enzymes within a Porous Matrix of Starch and Gelatin to Use in Inhibition Assay
3.1. Methods of Producing Preparations Based on Enzymes Immobilized in Starch and Gelatin Gels
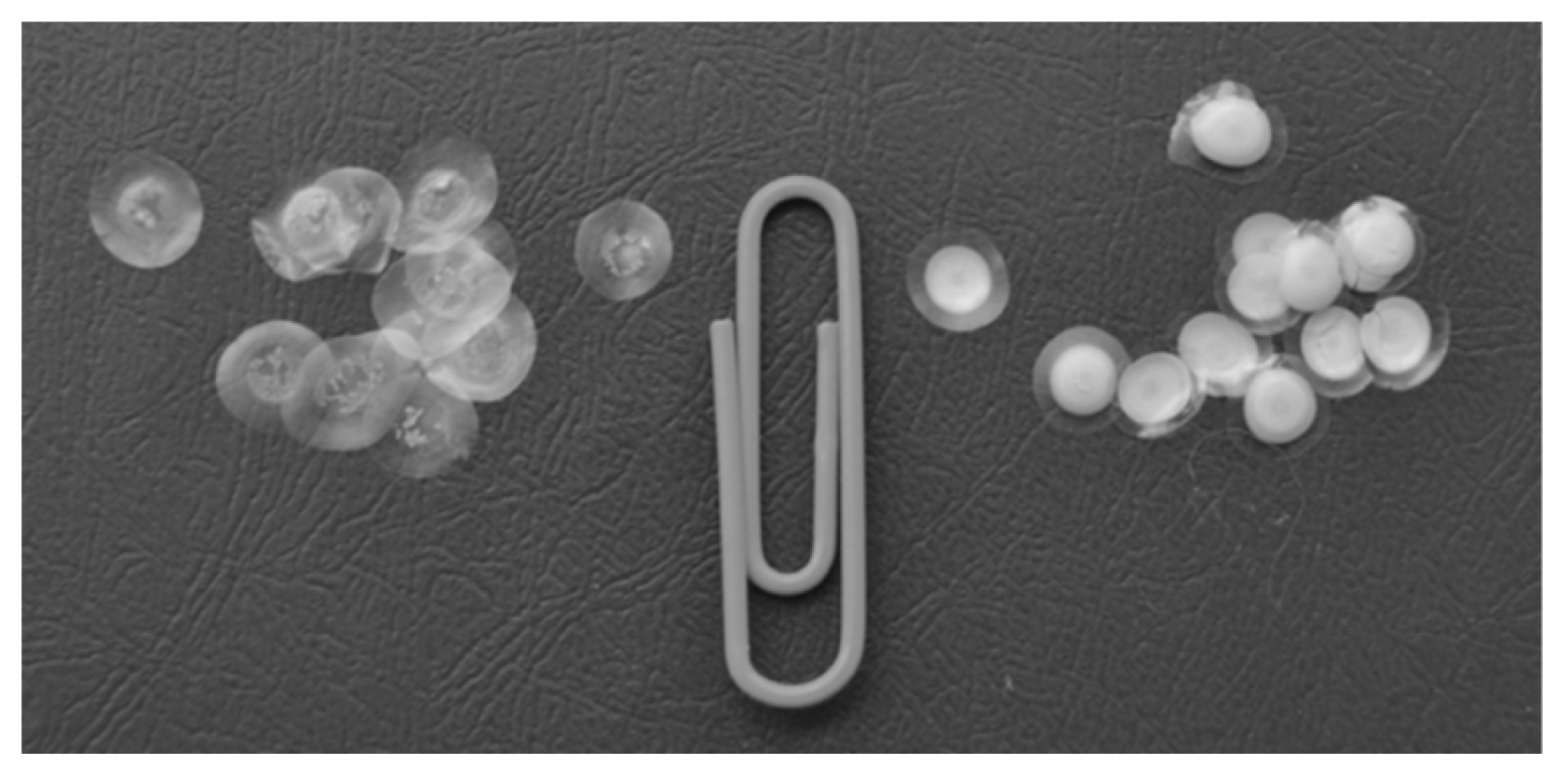
3.2. Characterization of Preparations Produced by Immobilizing Enzymes in Starch and Gelatin Gels
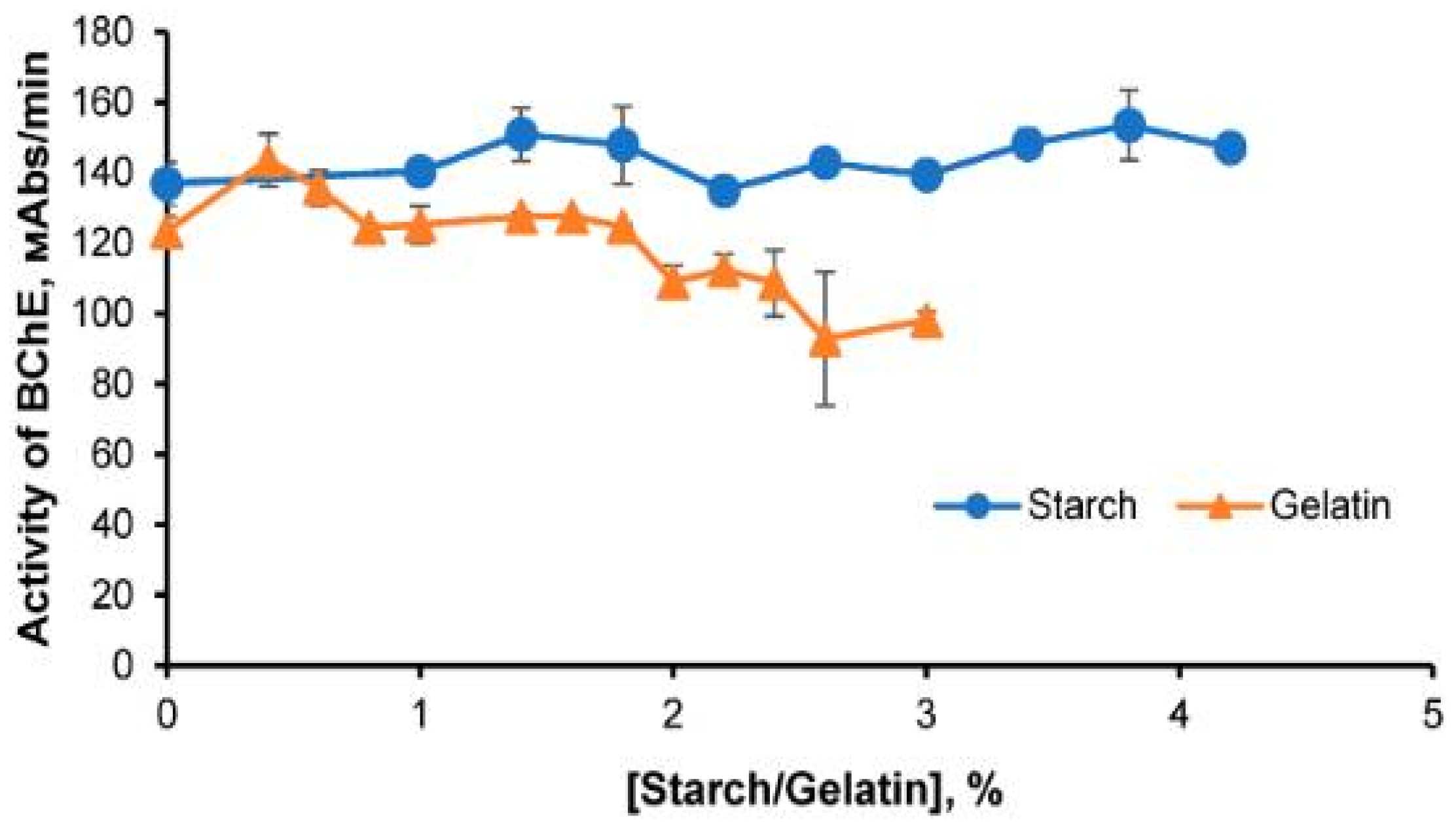
3.3. The Effect of the Gel Matrix on Stability of the Enzymes Immobilized in the Starch and Gelatin Gels
3.4. Using Enzymes Immobilized in Starch and Gelatin Gels in Inhibition Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, U.; Selamoglu, Z. Use of Enzymes in Dairy Industry: A Review of Current Progress. Arch. Razi Inst. 2020, 75, 131–136. [Google Scholar] [CrossRef]
- Contesini, F.J.; Melo, R.R.; Sato, H.H. An overview of Bacillus proteases: From production to application. Crit. Rev. Biotechnol. 2018, 38, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Shukla, P. Current Trends in Protein Engineering: Updates and Progress. Curr. Protein Pept. Sci. 2019, 20, 398–407. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, A.P. Protein engineering approaches to chemical biotechnology. Curr. Opin. Biotechnol. 2016, 42, 198–205. [Google Scholar] [CrossRef]
- Arduini, F.; Amine, A. Biosensors based on enzyme inhibition. In Biosensors Based on Aptamers and Enzymes; Advances in Biochemical Engineering/Biotechnology; Gu, M., Kim, H.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 140, pp. 299–326. [Google Scholar]
- Morales, M.A.; Halpern, J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef]
- Schachinger, F.; Chang, H.; Scheiblbrandner, S.; Ludwig, R. Amperometric Biosensors Based on Direct Electron Transfer Enzymes. Molecules 2021, 26, 4525. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Yoon, J. Enzymatic Electrochemical/Fluorescent Nanobiosensor for Detection of Small Chemicals. Biosensors 2023, 13, 492. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.-K. From Protein Engineering to Immobilization: Promising Strategies for the Upgrade of Industrial Enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Nidetzky, B. The Microenvironment in Immobilized Enzymes: Methods of Characterization and Its Role in Determining Enzyme Performance. Molecules 2019, 24, 3460. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Rehm, F.B.H.; Chen, S.; Rehm, B.H.A. Enzyme Engineering for In Situ Immobilization. Molecules 2016, 21, 1370. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Dubey, N.C.; Tripathi, B.P. Nature Inspired Multienzyme Immobilization: Strategies and Concepts. ACS Appl. Bio Mater. 2021, 4, 1077–1114. [Google Scholar] [CrossRef] [PubMed]
- Vidhya, S.; Murari, B.M. Sol-gel thin film based sensors and biosensors. Int. J. Pharm. Biol. Sci. 2016, 7, 297–310. [Google Scholar]
- Egorova, T.A.; Klunova, S.M.; Zhivukhin, E.A. Fundamentals of Biotechnology, 4th ed.; Publishing Center “Academy”: Moscow, Russia, 2003; pp. 85–102. [Google Scholar]
- Guisan, J.M. Immobilization of Enzymes and Cells, 3rd ed.; Humana Press: New York, NY, USA, 2013; pp. 77–96. Available online: http://link.springer.com/book/10.1007%2F978-1-62703-550-7 (accessed on 24 October 2023).
- Luan, N.M.; Teramura, Y.; Iwata, H. Immobilization of the soluble domain of human complement receptor 1 on agarose-encapsulated islets for the prevention of complement activation. Biomaterials 2010, 31, 8847–8853. [Google Scholar] [CrossRef]
- Allen, M.E.; Hindley, J.W.; Baxani, D.K.; Ces, O.; Elani, Y. Hydrogels as functional components in artificial cell systems. Nat. Rev. Chem. 2022, 6, 562–578. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Sig. Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. [Google Scholar] [CrossRef]
- Shokri, Z.; Seidi, F.; Karami, S.; Li, C.; Saeb, M.R.; Xiao, H. Laccase immobilization onto natural polysaccharides for biosensing and biodegradation. Carbohydr. Polym. 2021, 262, 117963. [Google Scholar] [CrossRef] [PubMed]
- Tembe, S.; Karve, M.; Inamdar, S.; Haram, S.; Melo, J.; D’Souza, S.F. Development of electrochemical biosensor based on tyrosinase immobilized in composite biopolymeric film. Anal. Biochem. 2006, 349, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, S.Y.; Savina, A.A.; Zaitsev, I.S. Biochemical aspects of lipase immobilization at polysaccharides for biotechnology. Adv. Colloid. Interface Sci. 2019, 272, 102016. [Google Scholar] [CrossRef] [PubMed]
- Ferraraccio, L.S.; Lisa, D.D.; Pastorino, L.; Bertoncello, P. Enzymes Encapsulated within Alginate Hydrogels: Bioelectrocatalysis and Electrochemiluminescence Applications. Anal. Chem. 2022, 94, 16122–16131. [Google Scholar] [CrossRef] [PubMed]
- De Wael, K.; De Belder, S.; Pilehvar, S.; Van Steenberge, G.; Herrebout, W.; Heering, H.A. Enzyme-Gelatin Electrochemical Biosensors: Scaling Down. Biosensors 2012, 2, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Rosseto, M.; Krein, D.D.C.; Balbé, N.P.; Dettmer, A. Starch–gelatin film as an alternative to the use of plastics in agriculture: A review. J. Sci. Food Agric. 2019, 99, 6671–6679. [Google Scholar] [CrossRef]
- Hathout, R.M.; Omran, M.K. Gelatin-based particulate systems in ocular drug delivery. Pharm. Dev. Technol. 2016, 21, 379–386. [Google Scholar] [CrossRef]
- Chakraborty, R.; Kalita, P.; Sen, S. Natural Starch in Biomedical and Food Industry: Perception and Overview. Curr. Drug Discov. Technol. 2019, 16, 355–367. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Ikada, Y.; Tabata, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Ikada, Y.; Tabata, Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001, 12, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Arora, B.; Bhatia, R.; Attri, P. 28—Bionanocomposites: Green Materials for a Sustainable Future. In New Polymer Nanocomposites for Environmental Remediation; Hussain, C.M., Mishra, A.K., Eds.; Elsevier: Wilmington, NC, USA, 2018; pp. 699–712. ISBN 978-0-12-811033-1. [Google Scholar] [CrossRef]
- Imberty, A.; Buleon, A.; Tran, V.; Peerez, S. Recent advances in knowledge of starch structure. Starch 1991, 43, 375–384. [Google Scholar] [CrossRef]
- French, D. Chemical and Physical Properties of Starch. J. Anim. Sci. 1973, 37, 1048–1061. [Google Scholar] [CrossRef]
- Birkett, A.M.; Brown, I.L. Resistant starch and health. In Technology of Functional Cereal Products; Hamaker, B.R., Ed.; CRC Press: West Palm Beach, FL, USA, 2008; pp. 63–85. [Google Scholar]
- Leloup, V.M.; Colonna, P.; Buleon, A. Influence of amylose-amylopectin ratio on gel properties. J. Cereal Sci. 1991, 13, 1–13. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Knill, C.J.; Liu, L.; Panesar, P.S. Starch and its derived products: Biotechnological and biomedical applications. In Renewable Resources for Functional Polymers and Biomaterials; Williams, P., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2011; Chapter 5; pp. 130–165. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and Functionality of Starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Djabourov, M. Architecture of gelatin gels. Contemp. Phys. 1988, 29, 273–297. [Google Scholar] [CrossRef]
- Bagal-Kestwal, D.R.; Pan, M.H.; Chiang, B.H. Properties and applications of gelatin, pectin, and carrageenan gels. In Bio Monomers for Green Polymeric Composite Materials; Visakh, P.M., Bayraktar, O., Menon, G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 117–140. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Structure–property relationships in food biopolymer gels and solutions. J. Rheol. 1995, 39, 1451–1463. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Milano, F.; Masi, A.; Madaghiele, M.; Sannino, A.; Salvatore, L.; Gallo, N. Current Trends in Gelatin-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1499. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Abdi, G.; Shekh, M.I.; Zendehdel, E.A.; Du, B.; Stadler, F.J. Gelatin Based Hydrogels for Tissue Engineering and Drug Delivery Applications. Mater. Res. Found. 2021, 81, 244–270. [Google Scholar] [CrossRef]
- Picout, D.R.; Ross-Murphy, S.B. Rheology of biopolymer solutions and gels. Sci. World J. 2003, 3, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, T.; White, S.J.; Zarbakhsh, A.; Heenan, R.K.; Howe, A.M. Small-angle scattering studies of sodium dodecyl sulfate interactions with gelatin. 1. Langmuir 1995, 11, 744–749. [Google Scholar] [CrossRef]
- Miao, Z.; Ding, K.; Wu, T.; Liu, Z.; Han, B.; An, G.; Miao, S.; Yang, G. Fabrication of 3D-networks of native starch and their application to produce porous inorganic oxide networks through a supercritical route. Microporous Mesoporous Mater. 2008, 111, 104–109. [Google Scholar] [CrossRef]
- Ohtsuka, A.; Watanabe, T.; Suzuki, T. Gel structure and water diffusion phenomena in starch gels studied by pulsed field gradient stimulated echo NMR. Carbohydr. Polym. 1994, 25, 95–100. [Google Scholar] [CrossRef]
- Gulnov, D.V.; Nemtseva, E.V.; Kratasyuk, V.A. Contrasting relationship between macro- and microviscosity of the gelatin- and starch-based suspensions and gels. Polym. Bull. 2016, 73, 3421–3435. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Lonshakova-Mukina, V.I.; Bezrukik, A.E.; Kratasyuk, V.A. Design of multicomponent reagents for enzymatic assays. Dokl. Biochem. Biophys. 2015, 461, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Lonshakova-Mukina, V.I.; Esimbekova, E.N.; Kratasyuk, V.A. Stabilization of Butyrylcholinesterase by the Entrapment into the Natural Polymer-Based Gels. Dokl. Biochem. Biophys. 2018, 479, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.N.; Kirillova, M.A.; Kratasyuk, V.A. Immobilization of Firefly Bioluminescent System: Development and Application of Reagents. Biosensors 2023, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.N.; Torgashina, I.G.; Nemtseva, E.V.; Antashkevich, A.A.; Sasova, P.Y.; Kratasyuk, V.A. Trypsin-Based Chemoenzymatic Assay for Detection of Pollutants and Safety Assessment of Food Additives. Chemosensors 2023, 11, 237. [Google Scholar] [CrossRef]
- Mehdi, W.A.; Mehde, A.A.; Özacar, M.; Özacar, Z. Characterization and immobilization of protease and lipase on chitin-starch material as a novel matrix. Int. J. Biol. Macromol. 2018, 117, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, L.; Lv, S.; Liu, X.; Wang, M.; Song, A.; Jia, X. Immobilization of Aspergillus niger xylanase on chitosan using dialdehyde starch as a coupling agent. Appl. Biochem. Biotechnol. 2010, 162, 24–32. [Google Scholar] [CrossRef]
- Dong, T.; Zhou, X.; Dai, Y.; Yang, X.; Zhang, W.; Yu, D.; Liu, T. Application of magnetic immobilized enzyme of nano dialdehyde starch in deacidification of rice bran oil. Enzyme Microb. Technol. 2022, 161, 110116. [Google Scholar] [CrossRef]
- Karacaoğlu, S.; Timur, S.; Telefoncu, A. Arginine Selective Biosensor Based on Arginase-Urease Immobilized in Gelatin. Artif. Cells Blood Substit. Biotechnol. 2003, 31, 357–363. [Google Scholar] [CrossRef]
- Mogharabi, M.; Nassiri-Koopaei, N.; Bozorgi-Koushalshahi, M.; Nafissi-Varcheh, N.; Bagherzadeh, G.; Faramarzi, M.A. Immobilization of laccase in alginate-gelatin mixed gel and decolorization of synthetic dyes. Bioinorg. Chem. Appl. 2012, 2012, 823830. [Google Scholar] [CrossRef]
- Wang, P.; Fan, X.; Cui, L.; Wang, Q.; Zhou, A. Decolorization of reactive dyes by laccase immobilized in alginate/gelatin blent with PEG. J. Env. Sci. 2008, 20, 1519–1522. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; Abd El Aziz, A.E.; Abdel Raouf, B.M.; Mohamed, S.A.; Nada, D. Antioxidant-biocompatible and stable catalase-based gelatin-alginate hydrogel scaffold with thermal wound healing capability: Immobilization and delivery approach. 3 Biotech 2022, 12, 73. [Google Scholar] [CrossRef]
- Hackenhaar, C.R.; Rosa, C.F.; Flores, E.E.E.; Santagapita, P.R.; Klein, M.P.; Hertz, P.F. Development of a biocomposite based on alginate/gelatin crosslinked with genipin for β-galactosidase immobilization: Performance and characteristics. Carbohydr. Polym. 2022, 291, 119483. [Google Scholar] [CrossRef] [PubMed]
- Sungur, S.; Emregul, E.; Gunendi, G.; Numanoglu, Y. New glucose biosensor based on glucose oxidase-immobilized gelatin film coated electrodes. J. Biomater. Appl. 2004, 18, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Kayastha, A.M.; Srinivasan. Characterization of gelatin-immobilized pigeonpea urease and preparation of a new urea biosensor. Biotechnol. Appl. Biochem. 2001, 34, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.N.; Kratasyuk, V.A.; Torgashina, I.G. Disk-shaped immobilized multicomponent reagent for bioluminescent analyses: Correlation between activity and composition. Enzym. Microb. Technol. 2007, 40, 343–346. [Google Scholar] [CrossRef]
- Lund, D. Influence of time, temperature, moisture, ingredients, and processing conditions on starch gelatinization. Crit. Rev. Food Sci. Nutr. 1984, 20, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef]
- Mathobo, V.M.; Silungwe, H.; Ramashia, S.E.; Anyasi, T.A. Effects of heat-moisture treatment on the thermal, functional properties and composition of cereal, legume and tuber starches-a review. J. Food Sci. Technol. 2021, 58, 412–426. [Google Scholar] [CrossRef]
- Zia-Ud-Din; Xiong, H.; Fei, P. Physical and chemical modification of starches: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2691–2705. [Google Scholar] [CrossRef]
- Li, J.; Kong, X.; Ai, Y. Modification of granular waxy, normal and high-amylose maize starches by maltogenic α-amylase to improve functionality. Carbohydr. Polym. 2022, 290, 119503. [Google Scholar] [CrossRef]
- Wang, D.; Mi, T.; Gao, W.; Yu, B.; Yuan, C.; Cui, B.; Liu, X.; Liu, P. Effect of modification by maltogenic amylase and branching enzyme on the structural and physicochemical properties of sweet potato starch. Int. J. Biol. Macromol. 2023, 239, 124234. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Zhu, J.; Ai, Y. Utilization of maltogenic α-amylase treatment to enhance the functional properties and reduce the digestibility of pulse starches. Food Hydrocoll. 2021, 120, 106932. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic modification of starch: A green approach for starch applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef]
- Ashogbon, A.O. Dual modification of various starches: Synthesis, properties and applications. Food Chem. 2021, 342, 128325. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wu, Y.; Tan, H. Gelatin-based Targeted Delivery Systems for Tissue Engineering. Curr. Drug Targets. 2023, 24, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; Nada, D.; Radwan, R.A.; Mohamed, S.A.; Gohary, N.A.E.L. Optimization of catalytic properties of Mucor racemosus lipase through immobilization in a biocompatible alginate gelatin hydrogel matrix for free fatty acid production: A sustainable robust biocatalyst for ultrasound-assisted olive oil hydrolysis. 3 Biotech 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Hang, H.; Wang, C.; Cheng, Y.; Li, N.; Song, L. Design and Properties of an Immobilization Enzyme System for Inulin Conversion. Appl. Biochem. Biotechnol. 2018, 184, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Lonshakova-Mukina, V.I.; Esimbekova, E.N.; Kratasyuk, V.A. Thermal Inactivation of Butyrylcholinesterase in Starch and Gelatin Gels. Catalysts 2021, 11, 492. [Google Scholar] [CrossRef]
- Lonshakova-Mukina, V.I.; Esimbekova, E.N.; Kratasyuk, V.A. A Multicomponent Butyrylcholinesterase Preparation for Enzyme Inhibition-Based Assay of Organophosphorus Pesticides. Catalysts 2022, 12, 643. [Google Scholar] [CrossRef]
- Lomakina, G.Y.; Bezrukikh, A.E.; Ugarova, N.N. Effect of Gelatin on Properties of Luciola Mingrelica Firefly Luciferase. Mosc. Univ. Chem. Bull. 2012, 67, 8–12. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Kirillova, M.A.; Kratasyuk, V.A. Complex of Reagents for the Quantitative Analysis of adenosine-5′-triphosphate. Patent RF 2654672, 21 June 2017. [Google Scholar]
- Santos, J.C.D.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.; Fernández-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, G.; Xu, D.; Cao, Y. Effects of ultrasound on the kinetics and thermodynamics properties of papain entrapped in modified gelatin. Food Hydrocoll. 2020, 105, 105757. [Google Scholar] [CrossRef]
- Kratasyuk, V.A.; Esimbekova, E.N. Bioluminescent Biomodul for Analyses of Various Media Toxicity and Method of Its Preparation. Patent RF 2413772, 10 March 2011. [Google Scholar]
- Kratasyuk, V.A.; Esimbekova, E.N. Express Method for Biotesting of Natural, Manufactoring Waters and Water Solutions. Patent RF 2413771, 10 March 2011. [Google Scholar]
- Denisov, I.A.; Lukyanenko, K.A.; Yakimov, A.S.; Kukhtevich, I.V.; Esimbekova, E.N.; Belobrov, P.I. Disposable luciferase-based microfluidic chip for rapid assay of water pollution. Luminescence 2018, 33, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Lukyanenko, K.A.; Denisov, I.A.; Yakimov, A.S.; Esimbekova, E.N.; Belousov, K.I.; Bukatin, A.S.; Kukhtevich, I.V.; Sorokin, V.V.; Evstrapov, A.A.; Belobrov, P.I. Analytical enzymatic reactions in microfluidic chips. Appl. Biochem. Microbiol. 2017, 53, 775–780. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Lonshakova-Mukina, V.I.; Kratasyuk, V.A. Enzyme Preparation Based on Immobilized Butyrylcholinesterase and Method of Its Preparation. Patent RF 2546245, 10 April 2015. [Google Scholar]
- Esimbekova, E.N.; Torgashina, I.G.; Kratasyuk, V.A. Comparative Study of Immobilized and Soluble NADH:FMN-oxidoreductase–luciferase coupled enzyme system. Biochemistry 2009, 74, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Malar, C.G.; Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on surface modification of nanocarriers to overcome diffusion limitations: An enzyme immobilization aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar] [CrossRef]
- Bezrukikh, A.E.; Esimbekova, E.N.; Kratasyuk, V.A. Thermoinactivation of coupled enzyme system of luminous bacteria NADH:FMN-oxidoreductase-luciferase in gelatin. Biology 2011, 1, 64–74. [Google Scholar]
- Govorun, A.E.; Esimbekova, E.N.; Kratasyuk, V.A. NAD(P)H:FMN-Oxidoreductase Functioning Under Macromolecular Crowding: In Vitro Modeling. Dokl. Biochem. Biophys. 2019, 486, 213–215. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Govorun, A.E.; Lonshakova-Mukina, V.I.; Kratasyuk, V.A. Gelatin and Starch: What Better Stabilizes the Enzyme Activity? Dokl. Biol. Sci. 2020, 491, 43–46. [Google Scholar] [CrossRef]
- Bezrukikh, A.E.; Esimbekova, E.N.; Nemtseva, E.V.; Kratasyuk, V.A.; Shimomura, O. Gelatin and starch as stabilizers of the coupled enzyme system of luminous bacteria NADH:FMN–oxidoreductase–luciferase. Anal. Bioanal. Chem. 2014, 406, 5743–5747. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Huang, Y.; Li, T. Applications of Gelatin in Biosensors: Recent Trends and Progress. Biosensors 2022, 12, 670. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Chang, Y.; Chen, S. Amperometric glucose sensor based on glucose oxidase immobilized on gelatin-multiwalled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 2011, 80, 114–120. [Google Scholar] [CrossRef]
- Zhu, Q.; Yuan, R.; Chai, Y.; Zhuo, Y.; Zhang, Y.; Li, X.; Wang, N. A Novel Amperometric Biosensor for Determination of Hydrogen Peroxide Based on Nafion and Polythionine as Well as Gold Nanoparticles and Gelatin as Matrixes. Anal. Lett. 2006, 39, 483–494. [Google Scholar] [CrossRef]
- Berto, M.; Diacci, C.; Theuer, L.; Lauro, M.D.; Simon, D.T.; Berggren, M.; Biscarini, F.; Beni, V.; Bortolotti, C.A. Label free urea biosensor based on organic electrochemical transistors. Flex. Print. Electron. 2018, 3, 024001. [Google Scholar] [CrossRef]
- Amine, A.; Mohammadi, H.; Bourais, I.; Palleschi, G. Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens. Bioelectron. 2006, 21, 1405–1423. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.N.; Kondik, A.M.; Kratasyuk, V.A. Bioluminescent enzymatic rapid assay of water integral toxicity. Environ. Monit. Assess. 2013, 185, 5909–5916. [Google Scholar] [CrossRef] [PubMed]



| Enzymes (Content) | Conditions | Immobilization Yield, % | Reference | |
|---|---|---|---|---|
| Starch/Gelatin | Starch | Gelatin | ||
| Red + Luc (0.1 mU of Red and 0.2 μg of Luc) | 3% potato starch gel, 4% gelatin gel | 100 | 17 | [71] |
| FLuc (5.5 × 1012 RU·mg−1) |
1% of gelatin, 0.5 mM DTT | - * | 60 | [59] |
| BChE (60 mU) | 3% potato starch gel, 1.4% gelatin gel | 90 | 100 | [58,85] |
| Try (11 U) | 3% potato starch gel | 100 | - ** | [60] |
| Enzyme Forms | Km App #, mg/mL | pH Optimum * | T,°C Optimum * | ||
|---|---|---|---|---|---|
| NADH | FMN | Tetradecanal | |||
| Free | (3.4 ± 0.7) × 10−2 | (0.6 ± 0.1) × 10−3 | (0.18 ± 0.04) × 10−6 | 6.7–6.9 | 20–25 |
| Immobilized in potato starch gel | (9.0 ± 1.8) × 10−2 | (1.3 ± 0.3) × 10−3 | (1.2 ± 0.2) × 10−6 | 5.8–8.0 | 5–50 |
| Immobilized in gelatin gel | (17.9 ± 3.5) × 10−2 | (2.8 ± 0.6) × 10−3 | (0.7 ± 0.1) × 10−6 | 6.6–7.3 | 15–50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esimbekova, E.N.; Torgashina, I.G.; Nemtseva, E.V.; Kratasyuk, V.A. Enzymes Immobilized into Starch- and Gelatin-Based Hydrogels: Properties and Application in Inhibition Assay. Micromachines 2023, 14, 2217. https://doi.org/10.3390/mi14122217
Esimbekova EN, Torgashina IG, Nemtseva EV, Kratasyuk VA. Enzymes Immobilized into Starch- and Gelatin-Based Hydrogels: Properties and Application in Inhibition Assay. Micromachines. 2023; 14(12):2217. https://doi.org/10.3390/mi14122217
Chicago/Turabian StyleEsimbekova, Elena N., Irina G. Torgashina, Elena V. Nemtseva, and Valentina A. Kratasyuk. 2023. "Enzymes Immobilized into Starch- and Gelatin-Based Hydrogels: Properties and Application in Inhibition Assay" Micromachines 14, no. 12: 2217. https://doi.org/10.3390/mi14122217
APA StyleEsimbekova, E. N., Torgashina, I. G., Nemtseva, E. V., & Kratasyuk, V. A. (2023). Enzymes Immobilized into Starch- and Gelatin-Based Hydrogels: Properties and Application in Inhibition Assay. Micromachines, 14(12), 2217. https://doi.org/10.3390/mi14122217








