Synthesis of Imidazole-Compound-Coated Copper Nanoparticles with Promising Antioxidant and Sintering Properties
Abstract
1. Introduction
2. Materials and Methods
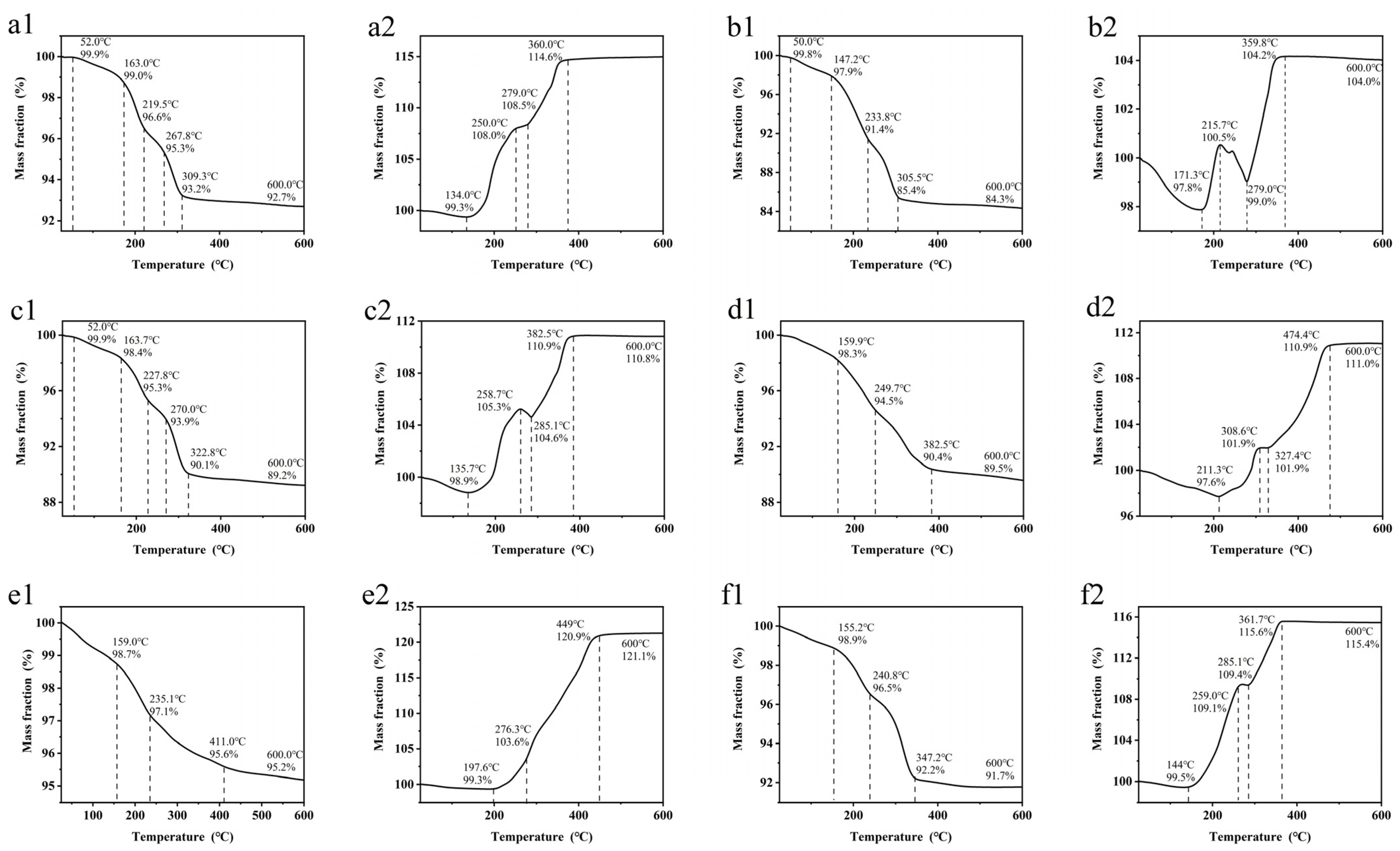
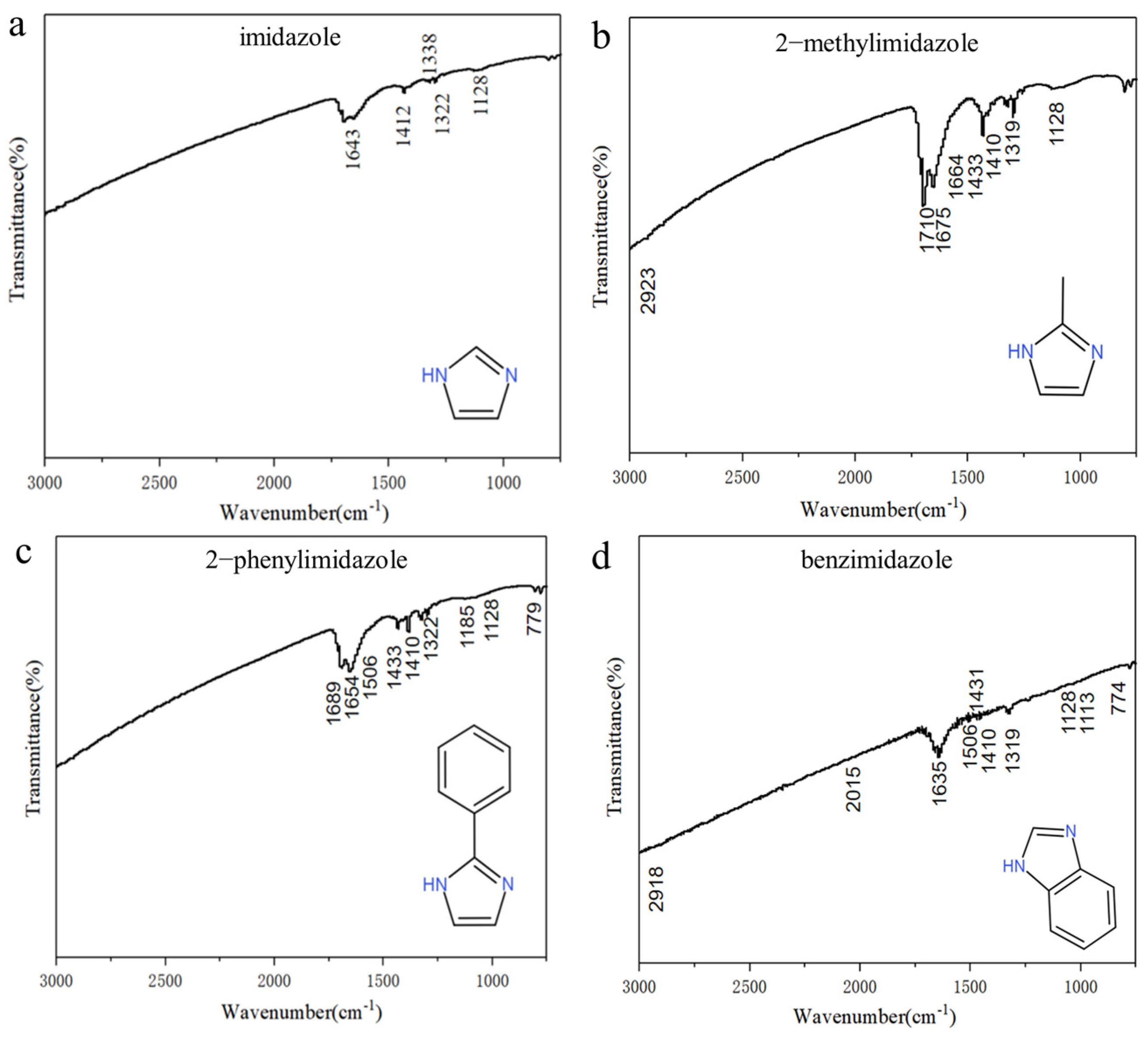
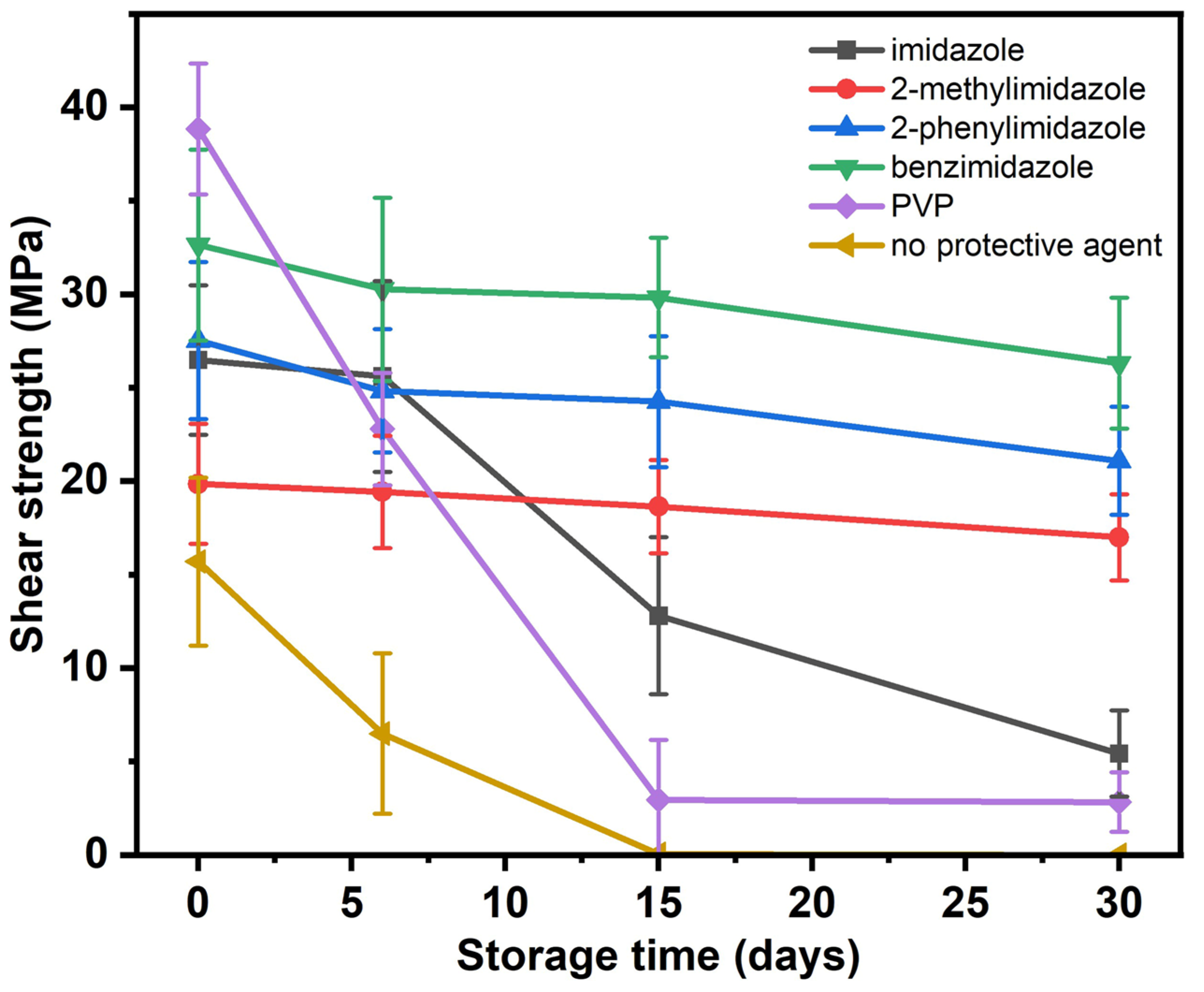
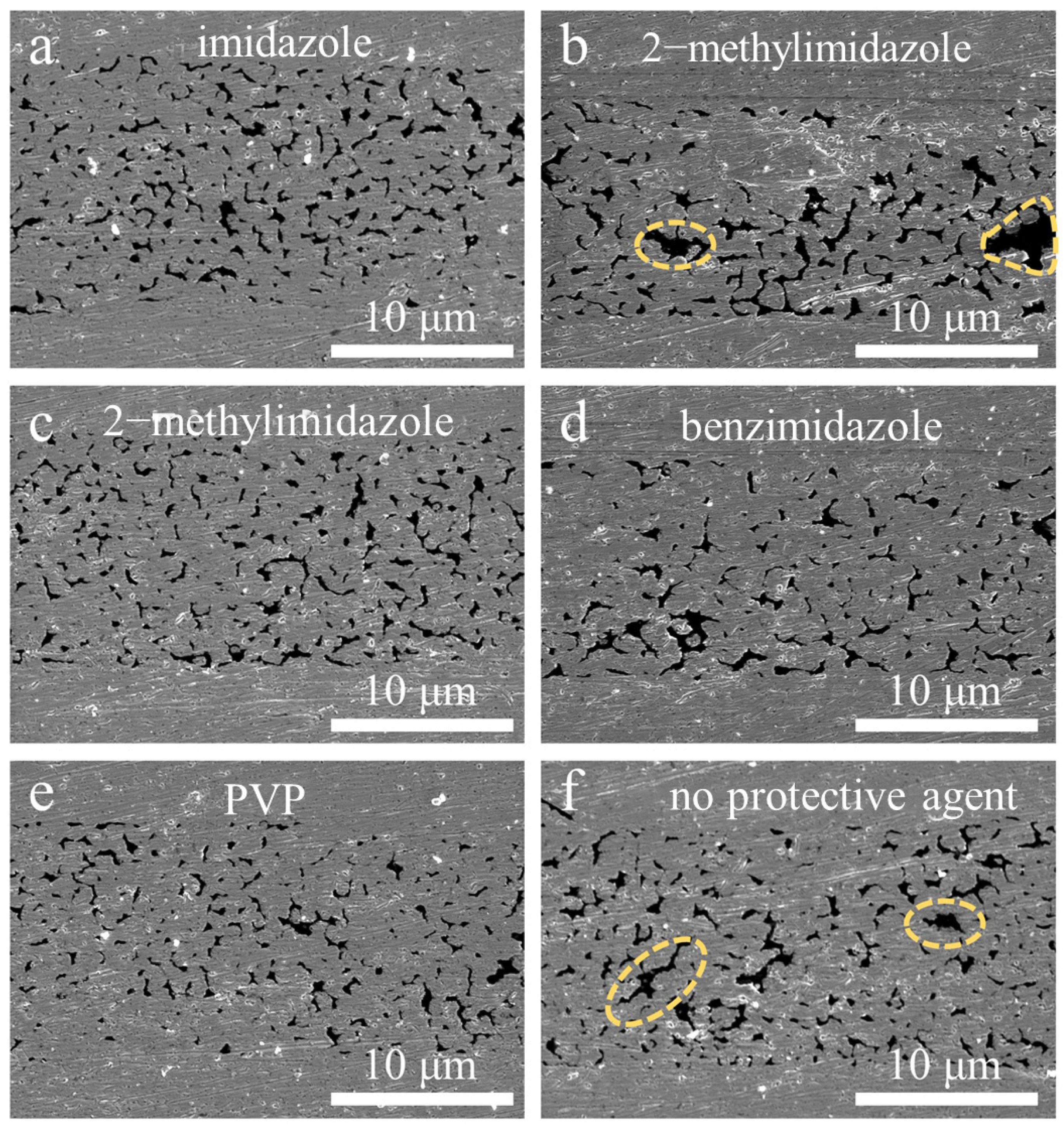
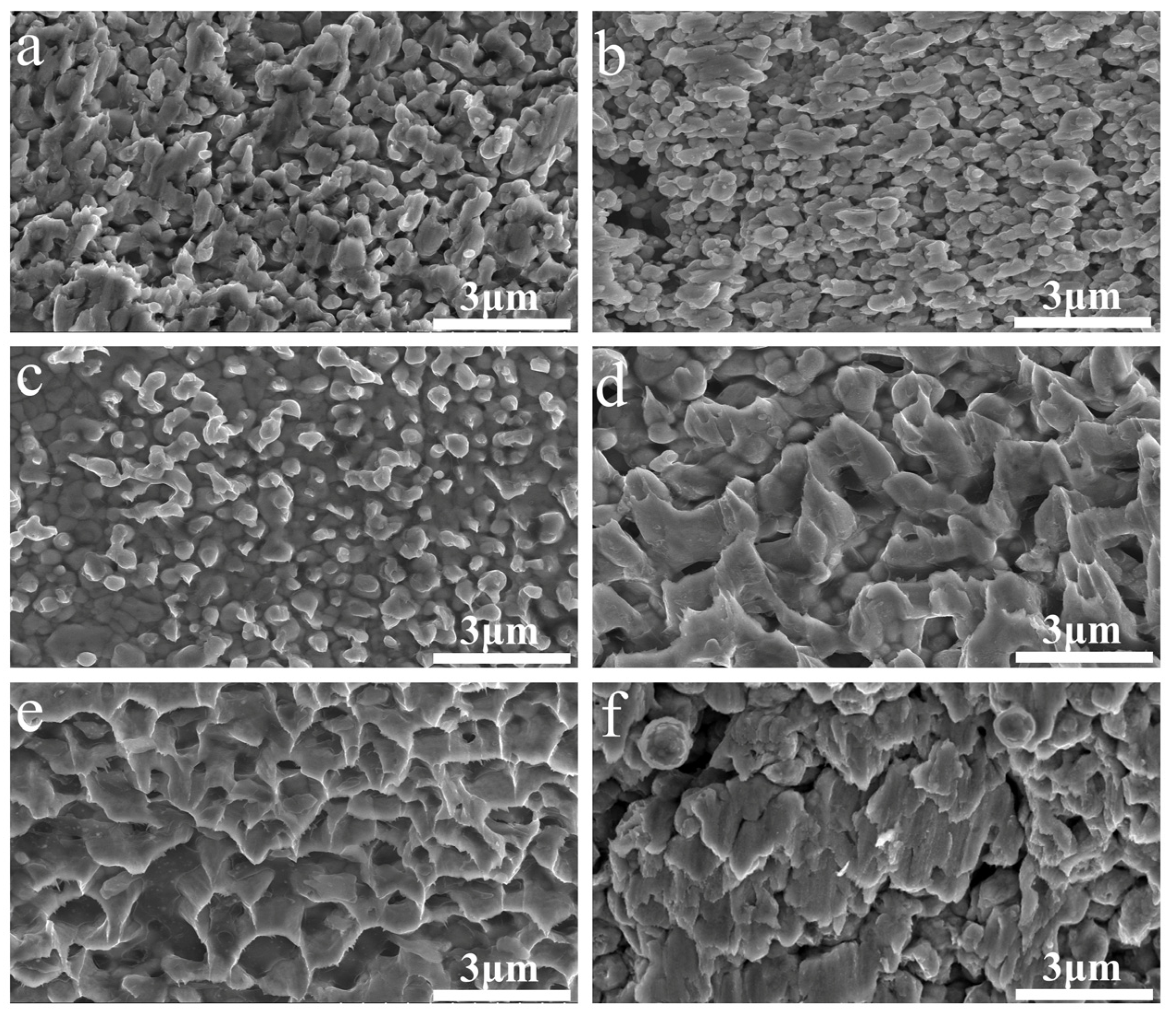
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A green method for the synthesis of Copper Nanoparticles using L-ascorbic acid. Mater. Rio Jan. 2014, 19, 197–203. [Google Scholar] [CrossRef]
- Yang, G.; Lai, H.; Lin, W.; Tong, J.; Peng, Z.; Cao, J.; Luo, J.; Zhang, Y.; Cui, C. Approaching the structure-property relationship of sintered metal nano/microparticles from the perspective of the agglomerate size effect. Powder Technol. 2022, 399, 117254. [Google Scholar] [CrossRef]
- Barai, K.; Tiwary, C.S.; Chattopadhyay, P.P.; Chattopadhyay, K. Synthesis of free standing nanocrystalline Cu by ball milling at cryogenic temperature. Mater. Sci. Eng. A 2012, 558, 52–58. [Google Scholar] [CrossRef]
- Yang, G.; Lai, H.; Lin, W.; Tong, J.; Cao, J.; Luo, J.; Zhang, Y.; Cui, C. A quantitative model to understand the microflow-controlled sintering mechanism of metal particles at nanometer to micron scale. Nanotechnology 2021, 32, 505721. [Google Scholar] [CrossRef]
- Liu, J.D.; Chen, H.T.; Ji, H.J.; Li, M.Y. Highly Conductive Cu-Cu Joint Formation by Low-Temperature Sintering of Formic Acid-Treated Cu Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 33289–33298. [Google Scholar] [CrossRef]
- Yamakawa, T.; Takemoto, T.; Shimoda, M.; Nishikawa, H.; Shiokawa, K.; Terada, N. Influence of Joining Conditions on Bonding Strength of Joints: Efficacy of Low-Temperature Bonding Using Cu Nanoparticle Paste. J. Electron. Mater. 2013, 42, 1260–1267. [Google Scholar] [CrossRef]
- Seong, M.-R.; Lee, G.-Y.; Kim, D.-K.; Kim, Y.-S.; Lee, C.S. Octanethiol coating of nano-sized copper powders using the vapor self-assembled monolayer method. Met. Mater. Int. 2009, 15, 963–966. [Google Scholar] [CrossRef]
- Musa, A.; Ahmad, M.B.; Hussein, M.Z.; Saiman, M.I.; Sani, H.A. Preparation, characterization and catalytic activity of biomaterial-supported copper nanoparticles. Res. Chem. Intermed. 2017, 43, 801–815. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, P.; Yan, H.; Chen, M. Systematically investigating solar absorption performance of plasmonic nanoparticles. Energy 2021, 216, 119254. [Google Scholar] [CrossRef]
- Ben Aissa, M.A.; Tremblay, B.; Andrieux-Ledier, A.; Maisonhaute, E.; Raouafi, N.; Courty, A. Copper nanoparticles of well-controlled size and shape: A new advance in synthesis and self-organization. Nanoscale 2015, 7, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Sampath, M.; Vijayan, R.; Ezhilarasu, T.; Tamilselvan, A.; Balasubramanian, S. Green Synthesis of Novel Jasmine Bud-Shaped Copper Nanoparticles. J. Nanotechnol. 2014, 2014, 626523. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Magdassi, S. Formation of air-stable copper-silver core-shell nanoparticles for inkjet printing. J. Mater. Chem. 2009, 19, 3057–3062. [Google Scholar] [CrossRef]
- Yang, G.; Lin, W.; Lai, H.; Zhong, C.; Zhang, Y.; Cui, C. Improved understanding of the enhancement of sintering of mixtures of Cu microparticles and Sn nanoparticles for electronic packaging. J. Mater. Sci-Mater. Electron. 2022, 33, 11467–11474. [Google Scholar] [CrossRef]
- Dou, Q.Q.; Li, Y.; Wong, K.W.; Ng, K.M. Facile synthesis of nearly monodisperse AgCu alloy nanoparticles with synergistic effect against oxidation and electromigration. J. Mater. Res. 2019, 34, 2095–2104. [Google Scholar] [CrossRef]
- Iflah, Y.; Zilbermann, I.; Pevzner, S.; Halevy, S.; Bochlin, Y.; Kadosh, Y.; Kaplan, A.; Korin, E.; Bettelheim, A. Growth Behavior of Copper and Platinum Nanoparticles in an Imidazolium Based Ionic Liquid. J. Electrochem. Soc. 2017, 164, H5026–H5030. [Google Scholar] [CrossRef][Green Version]
- Dang, T.; Le, T.; Fribourg-Blanc, E.; Dang, C. The influence of solvents and surfactants on the preparation of copper nanoparticles by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 025004. [Google Scholar] [CrossRef]
- Li, Y.J.; Tang, X.F.; Zhang, Y.T.; Li, J.; Lv, C.B.; Meng, X.L.; Huang, Y.L.; Hang, C.J.; Wang, C.Q. Cu nanoparticles of low polydispersity synthesized by a double-template method and their stability. Colloid. Polym. Sci. 2013, 292, 715–722. [Google Scholar] [CrossRef]
- Kanninen, P.; Johans, C.; Merta, J.; Kontturi, K. Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid. Interface Sci. 2008, 318, 88–95. [Google Scholar] [CrossRef]
- Yang, G.; Zou, Q.; Wang, P.; Lai, H.; Lai, T.; Zeng, X.; Li, Z.; Luo, J.; Zhang, Y.; Cui, C. Towards understanding the facile synthesis of well-covered Cu-Ag core-shell nanoparticles from a complexing model. J. Alloys Compd. 2021, 874, 159900. [Google Scholar] [CrossRef]
- Yang, G.; Wang, P.; Liu, Y.; Lu, S.; Luo, B.; Lai, T.; Ta, S.; Lin, T.; Luo, J.; Zhang, Y.; et al. Effect of Ag coating on the oxidation resistance, sintering properties, and migration resistance of Cu particles. J. Alloys Compd. 2022, 923, 166271. [Google Scholar] [CrossRef]
- Zuo, Y.; Shen, J.; Hu, Y.; Gao, R. Improvement of oxidation resistance and bonding strength of Cu nanoparticles solder joints of Cu-Cu bonding by phosphating the nanoparticle. J. Mater. Process Technol. 2018, 253, 27–33. [Google Scholar] [CrossRef]
- Dai, X.; Xu, W.; Zhang, T.; Shi, H.; Wang, T. Room temperature sintering of Cu-Ag core-shell nanoparticles conductive inks for printed electronics. Chem. Eng. J. 2019, 364, 310–319. [Google Scholar] [CrossRef]
- Aslam, M.; Gopakumar, G.; Shoba, T.L.; Mulla, I.S.; Vijayamohanan, K.; Kulkarni, S.K.; Urban, J.; Vogel, W. Formation of Cu and Cu2O Nanoparticles by Variation of the Surface Ligand: Preparation, Structure, and Insulating-to-Metallic Transition. J. Colloid. Interface Sci. 2002, 255, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-t.; Zhang, C.-y.; Yin, Y.-S. Rapid synthesis of copper nanoparticles by sodium hypophosphite reduction in ethylene glycol under microwave irradiation. J. Cryst. Growth 2004, 270, 722–728. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, W.; Xia, Z.; Wang, X.; Wang, Y.; Yue, Z.; Guo, F. Investigation of ethylene glycol, α-terpineol, and polyethylene glycol 400 on the sintering properties of Cu–Ag core–shell micro/nano-mixed paste. J. Mater. Sci. Mater. Electron. 2023, 34, 1585. [Google Scholar] [CrossRef]
- Dai, D.; Li, J.; Qian, J.; Wang, Z.; Zheng, K.; Yu, J.; Chen, X. The formation of Cu-Cu joints by low temperature sintering Cu NPs with copper formate layer and its oxidation enhancement. Mater. Lett. 2023, 339, 134087. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Sekiguchi, T.; Suganuma, K. Large-scale bare Cu bonding by 10 μm-sized Cu–Ag composite paste in low temperature low pressure air conditions. J. Sci. Adv. Mater. Devices 2023, 8, 100606. [Google Scholar] [CrossRef]
- Arai, S.; Nakajima, S.; Shimizu, M.; Horita, M.; Aizawa, M.; Kiyoshi, O. Direct Cu-Cu bonding by low-temperature sintering using three-dimensional nanostructured plated Cu films. Mater. Today Commun. 2023, 35, 105790. [Google Scholar] [CrossRef]
- Carano, M. The evolution of organic solderability preservatives (OSPs) from a temporary protectant to a leadership position in surface finishing chemistry. Circuit World 2011, 37, 12–19. [Google Scholar] [CrossRef]
- Jeong, H.; Min, K.D.; Lee, C.J.; Kim, J.H.; Jung, S.B. Mechanical reliability of Cu cored solder ball in flip chip package under thermal shock test. Microelectron. Reliab. 2020, 112, 105790. [Google Scholar] [CrossRef]
- Baek, S.; Jeong, G.W.; Son, J.H.; Kim, M.S.; Lee, H.B.R.; Kim, J.; Ko, Y.H. Interfacial reactions and mechanical properties of transient liquid-phase bonding joints in Cu/Sn/Ni(P) and Ni/Sn/(OSP)Cu structures for power modules. J. Mater. Sci. Mater. Electron. 2021, 32, 3324–3333. [Google Scholar] [CrossRef]
- Araujo Martinez, A.; Landee, C.P.; Dickie, D.A.; Wikaira, J.L.; Xiao, F.; Turnbull, M.M. Pyridyl-imidazole copper compounds. J. Coord. Chem. 2023, 76, 232–257. [Google Scholar] [CrossRef]
- Hardian, R.; Pogany, P.; Lee, Y.M.; Szekely, G. Molecular sieving using metal–polymer coordination membranes in organic media. J. Mater. Chem. A 2021, 9, 14400–14410. [Google Scholar] [CrossRef]
- Balela, M.D.; Amores, K.L. Formation of Oxidation-Stable Copper Nanoparticles in Water. Adv. Mater. Res. 2015, 1131, 255–259. [Google Scholar] [CrossRef]
- Yan, J.F.; Zou, G.S.; Hu, A.M.; Zhou, Y.N. Preparation of PVP coated Cu NPs and the application for low-temperature bonding. J. Mater. Chem. 2011, 21, 15981–15986. [Google Scholar]
- Li, J.; Yu, X.; Shi, T.; Cheng, C.; Fan, J.; Cheng, S.; Liao, G.; Tang, Z. Low-Temperature and Low-Pressure Cu-Cu Bonding by Highly Sinterable Cu Nanoparticle Paste. Nanoscale Res. Lett. 2017, 12, 255. [Google Scholar] [CrossRef]
- Yan, J.F.; Zou, G.S.; Zhang, Y.C.; Li, J.X.; Liu, L.; Wu, A.P.; Zhou, Y.N. Metal-Metal Bonding Process Using Cu + Ag Mixed Nanoparticles. Mater. Trans. 2013, 54, 879–883. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Y.B.; Liu, Z.Q.; Liu, Y.; Sun, R. Low pressure Cu-Cu bonding using MOD ink-modified Cu particle paste for die-attachment of power semiconductors. J. Mater. Sci. Mater. Electron. 2022, 33, 3576–3585. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Wu, H.Y.; Li, J.J.; Zhu, P.L.; Sun, R. Cu-Cu joint formation by low-temperature sintering of self-reducible Cu nanoparticle paste under ambient condition. Appl. Surf. Sci. 2021, 570, 7. [Google Scholar] [CrossRef]
- Li, J.J.; Yu, X.; Shi, T.L.; Cheng, C.L.; Fan, J.H.; Cheng, S.Y.; Li, T.X.; Liao, G.L.; Tang, Z.R. Depressing of Cu-Cu bonding temperature by composting Cu nanoparticle paste with Ag nanoparticles. J. Alloys Compd. 2017, 709, 700–707. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shirochi, T.; Yasuda, Y.; Morita, T. Metal-metal bonding process using metallic copper nanoparticles prepared in aqueous solution. Int. J. Adhes. Adhes. 2012, 33, 50–55. [Google Scholar] [CrossRef]
- Mou, Y.; Liu, J.X.; Cheng, H.; Peng, Y.; Chen, M.X. Facile Preparation of Self-Reducible Cu Nanoparticle Paste for Low Temperature Cu-Cu Bonding. JOM 2019, 71, 3076–3083. [Google Scholar] [CrossRef]
- Gao, Y.; Li, W.L.; Zhang, H.; Jiu, J.T.; Hu, D.W.; Suganuma, K. Size-Controllable Synthesis of Bimodal Cu Particles by Polyol Method and Their Application in Die Bonding for Power Devices. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 2190–2197. [Google Scholar] [CrossRef]
- Chang, S.J.; Tung, C.A.; Chen, B.W.; Chou, Y.C.; Li, C.C. Synthesis of non-oxidative copper nanoparticles. RSC Adv. 2013, 3, 24005–24008. [Google Scholar] [CrossRef]
- Bae, D.S.; Kim, E.J.; Bang, J.H.; Kim, S.W.; Han, K.S.; Lee, J.K.; Kim, B.I.; Adair, J.H. Synthesis and characterization of silver nanoparticles by a reverse micelle process. Met. Mater. Int. 2005, 11, 291–294. [Google Scholar] [CrossRef]


| Protective Agent | Resistivity (μΩ·cm) |
|---|---|
| imidazole | 14.7 |
| 2-methylimidazole | 10.63 |
| 2-phenylimidazole | 5.69 |
| benzimidazole | 4.86 |
| PVP | 15.65 |
| without protective agent | 30.15 |
| Diameter (nm) | Organic Additive | Sinter Condition (Atmosphere, Pressure, Temperature) | Shear Strength (MPa) | Ref. |
|---|---|---|---|---|
| 20–110 | PVP | air, 5 MPa, 220 °C | 13.5 | [35] |
| 40–80 | PVP | Ar-H2, 1.08 MPa, 300 °C | 31.88 | [36] |
| 30–270 | alkylamine | air, 0 MPa, 350 °C | 26 | [6] |
| 30 | PVP | mixed Ar-H2, 10 MPa, 320 °C | 31.2 | [5] |
| 50–200 | PVP | air, 5 MPa, 250 °C | 20 | [37] |
| 100 | octylamine | N2, 0.4 MPa, 300 °C | 17.1 | [38] |
| 60–160 | 1-amino-2-propanol | air, 10 MPa, 250 °C | 52.01 | [39] |
| 30–80 | PVP | mixed Ar-H2, 1.12 MPa, 250 °C | 25.41 | [40] |
| 54–64 | CTAB | H2, 1.2 MPa, 400 °C | 37.7 | [41] |
| 3.5–9.5 | isopropanolamine | Ar, 36.2 MPa 250 °C, | 36.2 | [42] |
| 80–200 | PVP | air, 0.4 MPa, 300 °C | 20 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yu, X.; Chen, Z.; Wu, S.; Lai, H.; Ta, S.; Lin, T.; Yang, G.; Cui, C. Synthesis of Imidazole-Compound-Coated Copper Nanoparticles with Promising Antioxidant and Sintering Properties. Micromachines 2023, 14, 2079. https://doi.org/10.3390/mi14112079
Zhang Y, Yu X, Chen Z, Wu S, Lai H, Ta S, Lin T, Yang G, Cui C. Synthesis of Imidazole-Compound-Coated Copper Nanoparticles with Promising Antioxidant and Sintering Properties. Micromachines. 2023; 14(11):2079. https://doi.org/10.3390/mi14112079
Chicago/Turabian StyleZhang, Yu, Xianchong Yu, Ziyuan Chen, Song Wu, Haiqi Lai, Shiwo Ta, Tingyu Lin, Guannan Yang, and Chengqiang Cui. 2023. "Synthesis of Imidazole-Compound-Coated Copper Nanoparticles with Promising Antioxidant and Sintering Properties" Micromachines 14, no. 11: 2079. https://doi.org/10.3390/mi14112079
APA StyleZhang, Y., Yu, X., Chen, Z., Wu, S., Lai, H., Ta, S., Lin, T., Yang, G., & Cui, C. (2023). Synthesis of Imidazole-Compound-Coated Copper Nanoparticles with Promising Antioxidant and Sintering Properties. Micromachines, 14(11), 2079. https://doi.org/10.3390/mi14112079






