Acoustic Bubble and Magnetic Actuation-Based Microrobot for Enhanced Multiphase Drug Delivery Efficiency
Abstract
:1. Introduction
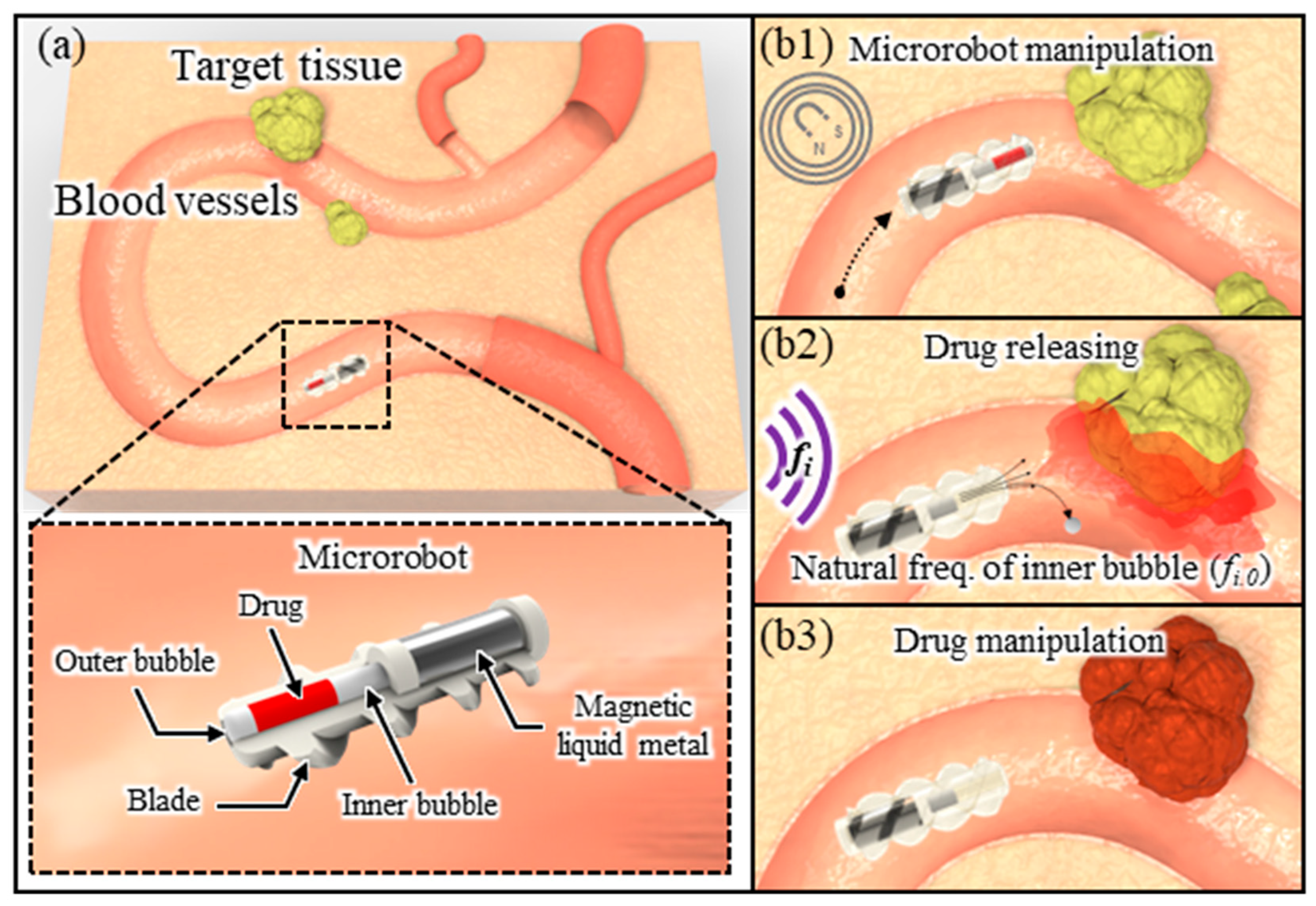
2. Principle
3. Results and Discussion
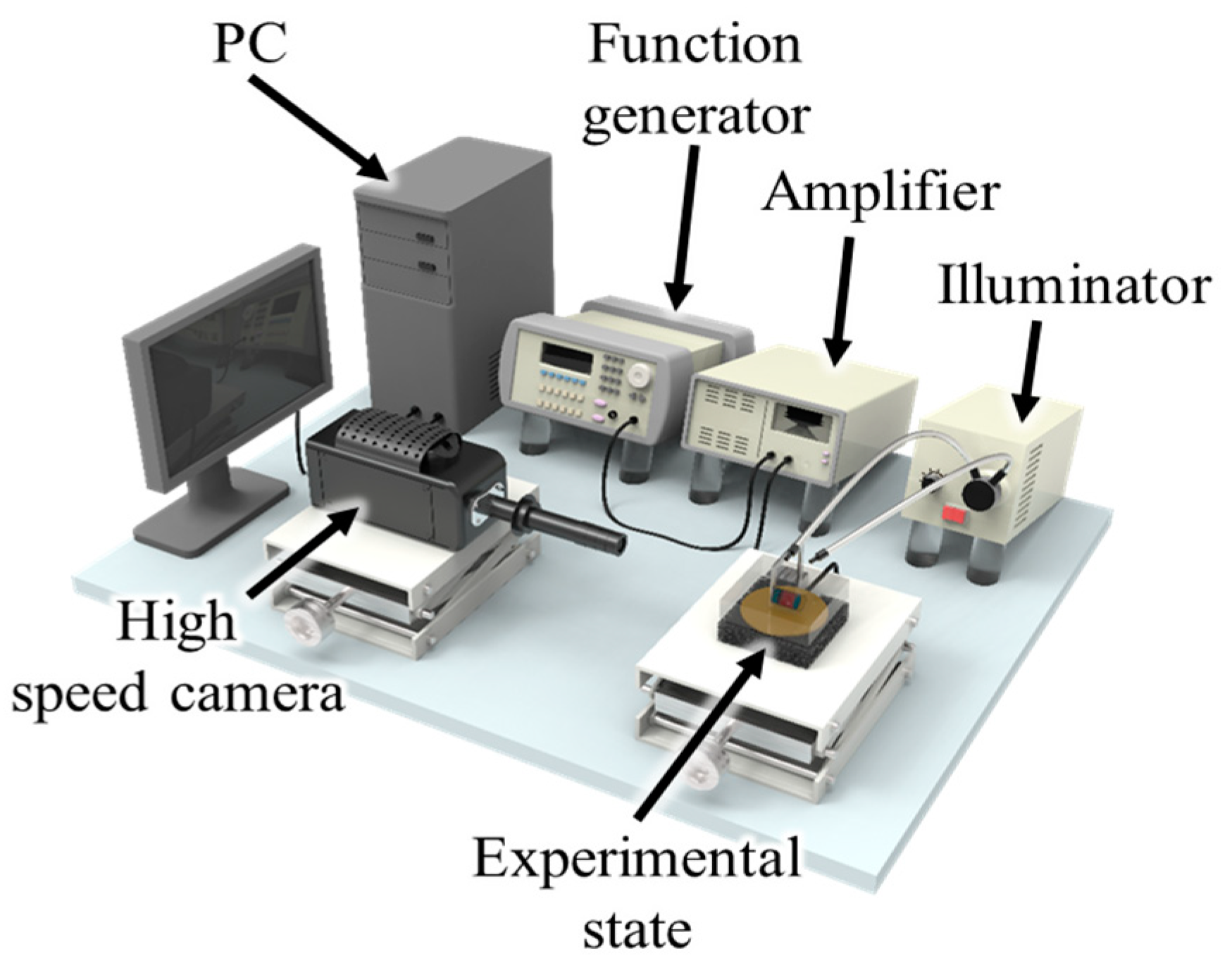
3.1. Experiment Setup and Methods
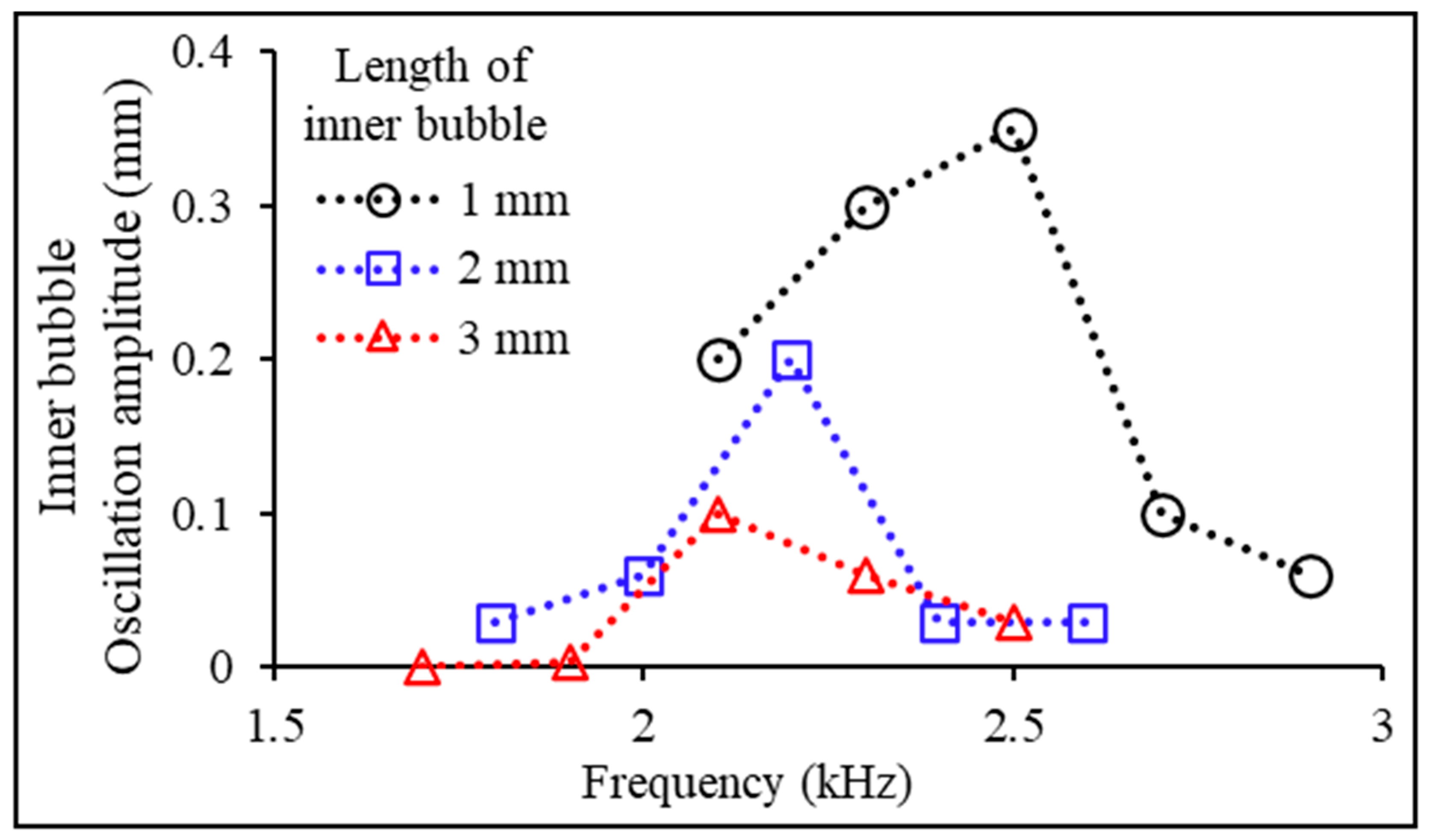
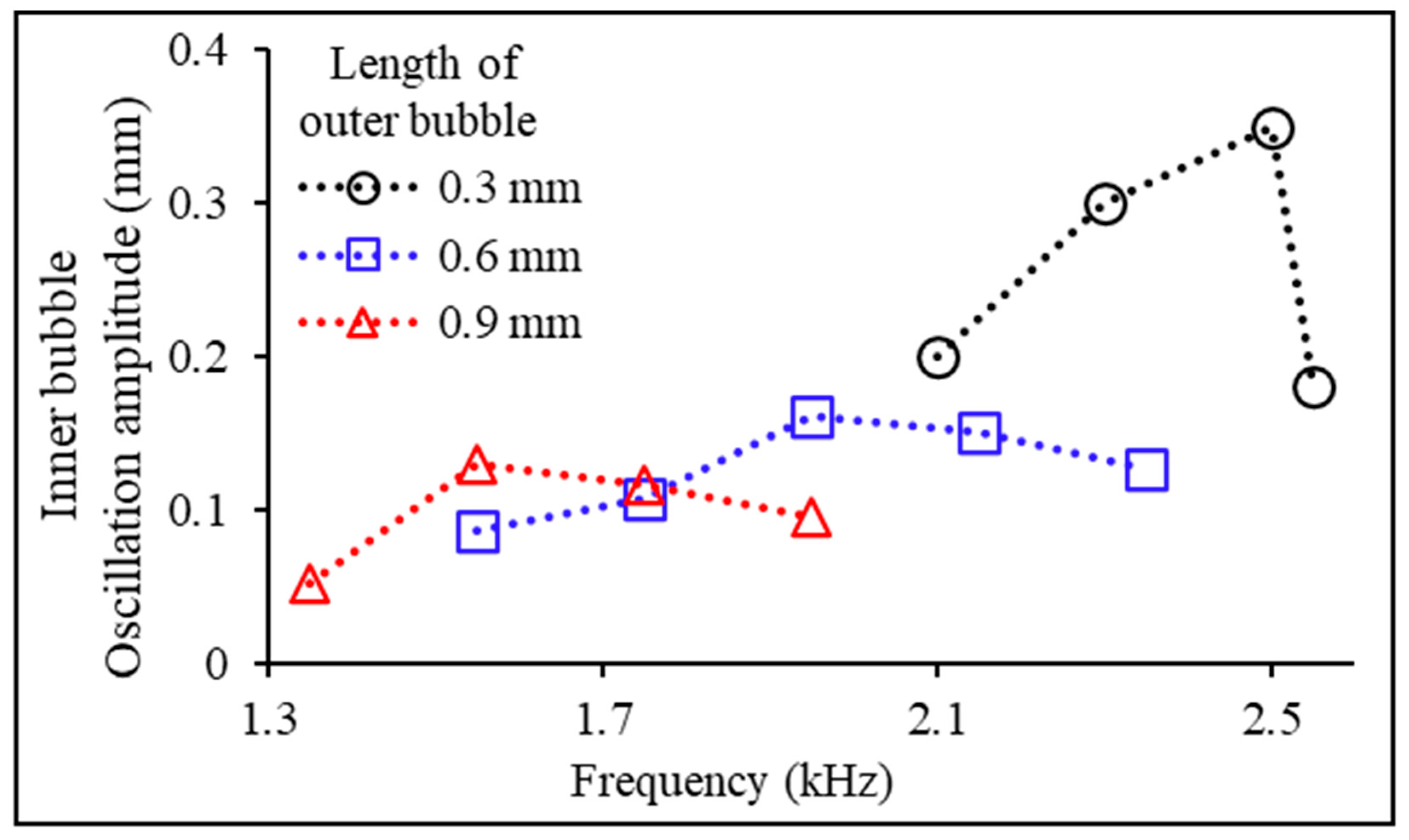
3.2. Experimental Variables: Bubble Length
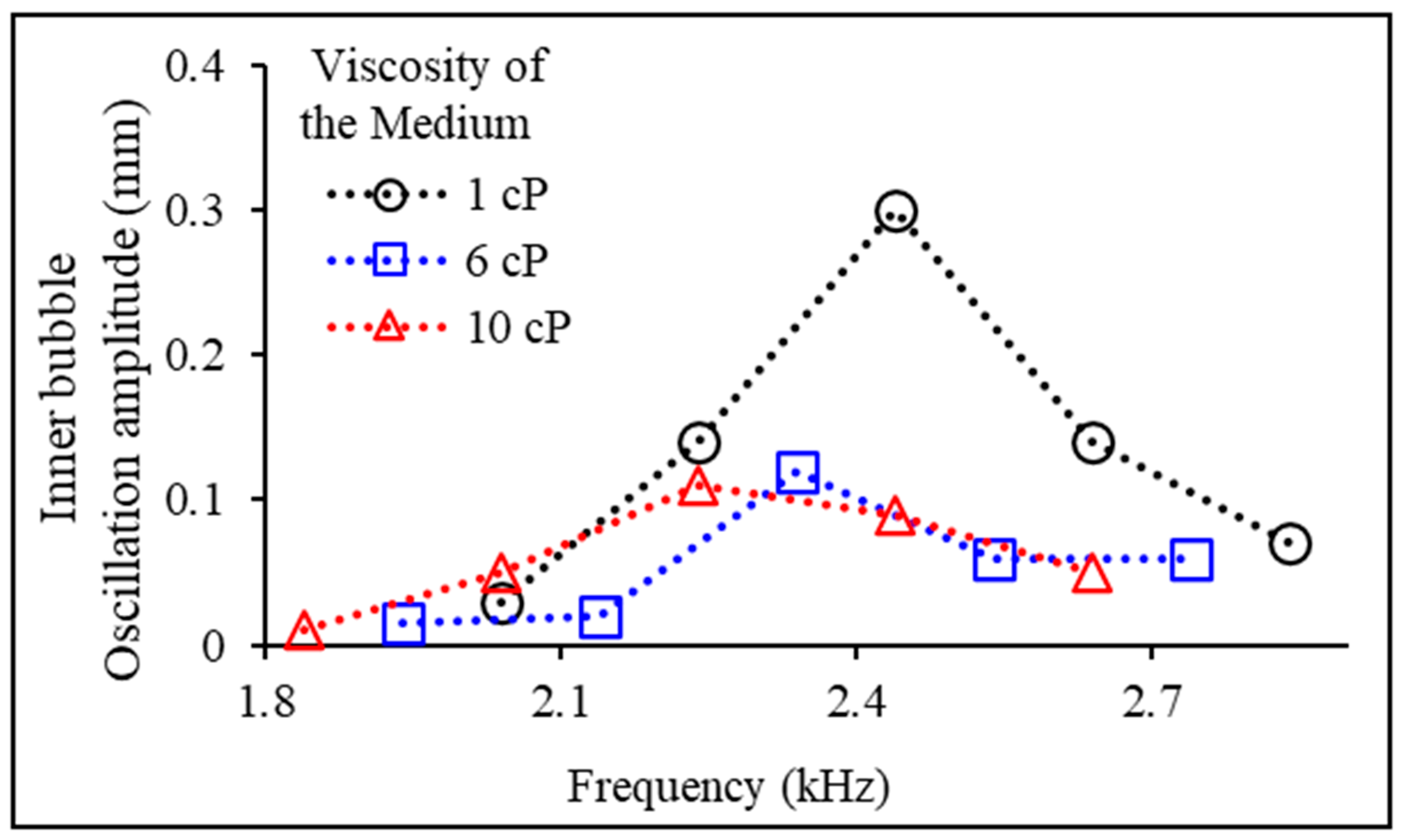
3.3. Experimental Variables: Experimental Fluid Viscosity
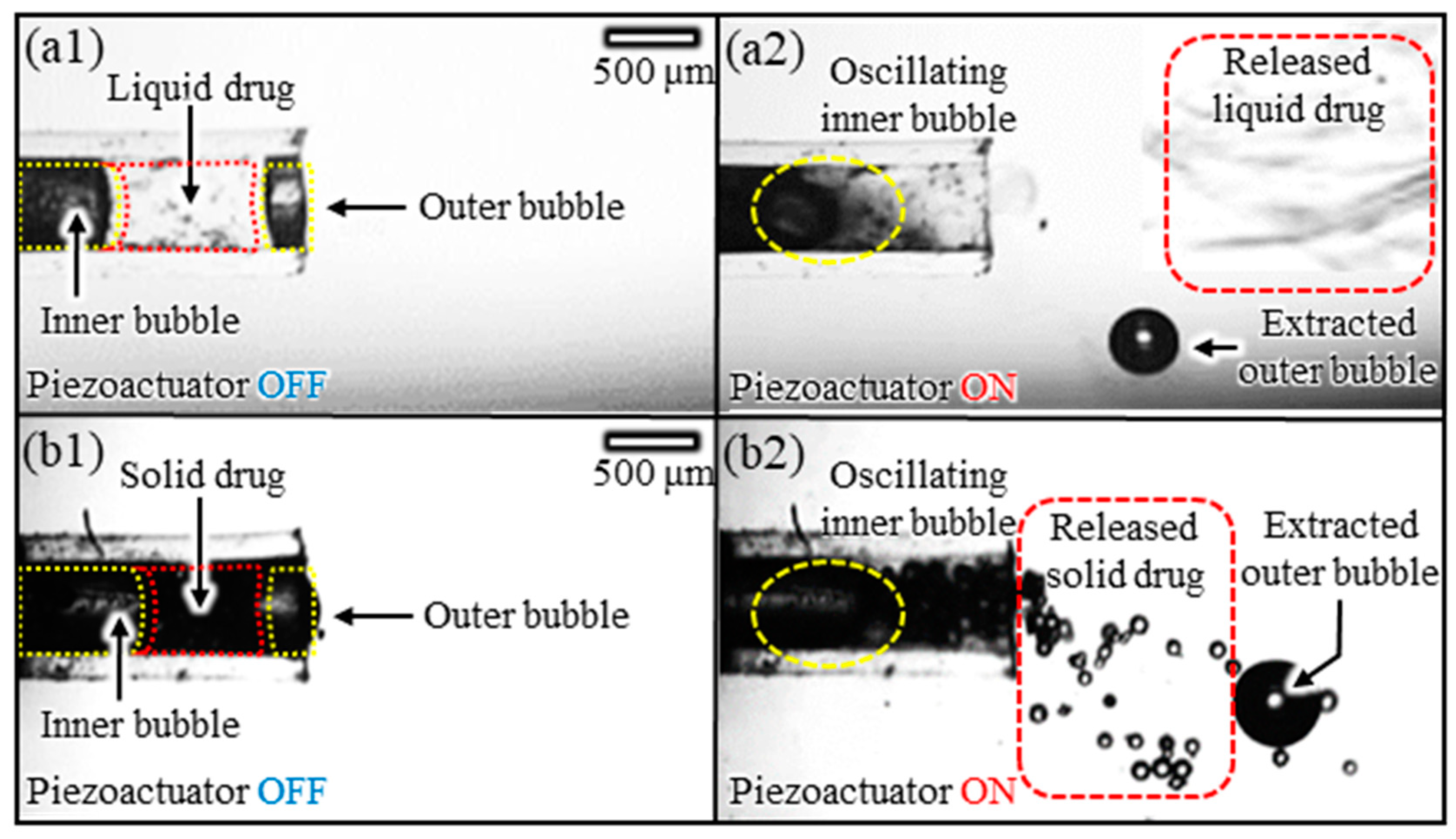
3.4. Multiphase Drug Release Experiments
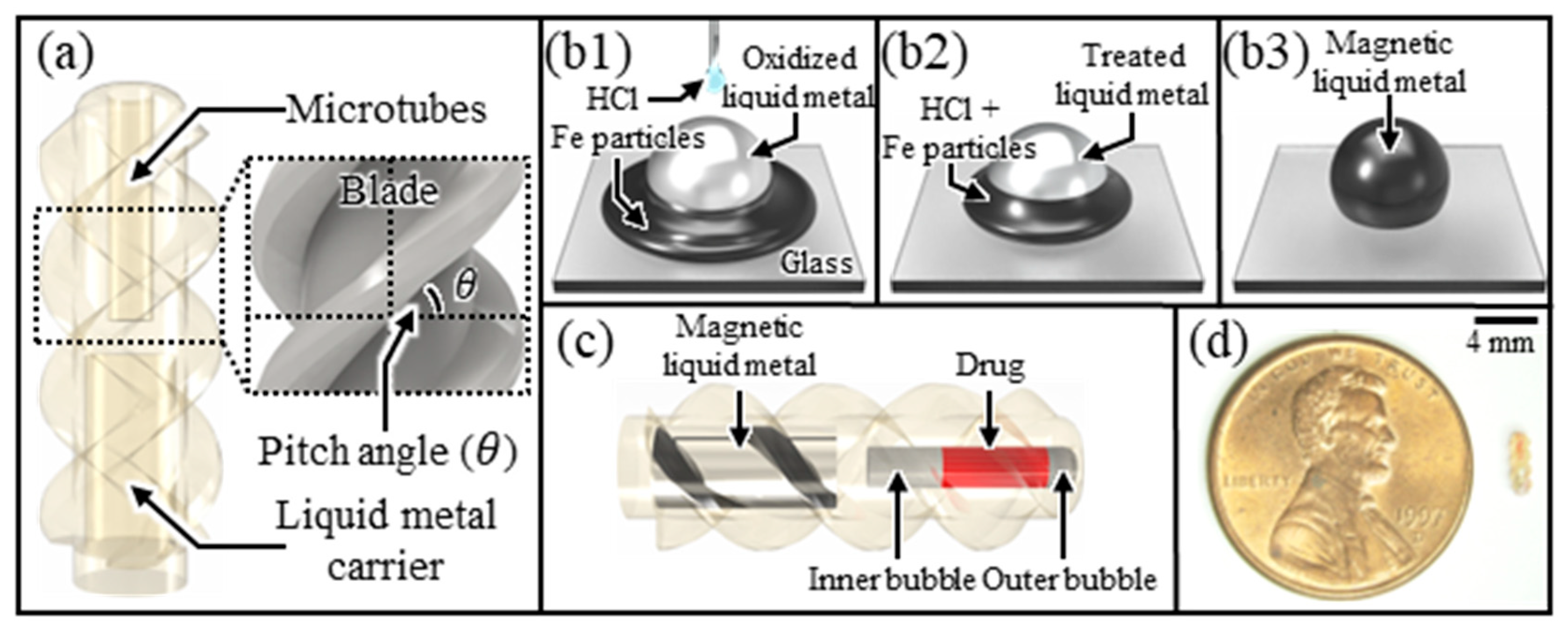
3.5. Design and Fabrication of Microrobots
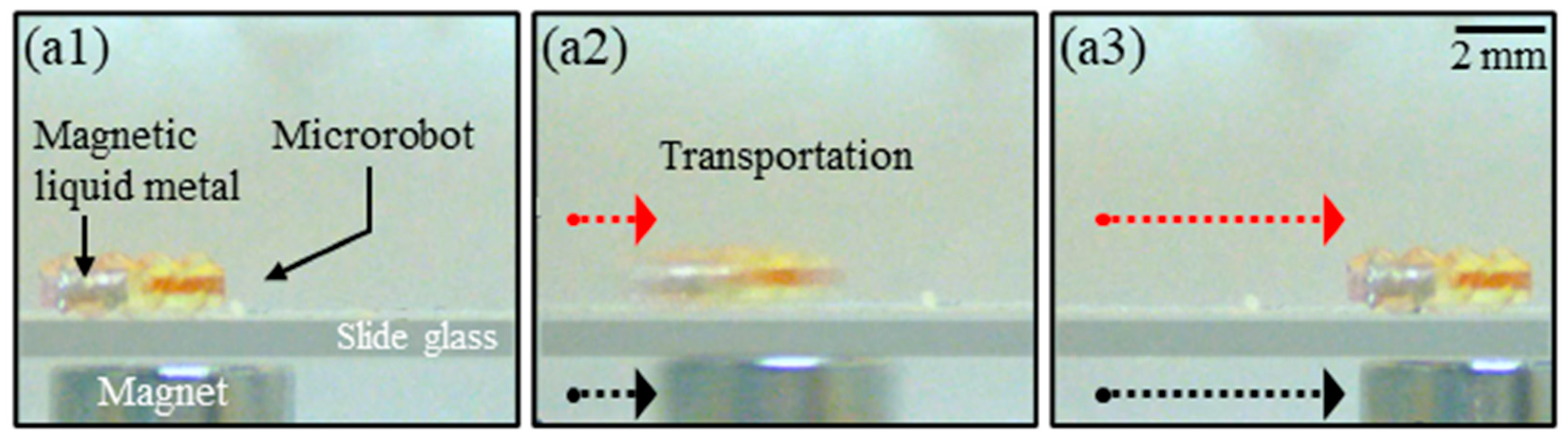
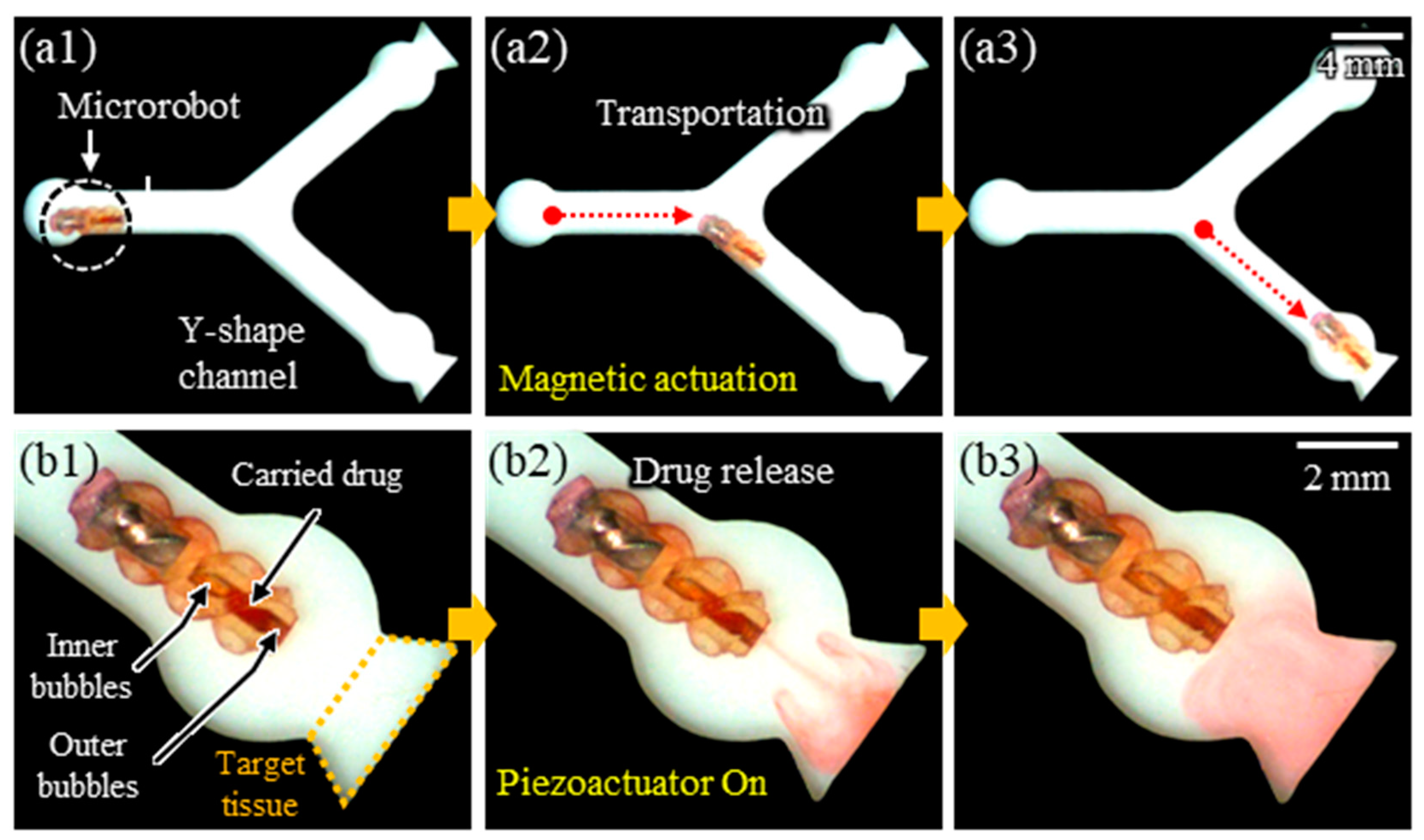
3.6. Experimental Demonstrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Bradbury, J. Beyond Pills and Jabs: Researchers Develop New Ways to Get Drugs to the Right Place at the Right Time. Lancet 2003, 362, 1984–1985. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the War on Cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and Severity of Self-Reported Chemotherapy Side Effects in Routine Care: A Prospective Cohort Study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound Microbubble Contrast Agents: Fundamentals and Application to Gene and Drug Delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced Materials and Processing for Drug Delivery: The Past and the Future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Abribat, T. The Rise and Rise of Drug Delivery. Nat. Rev. Drug Discov. 2005, 4, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.A. Nanostructure-Mediated Drug Delivery. Nanomedicine 2005, 1, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, W.; Xu, L.P.; Zhang, X.; Wang, S. Fuel-Free Synthetic Micro-/Nanomachines. Adv. Mater. 2017, 29, 1603250. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lee, J.; Nelson, B.J.; Zhang, L.; Choi, H. Fabrication and Manipulation of Ciliary Microrobots with Non-Reciprocal Magnetic Actuation. Sci. Rep. 2016, 6, 30713. [Google Scholar] [CrossRef]
- Peyer, K.E.; Tottori, S.; Qiu, F.; Zhang, L.; Nelson, B.J. Magnetic Helical Micromachines. Chemistry 2013, 19, 28–38. [Google Scholar] [CrossRef]
- Sourabh Shivaji, B.; Afaqahmed Mushtaqahmed, J.; Umesh Suresh, G. Medical Robotics and Automation. Int. J. Mech. Eng. Rob. Res. 2013, 2, 112–118. [Google Scholar]
- Jang, D.; Jeong, J.; Song, H.; Chung, S.K. Targeted Drug Delivery Technology Using Untethered Microrobots: A Review. J. Micromech. Microeng. 2019, 29, 053002. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J. Synthetic Micro/Nanomotors in Drug Delivery. Nanoscale 2014, 6, 10486–10494. [Google Scholar] [CrossRef]
- Luo, M.; Feng, Y.; Wang, T.; Guan, J. Micro-/Nanorobots at Work in Active Drug Delivery. Adv. Funct. Mater. 2018, 28, 1706100. [Google Scholar] [CrossRef]
- Diller, E. Micro-Scale Mobile Robotics. Found. Trends Robot. 2011, 2, 143–259. [Google Scholar] [CrossRef]
- Chen, X.Z.; Hoop, M.; Mushtaq, F.; Siringil, E.; Hu, C.; Nelson, B.J.; Pané, S. Recent Developments in Magnetically Driven Micro- and Nanorobots. Appl. Mater. Today 2017, 9, 37–48. [Google Scholar] [CrossRef]
- Choi, H.; Cha, K.; Jeong, S.; Park, J.O.; Park, S. 3-D Locomotive and Drilling Microrobot Using Novel Stationary EMA System. IEEE/ASME Trans. Mechatron. 2013, 18, 1221–1225. [Google Scholar] [CrossRef]
- Pawashe, C.; Floyd, S.; Sitti, M. Modeling and Experimental Characterization of an Untethered Magnetic Micro-Robot. Int. J. Rob. Res. 2009, 28, 1077–1094. [Google Scholar] [CrossRef]
- Yesin, K.B.; Vollmers, K.; Nelson, B.J. Modeling and Control of Untethered Biomicrorobots in a Fluidic Environment Using Electromagnetic Fields. Int. J. Robot. Res. 2006, 25, 527–536. [Google Scholar] [CrossRef]
- Jeong, S.; Choi, H.; Choi, J.; Yu, C.; Park, J.-O.; Park, S. Novel Electromagnetic Actuation (EMA) Method for 3-Dimensional Locomotion of Intravascular Microrobot. Sens. Actuators A Phys. 2010, 157, 118–125. [Google Scholar] [CrossRef]
- Pirmoradi, F.N.; Jackson, J.K.; Burt, H.M.; Chiao, M. A Magnetically Controlled MEMS Device for Drug Delivery: Design, Fabrication, and Testing. Lab. Chip 2011, 11, 3072–3080. [Google Scholar] [CrossRef]
- Yi, Y.; Zaher, A.; Yassine, O.; Kosel, J.; Foulds, I.G. A Remotely Operated Drug Delivery System with an Electrolytic Pump and a Thermo-Responsive Valve. Biomicrofluidics 2015, 9, 052608. [Google Scholar] [CrossRef]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-Inspired Magnetic Swimming Microrobots for Biomedical Applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef]
- Ceylan, H.; Giltinan, J.; Kozielski, K.; Sitti, M. Mobile Microrobots for Bioengineering Applications. Lab. Chip 2017, 17, 1705–1724. [Google Scholar] [CrossRef]
- Honda, T.; Arai, K.I.; Ishiyama, K. Micro Swimming Mechanisms Propelled by External Magnetic Fields. IEEE Trans. Magn. 1996, 32, 5085–5087. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B.J. Artificial Bacterial Flagella: Fabrication and Magnetic Control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 2012, 8, 460–467. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and PH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Chen, G.; Hoffman, A.S. Graft Copolymers That Exhibit Temperature-Induced Phase Transitions over a Wide Range of PH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From Controlled Release to PH-Responsive Drug Delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Li, H.; Go, G.; Ko, S.Y.; Park, J.O.; Park, S. Magnetic Actuated PH-Responsive Hydrogel-Based Soft Micro-Robot for Targeted Drug Delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Jeong, J.; Jang, D.; Kim, D.; Lee, D.; Chung, S.K. Acoustic Bubble-Based Drug Manipulation: Carrying, Releasing and Penetrating for Targeted Drug Delivery Using an Electromagnetically Actuated Microrobot. Sens. Actuators A Phys. 2020, 306, 111973. [Google Scholar] [CrossRef]
- Ko, S.H.; Lee, S.J.; Kang, K.H. A Synthetic Jet Produced by Electrowetting-Driven Bubble Oscillations in Aqueous Solution. Appl. Phys. Lett. 2009, 94, 194102. [Google Scholar] [CrossRef]
- Glezer, A.; Amitay, M. SYNTHETIC JETS. Annu. Rev. Fluid Mech. 2002, 34, 503–529. [Google Scholar] [CrossRef]
- Dijkink, R.J.; Van Der Dennen, J.P.; Ohl, C.D.; Prosperetti, A. The “Acoustic Scallop”: A Bubble-Powered Actuator. J. Micromech. Microeng. 2006, 16, 1653–1659. [Google Scholar] [CrossRef]
- Feng, J.; Yuan, J.; Cho, S.K. Micropropulsion by an Acoustic Bubble for Navigating Microfluidic Spaces. Lab. Chip 2015, 15, 1554–1562. [Google Scholar] [CrossRef]
- Chen, X.M.; Prosperetti, A. Thermal Processes in the Oscillations of Gas Bubbles in Tubes. J. Acoust. Soc. Am. 1998, 104, 1389–1398. [Google Scholar] [CrossRef]
- Geng, X.; Yuan, H.; Oguz, H.N.; Prosperetti, A. The Oscillation of Gas Bubbles in Tubes: Experimental Results. J. Acoust. Soc. Am. 1999, 106, 674–681. [Google Scholar] [CrossRef]
- Oguz, H.N.; Prosperetti, A. The Natural Frequency of Oscillation of Gas Bubbles in Tubes. J. Acoust. Soc. Am. 1998, 103, 3301–3308. [Google Scholar] [CrossRef]
- Xu, T.; Yu, J.; Vong, C.I.; Wang, B.; Wu, X.; Zhang, L. Dynamic Morphology and Swimming Properties of Rotating Miniature Swimmers with Soft Tails. IEEE/ASME Trans. Mechatron. 2019, 24, 924–934. [Google Scholar] [CrossRef]
- Jeong, J.; Seo, J.; Lee, J.B.; Chung, S.K.; Kim, D. Electromagnet Polarity Dependent Reversible Dynamic Behavior of Magnetic Liquid Metal Marble. Mater. Res. Express 2020, 7, 015708. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for BioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent Progress on Parylene C Polymer for Biomedical Applications: A Review. Prog. Org. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, Y.; Jeong, J.; Jang, D.; Kim, D.; Chung, S. Acoustic Bubble and Magnetic Actuation-Based Microrobot for Enhanced Multiphase Drug Delivery Efficiency. Micromachines 2023, 14, 2169. https://doi.org/10.3390/mi14122169
Park J, Kim Y, Jeong J, Jang D, Kim D, Chung S. Acoustic Bubble and Magnetic Actuation-Based Microrobot for Enhanced Multiphase Drug Delivery Efficiency. Micromachines. 2023; 14(12):2169. https://doi.org/10.3390/mi14122169
Chicago/Turabian StylePark, Jihyeok, Youngkwang Kim, Jinwon Jeong, Deasung Jang, Daegeun Kim, and Sangkug Chung. 2023. "Acoustic Bubble and Magnetic Actuation-Based Microrobot for Enhanced Multiphase Drug Delivery Efficiency" Micromachines 14, no. 12: 2169. https://doi.org/10.3390/mi14122169
APA StylePark, J., Kim, Y., Jeong, J., Jang, D., Kim, D., & Chung, S. (2023). Acoustic Bubble and Magnetic Actuation-Based Microrobot for Enhanced Multiphase Drug Delivery Efficiency. Micromachines, 14(12), 2169. https://doi.org/10.3390/mi14122169




