Synthesis and Catalytic Degradation of PEF, ENR, and CIP by g-C3N4/TCNQ/Eu Composite
Abstract
:1. Introduce
2. Experiment
2.1. Composite Material Synthesis
2.2. Experiments on the Catalytic Degradation of PEF, ENR and CIP by g-C3N4/TCNQ/Eu Composite
2.3. Identification of Intermediate Products
2.4. Conditions for Chromatographic Analysis and Mass Spectrometry
3. Result and Analysis
3.1. Characterization Results Analysis of g-C3N4/TCNQ/Eu
3.1.1. X-ray Diffractometer
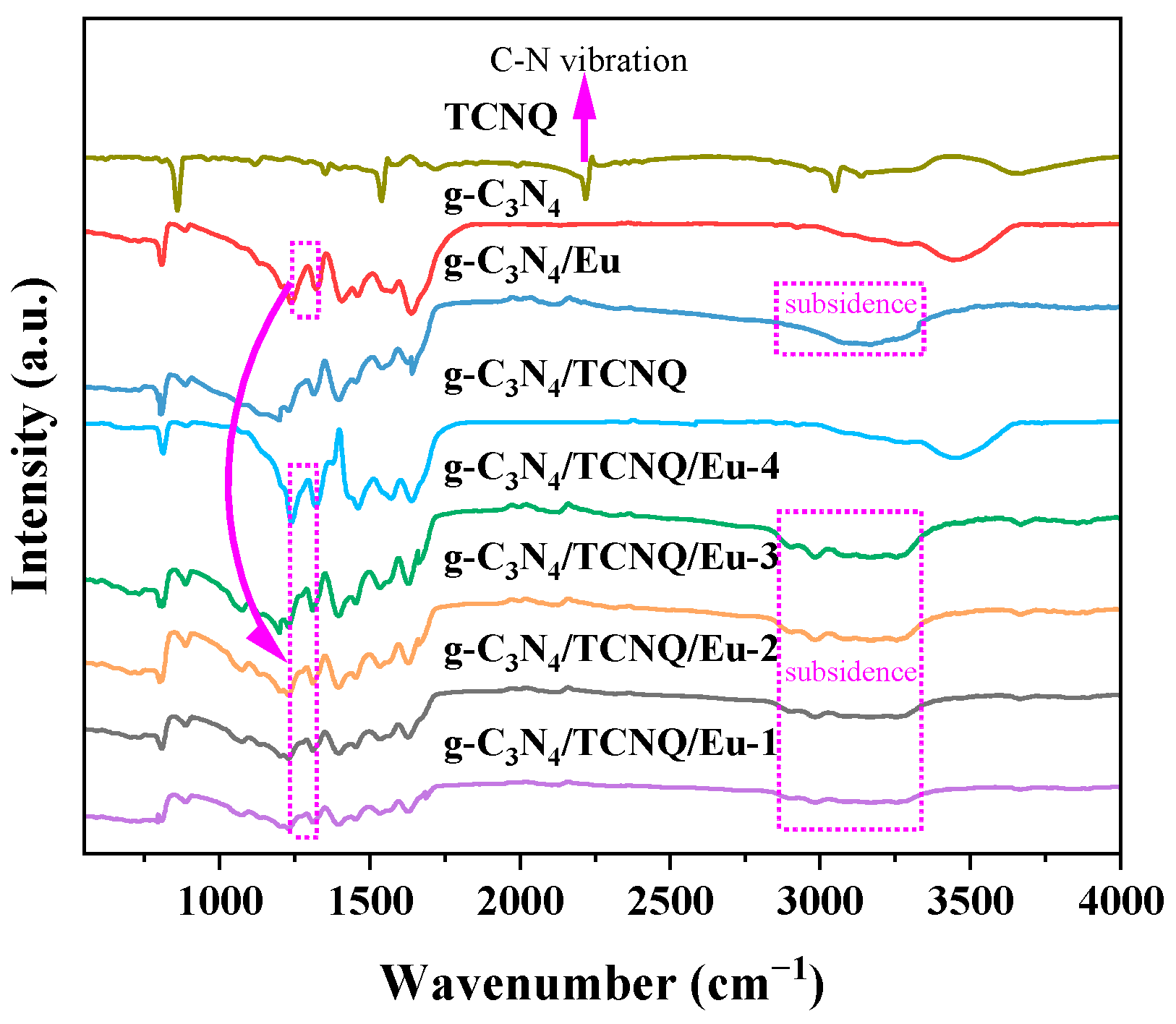
3.1.2. Fourier Transform Infrared Spectroscopy
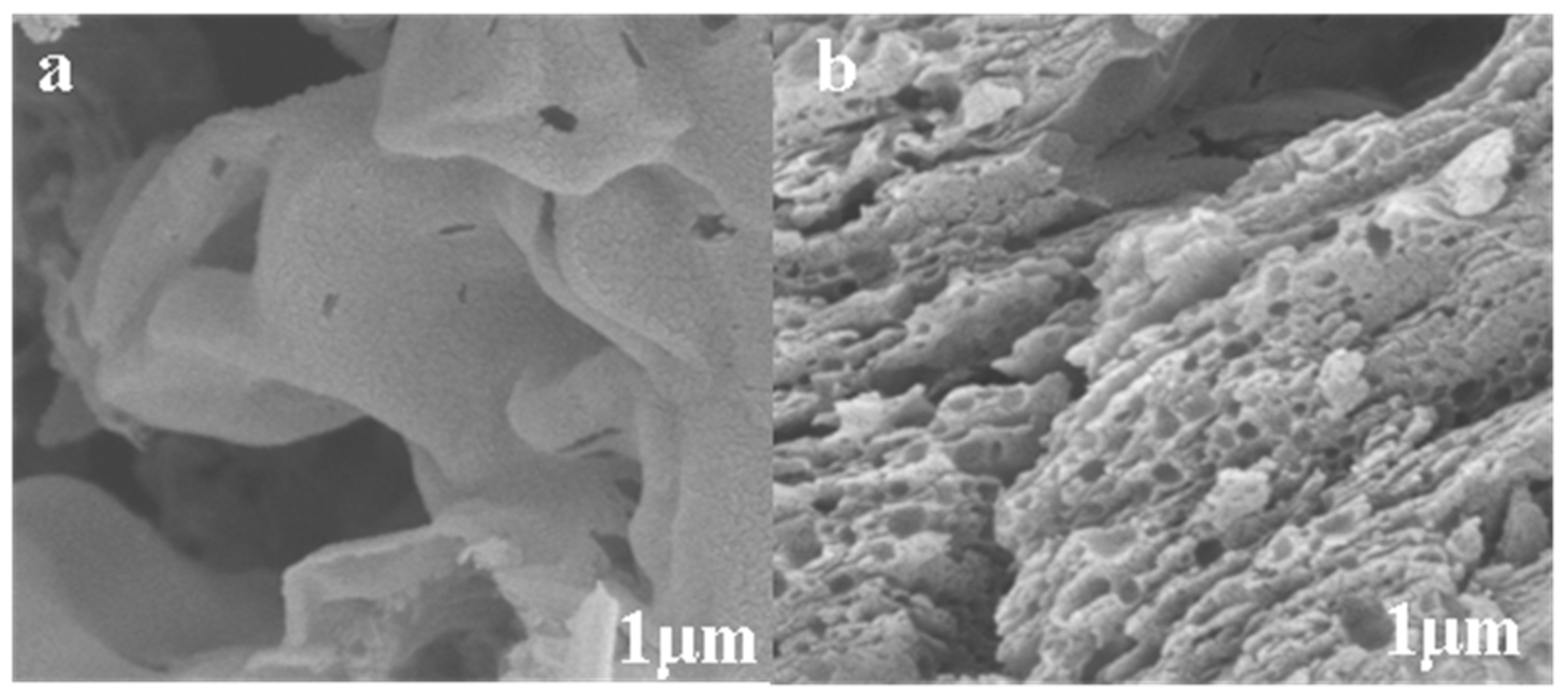
3.1.3. Scanning Electron Microscope
3.1.4. Transmission Electron Microscope
3.1.5. X-ray Photoelectric Energy Spectrum
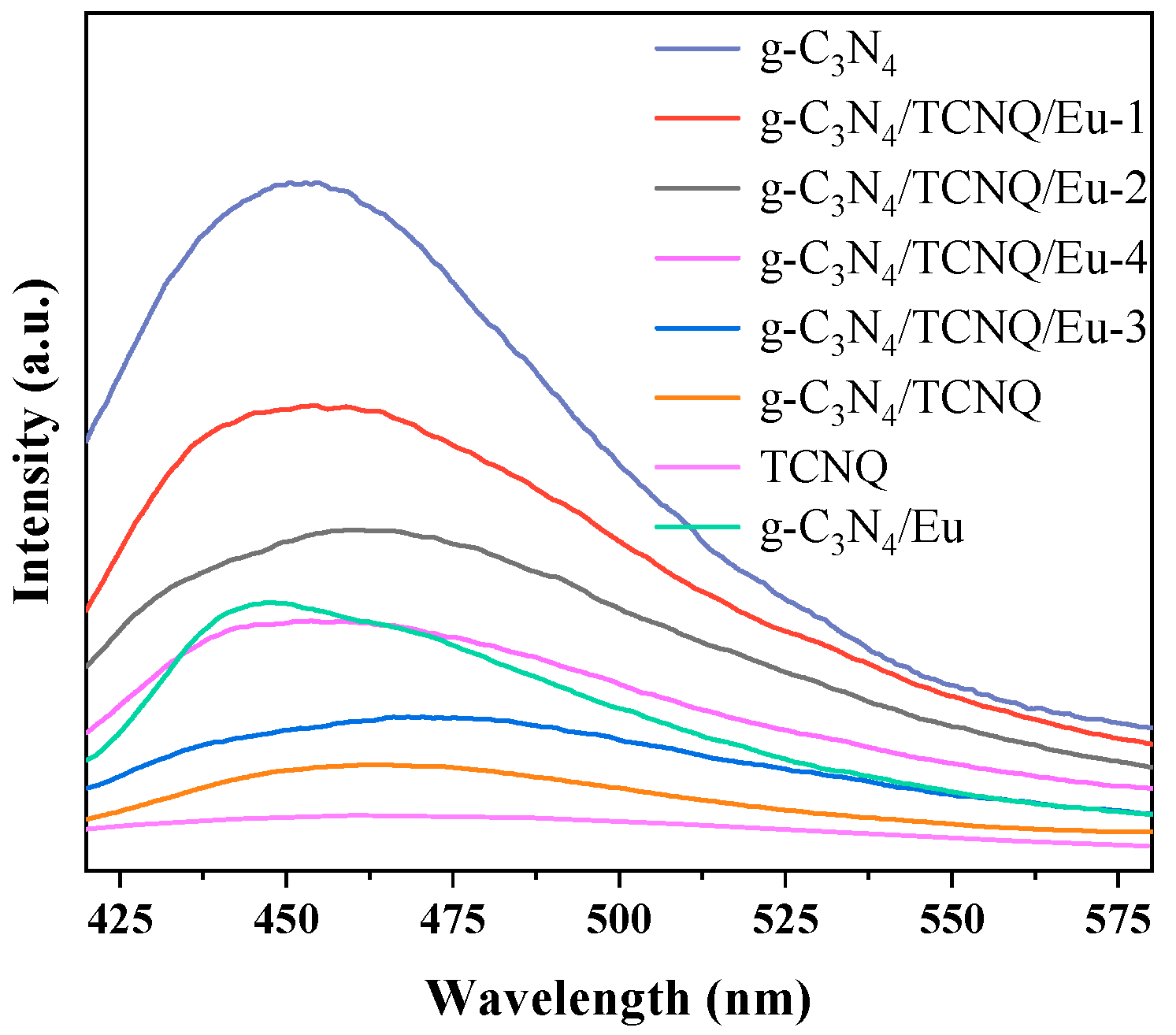
3.1.6. Photoluminescence (PL) Spectrum
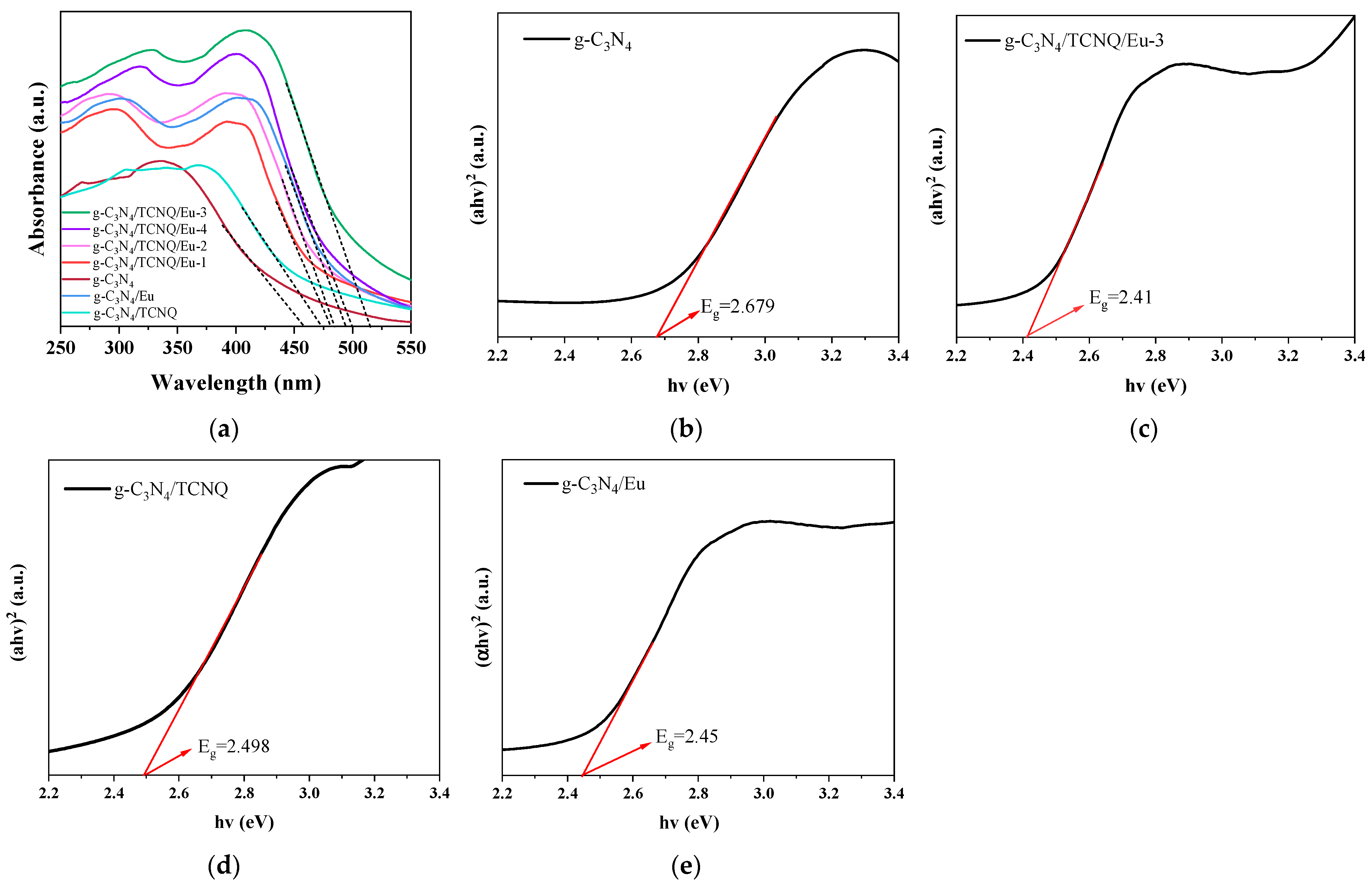
3.1.7. UV-Vis Diffuse Reflectance Spectroscopy
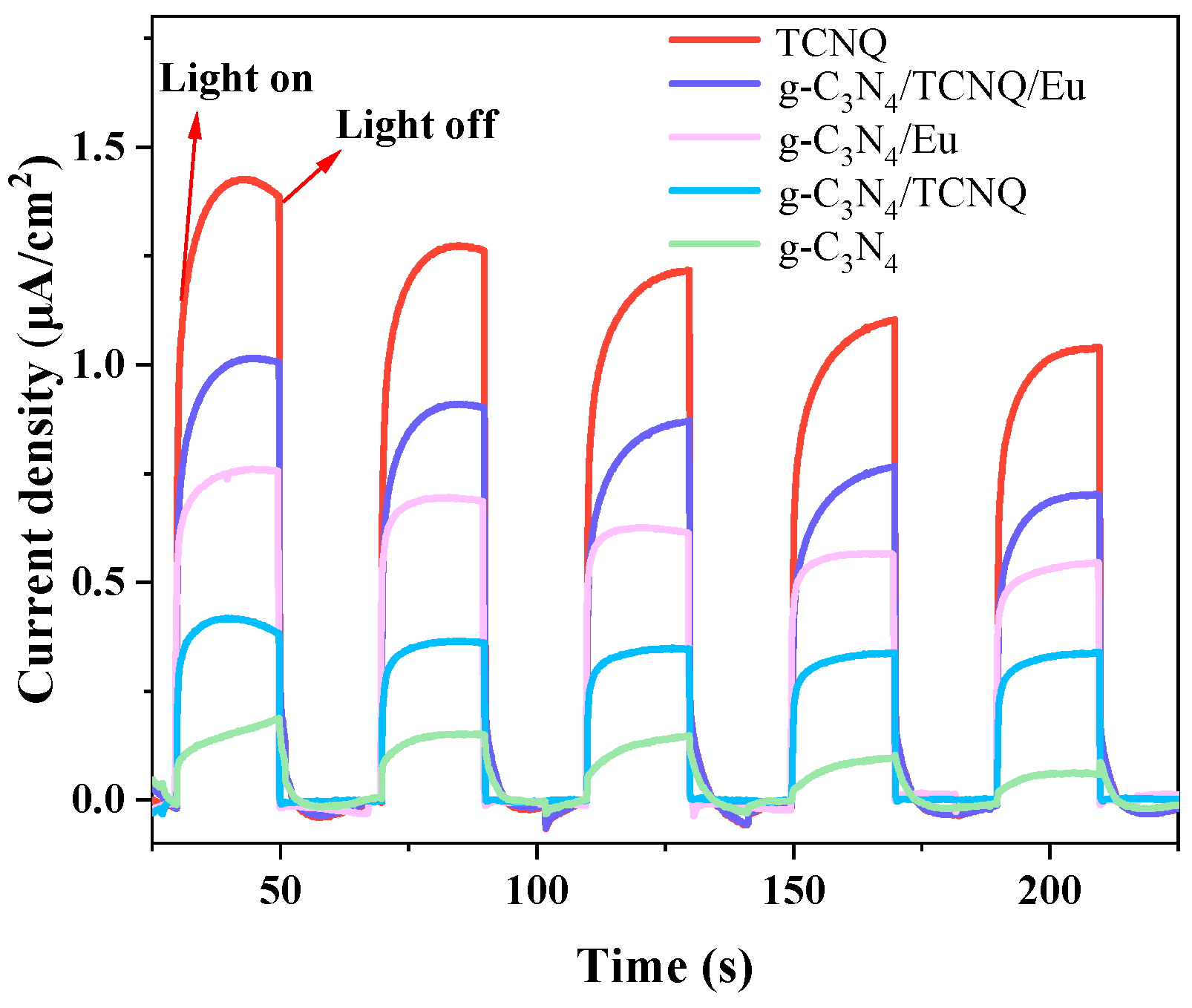
3.1.8. Photocurrent Test
3.2. Photocatalytic Performance Analysis of g-C3N4/TCNQ/Eu Composite
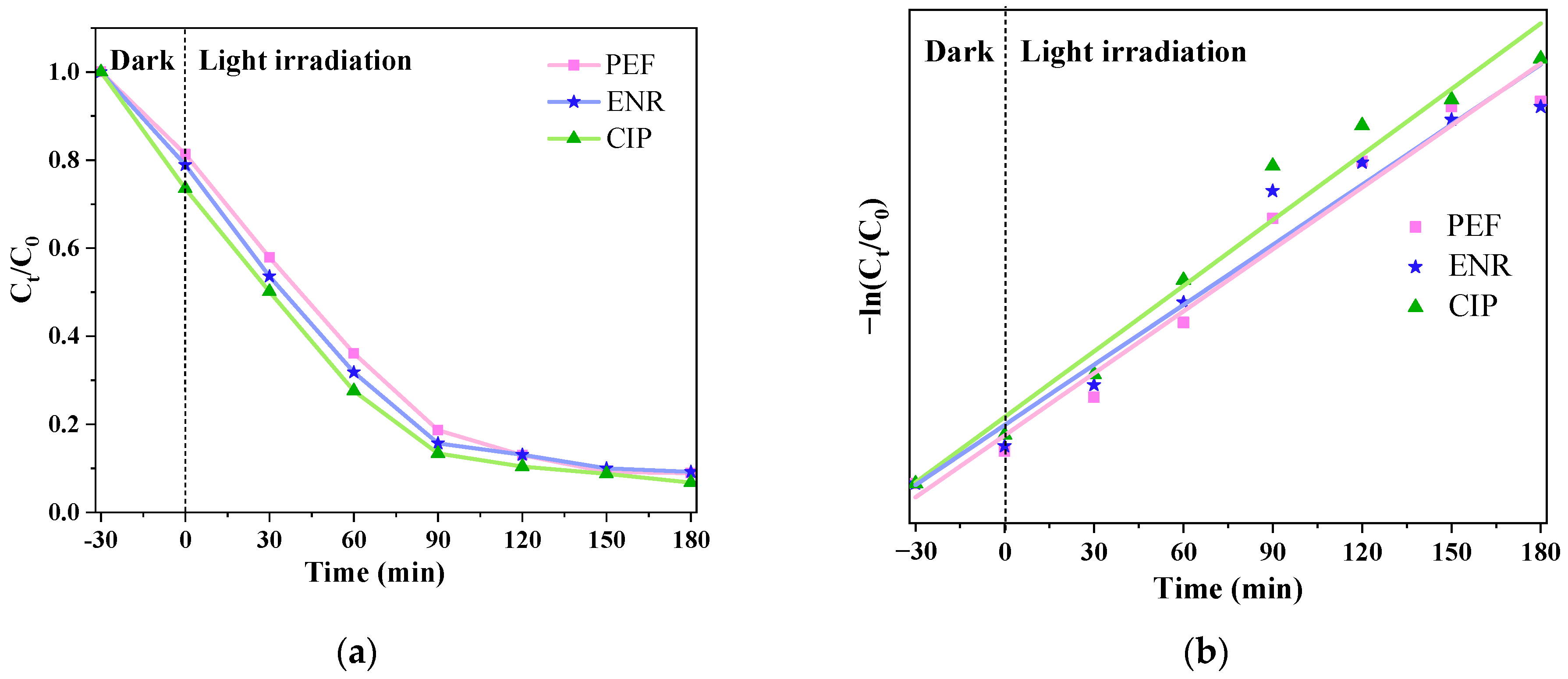
3.2.1. Different Medications’ Effects on the Catalytic Effect
3.2.2. Different Amounts of Copolymer Doping’s Effects on CIP’s Catalytic Degradation
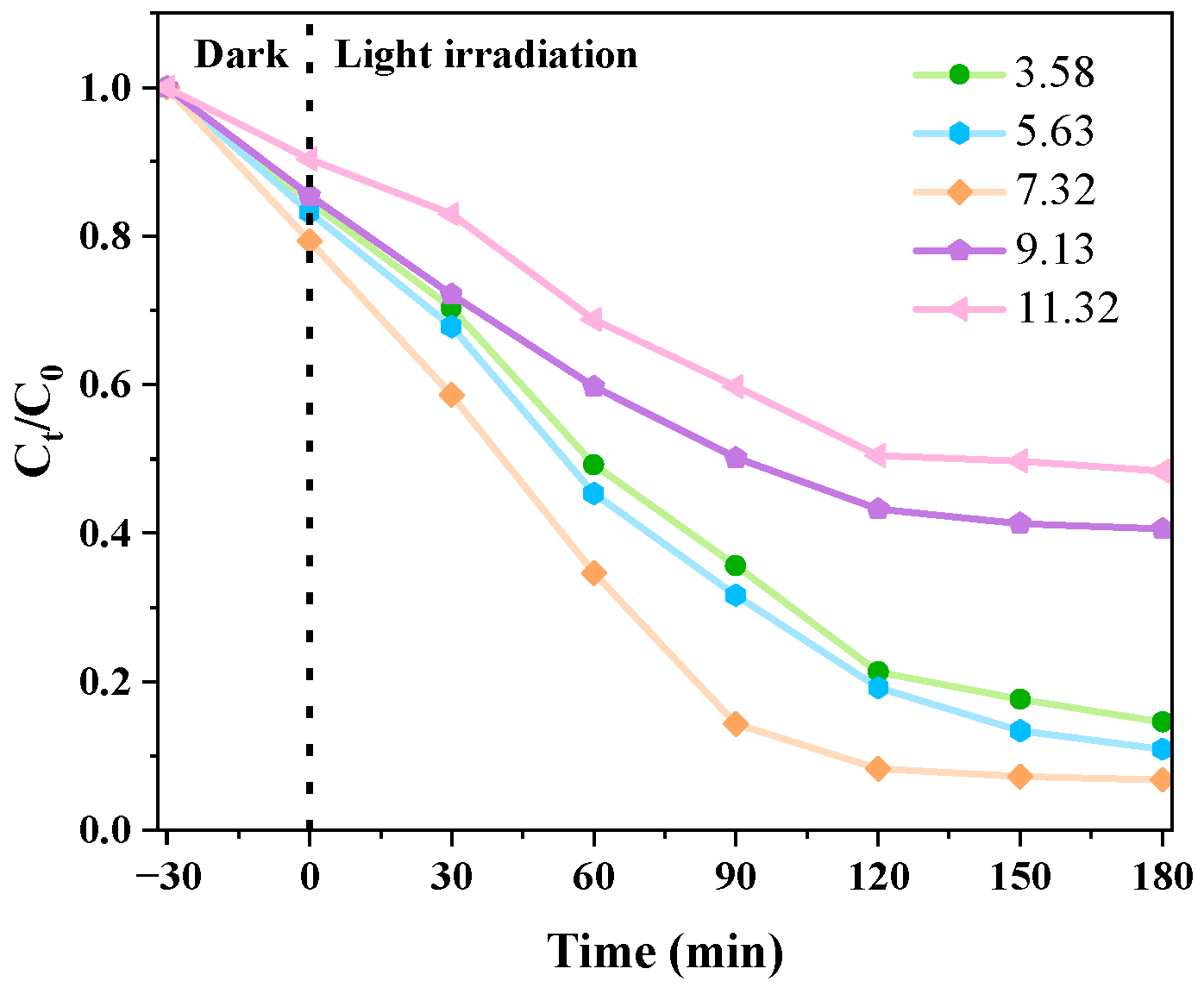
3.2.3. Different pH Levels’ Effects on the Catalytic Impact of CIP Degradation
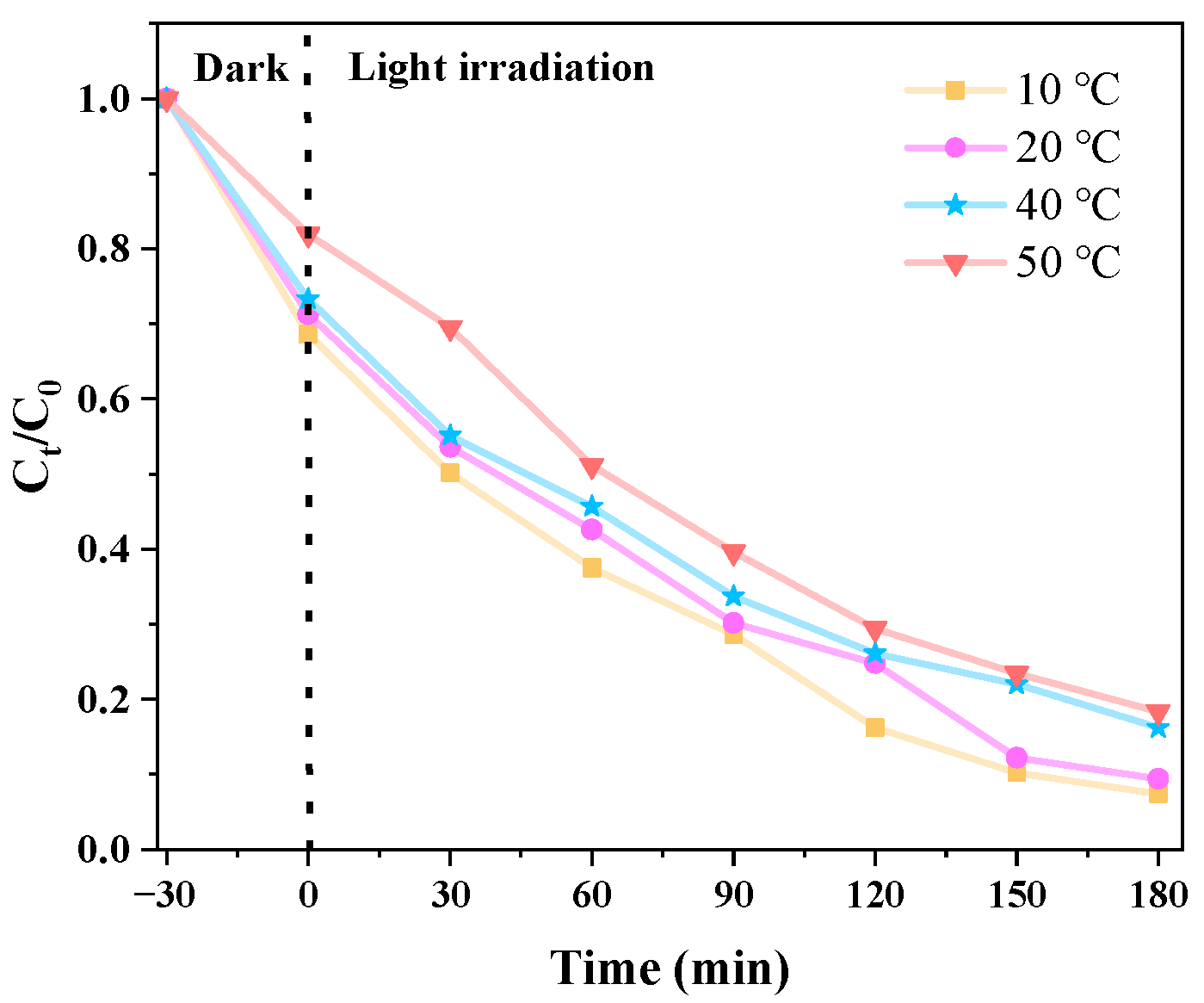
3.2.4. Effects of Different Temperatures on Catalytic Degradation of CIP
3.3. Kinetic Analysis of Photocatalytic Degradation of CIP
3.4. Analysis of Reuse Experimental Results of CIP Degradation by Catalyst
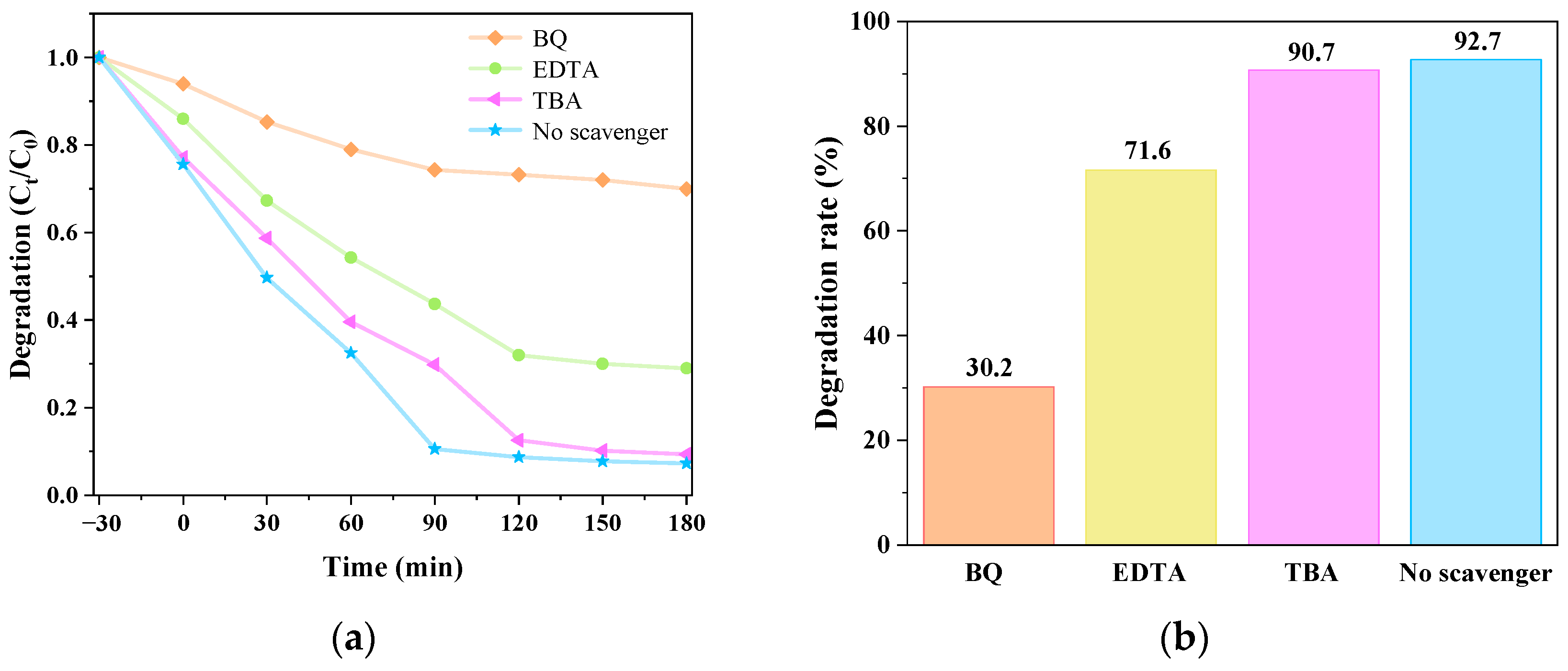
3.5. Analysis of Free Radical Capture Experimental Data of Catalyst-Mediated CIP Degradation
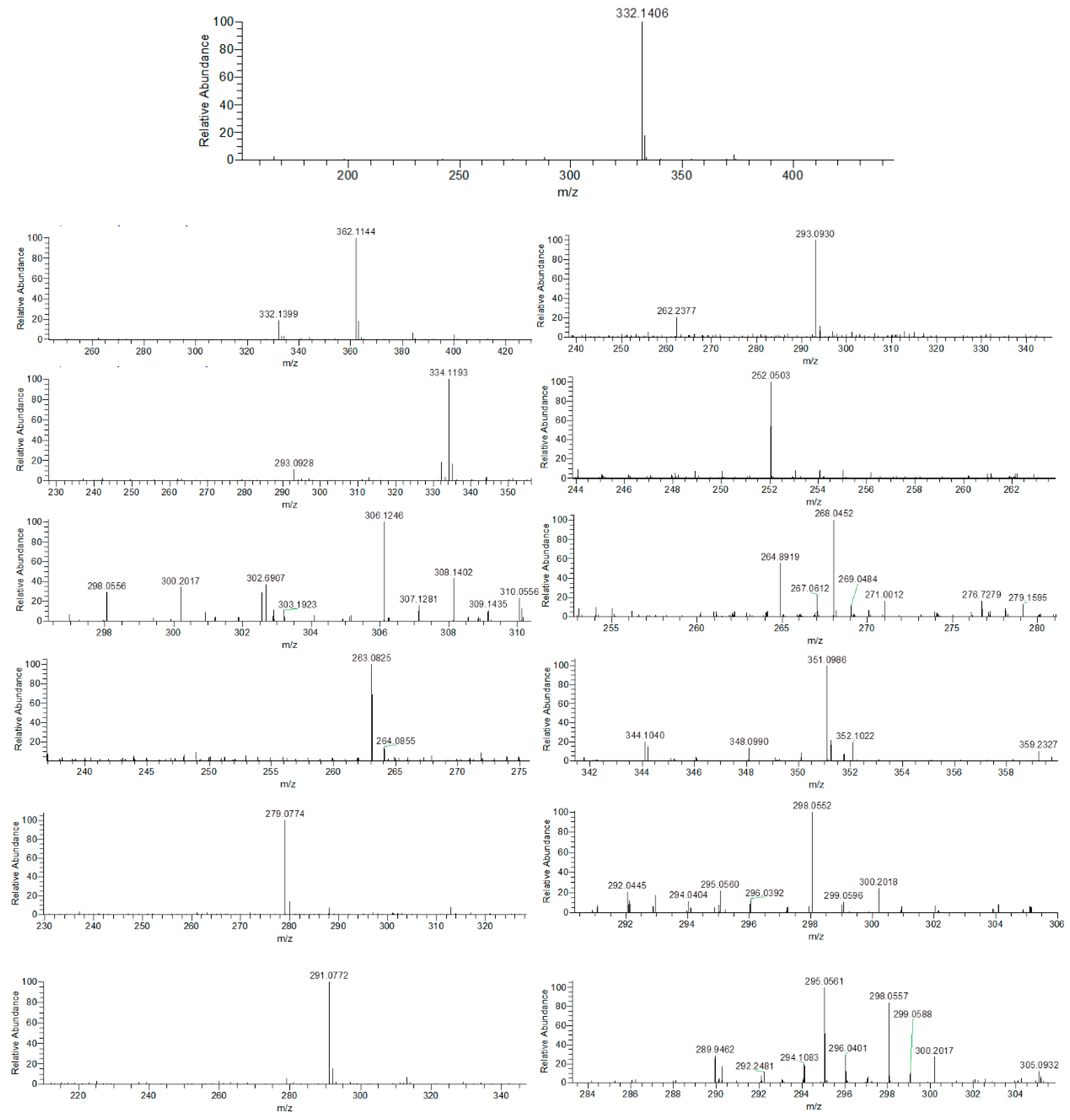
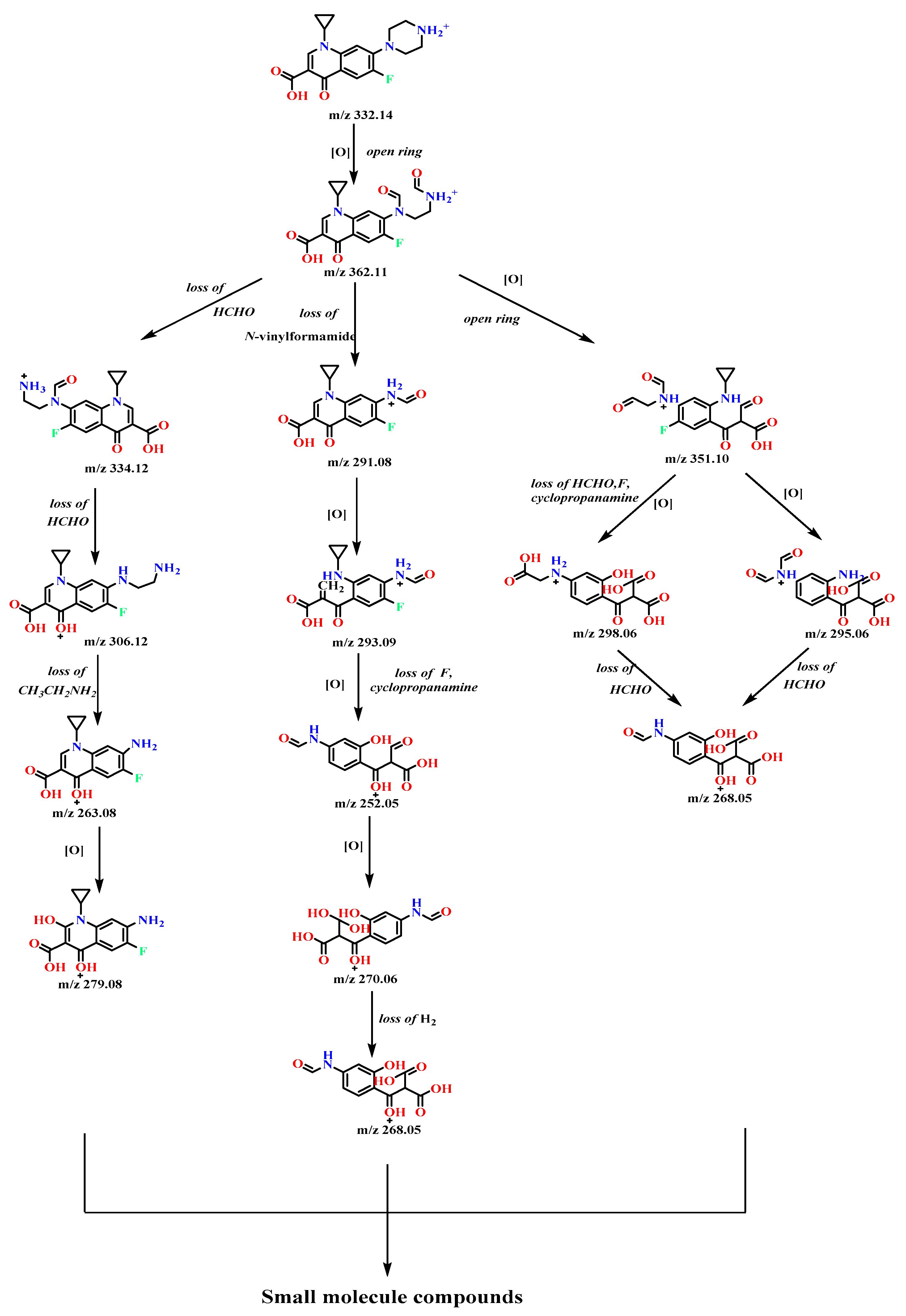
3.6. Identification of Intermediate Products of CIP Degradation by Catalyst
3.7. Photocatalytic Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soukiazis, E.; Proenca, S.; Cerqueira, P.A. The Interconnections between Renewable Energy, Economic Development and Environmental Pollution: A Simultaneous Equation System Approach. Energy J. 2019, 40, 1–24. [Google Scholar] [CrossRef]
- Bai, X.; Lutz, A.; Carroll, R.; Keteles, K.; Dahlin, K.; Murphy, M.; Nguyen, D. Occurrence, distribution, and seasonality of emerging contaminants in urban watersheds. Chemosphere 2018, 200, 133–142. [Google Scholar] [CrossRef]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Dunsmuir, D.; Businge, S.; Tumusiime, R.; Karugaba, J.; Wiens, M.O.; Görges, M.; Kissoon, N.; Orach, S.; Kasyaba, R.; et al. Evaluation of a digital triage platform in Uganda: A quality improvement initiative to reduce the time to antibiotic administration. PLoS ONE 2020, 15, e0240092. [Google Scholar] [CrossRef]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2020, 10, 20784–20793. [Google Scholar] [CrossRef]
- Sharma, P.C.; Goyal, R.; Sharma, A.; Sharma, D.; Saini, N.; Rajak, H.; Sharma, S.; Thakur, V.K. Insights on fluoroquinolones in cancer therapy: Chemistry and recent developments. Mater. Today Chem. 2020, 17, 100296. [Google Scholar] [CrossRef]
- Aylaz, G.; Okan, M.; Duman, M.; Aydin, H.M. Study on Cost-Efficient Carbon Aerogel to Remove Antibiotics from Water Resources. ACS Omega 2020, 5, 16635–16644. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cai, A.; Li, T.T. Covalent Organic Framework-Semiconductor-Based Heterostructures for Photocatalytic Applications. ChemSusChem 2023, 16, e202300021. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Chandrasekaran, A. Recent advances in TiO2/ZnS-based binary and ternary photocatalysts for the degradation of organic pollutants. Sci. Total Environ. 2023, 868, 161525. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, W.; Su, X.; Li, Z.; Ding, X.; Du, X.; Ren, Y.; Zhang, H.; Feng, J.; Wei, T. A regenerable Cu2O/BiOBr S-scheme heterojunction photocatalysts for efficient photocatalytic degradation of mixed organic pollutants. Sep. Purif. Technol. 2023, 313, 123447. [Google Scholar] [CrossRef]
- Awad, M.E.; Farrag, A.M.; El-Bindary, A.A.; El-Bindary, M.A.; Kiwaan, H.A. Photocatalytic degradation of Rhodamine B dye using low-cost pyrofabricated titanium dioxide quantum dots-kaolinite nanocomposite. Appl. Organomet. Chem. 2023, 37, e7113. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, H.; Nie, R. Synthesis and biomedical applications of graphitic carbon nitride quantum dots. J. Mater. Chem. B 2019, 7, 5432–5448. [Google Scholar] [CrossRef]
- Li, H.; Huang, G.; Xu, H.; Yang, Z.; Xu, X.; Li, J.; Qu, A.; Chen, Y. Enhancing photodegradation activity of g-C3N4 via decorating with S-doped carbon nitride quantum dots by in situ polymerization. J. Solid State Chem. 2020, 292, 121705. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Wang, D.; Zhang, Q.; Zeng, J.; Zhang, R. Facile synthesis of nitrogen-defective g-C3N4 for superior photocatalytic degradation of rhodamine B. RSC Adv. 2021, 11, 30503–30509. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xu, J.; Wang, X.; Li, L.; Antonietti, M.; Shalom, M. Phenyl-Modified Carbon Nitride Quantum Dots with Distinct Photoluminescence Behavior. Angew. Chem. Int. Ed. 2016, 55, 3672–3676. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Yao, W.; Chen, K.; Chen, D. One step synthesis of P-doped g-C3N4 with the enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2018, 430, 309–315. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Sun, H.; Li, M.; Shi, W. High-efficiency photocatalytic water splitting by a N-doped porous g-C3N4 nanosheet polymer photocatalyst derived from urea and N,N-dimethylformamide. Inorg. Chem. Front. 2020, 7, 1770–1779. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Dong, F.; Reshak, A.H.; Ye, L.; Pinna, N.; Zeng, C.; Zhang, T.; Huang, H. Chlorine intercalation in graphitic carbon nitride for efficient photocatalysis. Appl. Catal. B Environ. 2017, 203, 465–474. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Yang, S.; Li, X.; Lin, X. Ag3PO4 nanocrystals and g-C3N4 quantum dots decorated Ag2WO4 nanorods: Ternary nanoheterostructures for photocatalytic degradation of organic contaminants in water. RSC Adv. 2019, 9, 8065–8072. [Google Scholar] [CrossRef]
- Yan, W.; Yan, L.; Jing, C. Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms. Appl. Catal. B Environ. 2019, 244, 475–485. [Google Scholar] [CrossRef]
- El-Bindary, A.A.; El-Marsafy, S.M.; El-Maddah, A.A. Enhancement of the photocatalytic activity of ZnO nanoparticles by silver doping for the degradation of AY99 contaminants. J. Mol. Struct. 2019, 1191, 76–84. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Al-Qahtani, S.D.; Alshareef, M.; El-Desouky, M.G.; El-Bindary, A.A.; El-Metwaly, N.M.; El-Bindary, M.A. Highly efficient adsorption and removal bio-staining dye from industrial wastewater onto mesoporous Ag-MOFs. Process Saf. Environ. Prot. 2023, 172, 395–407. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Sui, G.; Du, L.; Zhuang, Y.; Zhang, Y.; Zou, Y. Removal of volatile organic compounds from air using supported ionic liquid membrane containing ultraviolet-visible light-driven Nd-TiO2 nanoparticles. J. Mol. Struct. 2021, 1231, 130023. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Liu, J.; Huang, L. Synthesis and photocatalytic performance of europium-doped graphitic carbon nitride. J. Rare Earths 2013, 31, 1085–1091. [Google Scholar] [CrossRef]
- Babkin, R.Y.; Lamonova, K.V.; Orel, S.M.; Prudnikov, A.M.; Pashkevich, Y.G.; Gornostaeva, O.V.; Viagin, O.G.; Maksimchuk, P.O.; Malyukin, Y.V. Formation mechanism of luminescence spectra of carbon nitride films doped by europium chloride CNx: EuCl3. J. Lumin. 2017, 186, 247–254. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Xu, J.; Zhao, S.; Liu, Z.; Li, Y. Rare earth element, Sm, modified graphite phase carbon nitride heterostructure for photocatalytic hydrogen production. New J. Chem. 2019, 43, 1716–1724. [Google Scholar] [CrossRef]
- Nafady, A.; Sabri, Y.M.; Kandjani, A.E.; Alsalme, A.M.; Bond, A.M.; Bhargava, S. Preferential synthesis of highly conducting Tl(TCNQ) phase II nanorod networks via electrochemically driven TCNQ/Tl(TCNQ) solid-solid phase transformation. J. Solid State Electrochem. 2016, 20, 3303–3314. [Google Scholar] [CrossRef]
- Vickers, E.B.; Giles, I.D.; Miller, J.S. M[TCNQ]y-Based Magnets (M = Mn, Fe, Co, Ni; TCNQ = 7,7,8,8-tetracyano-p-quinodimethane). Chem. Mater. 2005, 17, 1667–1672. [Google Scholar] [CrossRef]
- Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Seiler, P.; Gross, M.; Diederich, F. A novel reaction of 7,7,8,8-tetracyanoquinodimethane (TCNQ): Charge-transfer chromophores by [2 + 2] cycloaddition with alkynes. Chem. Commun. 2007, 45, 4731–4733. [Google Scholar] [CrossRef]
- Kosaka, W.; Liu, Z.; Miyasaka, H. Layered ferrimagnets constructed from charge-transferred paddlewheel [Ru2] units and TCNQ derivatives: The importance of interlayer translational distance in determining magnetic ground state. Dalton Trans. 2018, 47, 11760–11768. [Google Scholar] [CrossRef]
- La, D.D.; Ramanathan, R.; Rananaware, A.; Bansal, V.; Bhosale, S.V. Nanostructured charge transfer complex of CuTCNQF4 for efficient photo-removal of hexavalent chromium. RSC Adv. 2016, 6, 33931–33936. [Google Scholar] [CrossRef]
- Hoshyargar, F.; Shafiei, M.; Piloto, C.; Motta, N.; O’Mullane, A.P. Investigation of the room temperature gas sensing properties of metal–organic charge transfer complex CuTCNQF4. J. Mater. Chem. C 2016, 4, 11173–11179. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, L.; Fan, D.; Lv, X.; Li, Y.; Yan, Z. Synthesis of boron-doped g-C3N4 with enhanced electro-catalytic activity and stability. Chem. Phys. Lett. 2017, 672, 26–30. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, W.; Lv, Y.; Bai, X.; Liu, Y.; Jiang, W.; Zhu, Y. Enhancement of mineralization ability of C3N4 via a lower valence position by a tetracyanoquinodimethane organic semiconductor. J. Mater. Chem. A 2014, 2, 11432–11438. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, W.; Guo, R.; Huang, C.; Pan, W.; Liu, P. An exploration on in-situ synthesis of europium doped g-C3N4 for photocatalytic water splitting. Energy Procedia 2019, 158, 1553–1558. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Tang, Y.; Müllen, K.; Feng, X. Titania nanosheet-mediated construction of a two-dimensional titania/cadmium sulfide heterostructure for high hydrogen evolution activity. Adv. Mater. 2014, 26, 734–738. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J. Hollow-Structured Mesoporous Materials: Chemical Synthesis, Functionalization and Applications. Adv. Mater. 2014, 26, 3176–3205. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lei, W.; Pan, X.; Lu, B.; Ye, Z. A nine-fold enhancement of visible-light photocatalytic hydrogen production of g-C3N4 with TCNQ by forming a conjugated structure. RSC Adv. 2020, 10, 20110–20117. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Tian, L.; She, C.; Jiang, F.; Zheng, H.; Li, W.; Wu, G.; Long, D.; Li, Q. Electrochemical Behavior of Europium(III)-Europium(II) in LiF-NaF-KF Molten Salt. Electrochimica Acta 2014, 147, 114–120. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tian, Q.; Zhang, Y.; Li, Y.; Yang, X.; Zheng, H.; Chen, L.; Li, F. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water Res. 2021, 210, 117985. [Google Scholar] [CrossRef] [PubMed]
- Thanh Truc, N.T.; Pham, T.-D.; Van Thuan, D.; Son, L.T.; Tran, D.T.; Nguyen, M.V.; Nguyen, V.N.; Dang, N.M.; Trang, H.T. Superior activity of Cu-NiWO4/g-C3N4 Z direct system for photocatalytic decomposition of VOCs in aerosol under visible light. J. Alloys Compd. 2019, 798, 12–18. [Google Scholar] [CrossRef]
- Geng, K.; Wu, Y.; Jiang, G.; Liu, K.; Jiang, L. RuC@g-C3N4(H+)/TiO2 visible active photocatalyst: Facile fabrication and Z-scheme carrier transfer mechanism. Mol. Catal. 2018, 458, 33–42. [Google Scholar] [CrossRef]



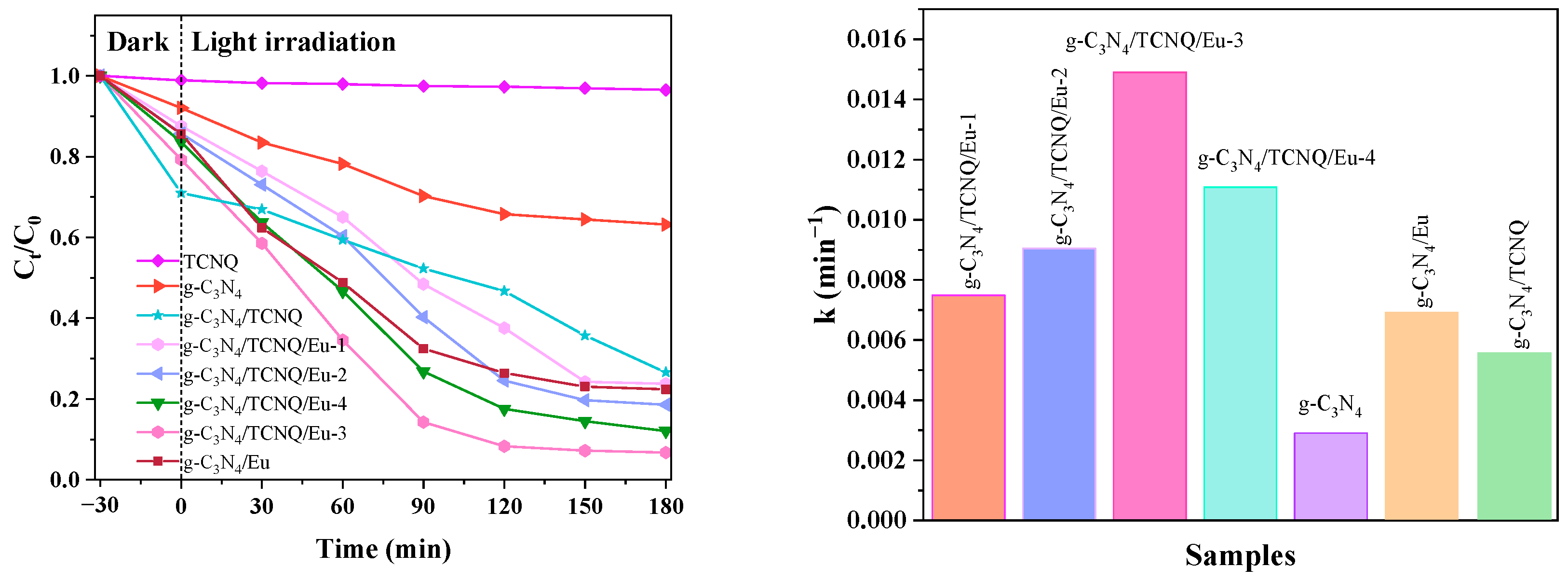
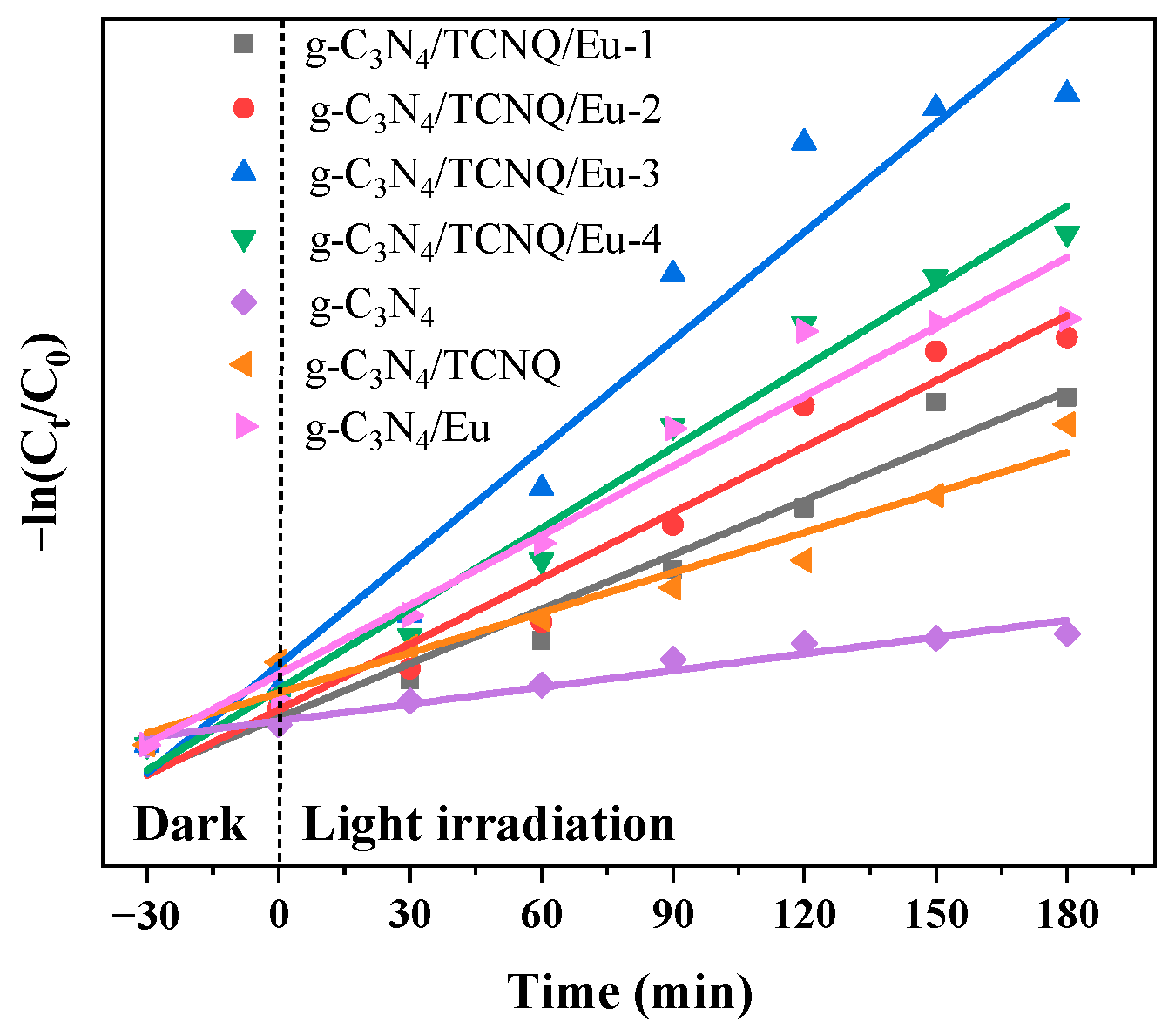


| Sample Name | Regression Equation | k | R2 |
|---|---|---|---|
| g-C3N4 | y = 0.0029x + 0.0885 | 0.0029 | 0.9598 |
| g-C3N4/TCNQ/Eu-1 | y = 0.0075x + 0.1109 | 0.0075 | 0.9668 |
| g-C3N4/TCNQ/Eu-2 | y = 0.0091x + 0.1455 | 0.0091 | 0.9641 |
| g-C3N4/TCNQ/Eu-3 | y = 0.0149x + 0.3297 | 0.0149 | 0.9513 |
| g-C3N4/TCNQ/Eu-4 | y = 0.0111x + 0.2290 | 0.0111 | 0.9796 |
| g-C3N4/Eu | y = 0.0096x + 0.2290 | 0.0096 | 0.9518 |
| g-C3N4/TCNQ | y = 0.0055x + 0.2143 | 0.0055 | 0.9578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Geng, J.; Shen, J.; Shi, Q.; Lv, J.; Lv, Y.; Song, C. Synthesis and Catalytic Degradation of PEF, ENR, and CIP by g-C3N4/TCNQ/Eu Composite. Micromachines 2023, 14, 2146. https://doi.org/10.3390/mi14122146
Chen H, Geng J, Shen J, Shi Q, Lv J, Lv Y, Song C. Synthesis and Catalytic Degradation of PEF, ENR, and CIP by g-C3N4/TCNQ/Eu Composite. Micromachines. 2023; 14(12):2146. https://doi.org/10.3390/mi14122146
Chicago/Turabian StyleChen, Hongyue, Jianxin Geng, Jinghui Shen, Qi Shi, Jingxue Lv, Yuguang Lv, and Chaoyu Song. 2023. "Synthesis and Catalytic Degradation of PEF, ENR, and CIP by g-C3N4/TCNQ/Eu Composite" Micromachines 14, no. 12: 2146. https://doi.org/10.3390/mi14122146
APA StyleChen, H., Geng, J., Shen, J., Shi, Q., Lv, J., Lv, Y., & Song, C. (2023). Synthesis and Catalytic Degradation of PEF, ENR, and CIP by g-C3N4/TCNQ/Eu Composite. Micromachines, 14(12), 2146. https://doi.org/10.3390/mi14122146





