Experimental Investigation of Electrochemical Capacitive Responses versus Pore Geometries through Artificial Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
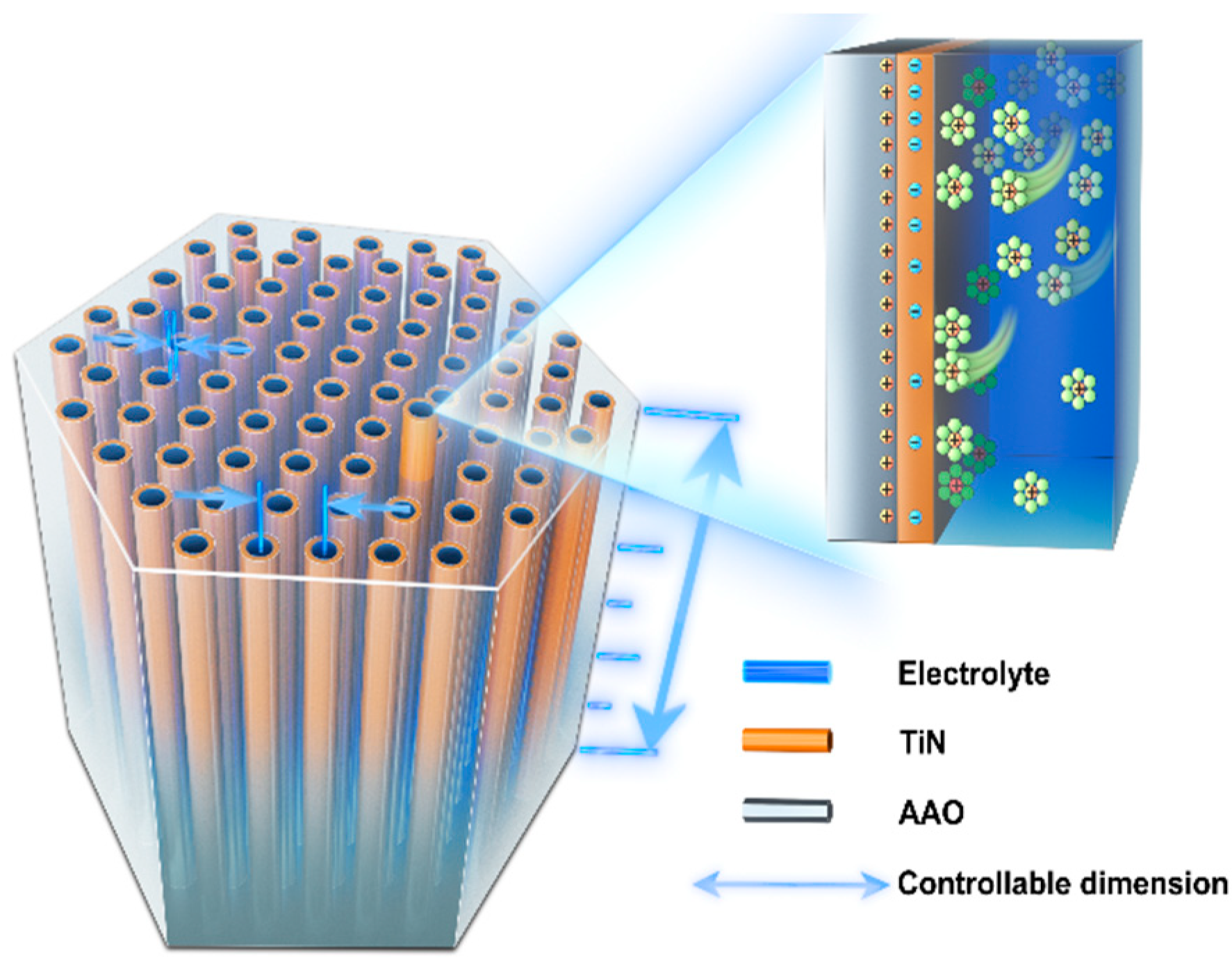
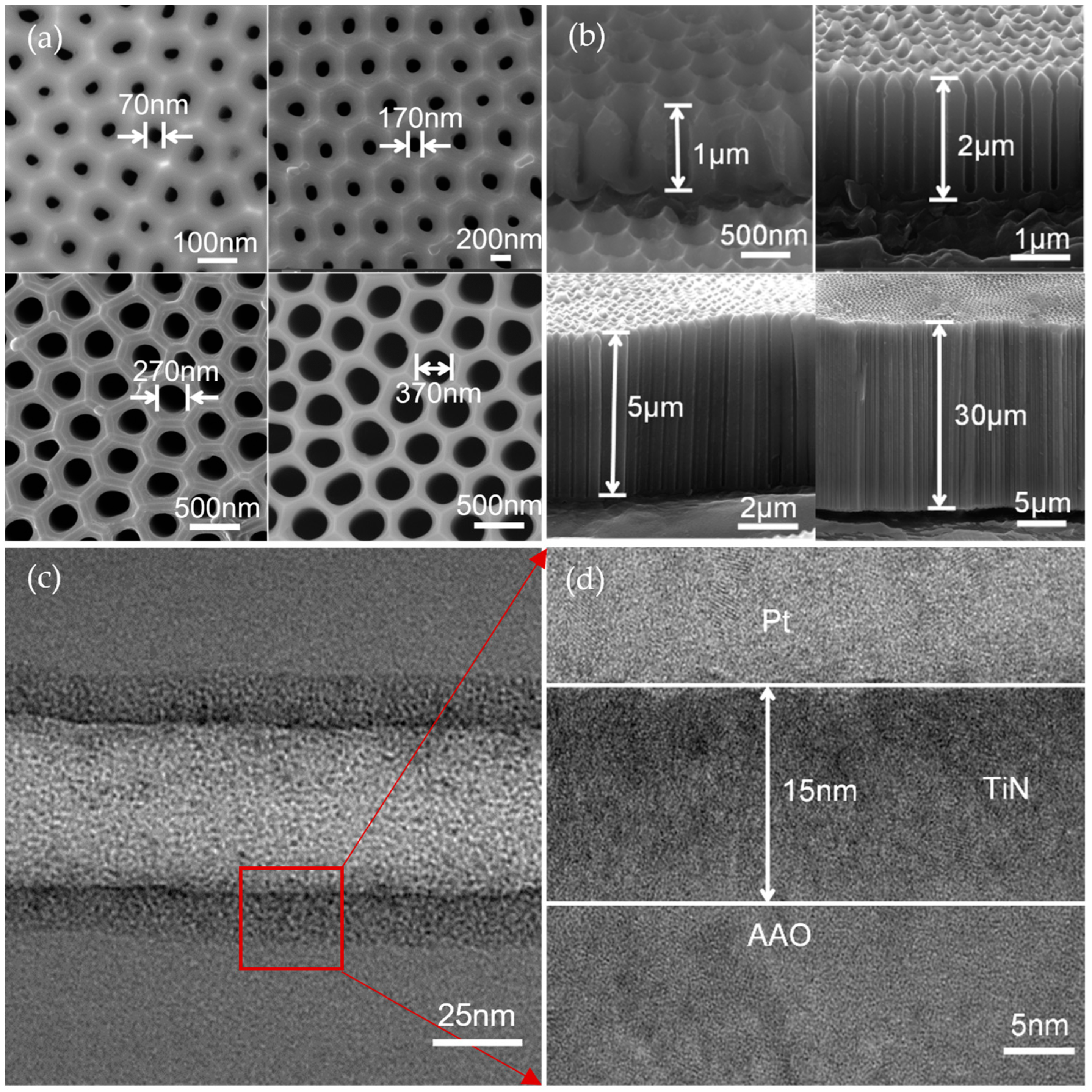
2.1. Preparation of TiN/AAO Porous Array Electrodes
2.2. Characterization Method of the TiN/AAO Electrode
2.3. Characterization of the TiN/AAO Porous Array Electrode
3. Results and Discussion
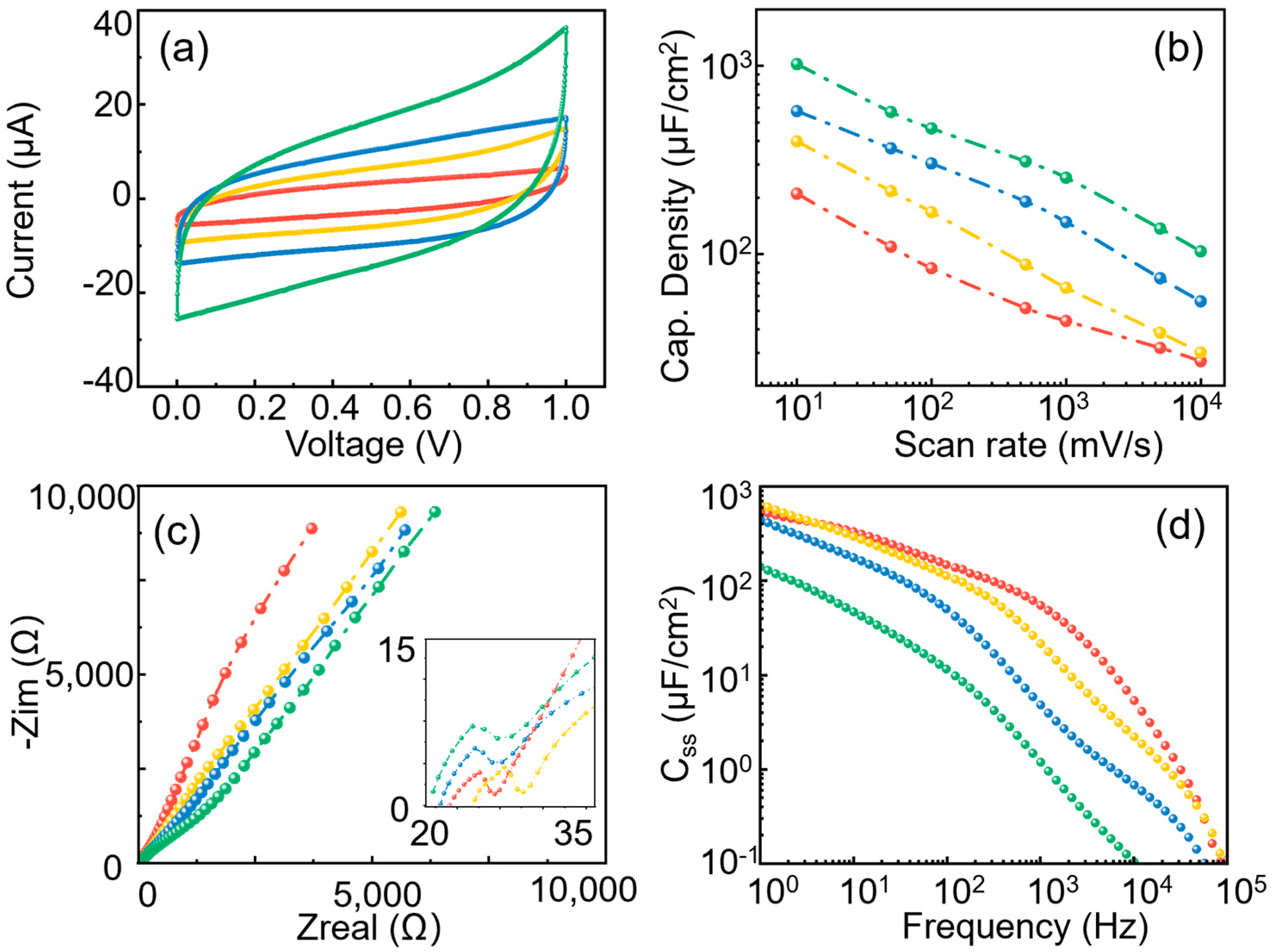
3.1. Effect of Pore Depth
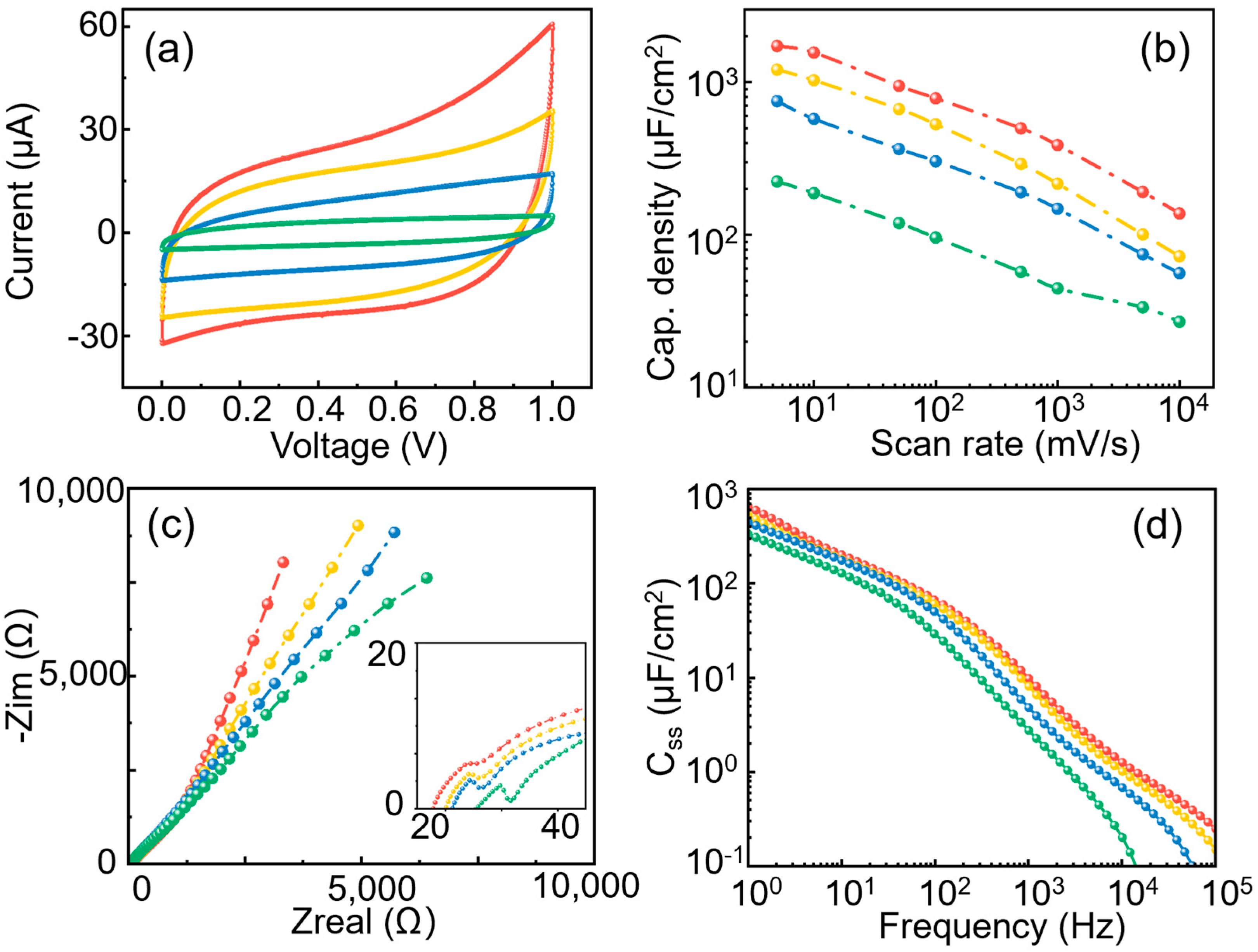
3.2. Effect of Pore Diameter
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saini, P. A Historical Review of Electrode Materials and Electrolytes for Electrochemical Double Layer Supercapacitors and Pseudocapacitors. Indian J. Pure Appl. Phys. 2023, 61, 268–290. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xu, S.; Xie, Y.; Sanghadasa, M.; Wang, X.; Lin, L. A Review of On-Chip Micro Supercapacitors for Integrated Self-Powering Systems. J. Microelectromech. Syst. 2017, 26, 949–965. [Google Scholar] [CrossRef]
- Huang, J.; Gao, Y.; Luo, J.; Wang, S.; Li, C.; Chen, S.; Zhang, J. Editors’ Choice-Review-Impedance Response of Porous Electrodes: Theoretical Framework, Physical Models and Applications. J. Electrochem. Soc. 2020, 167, 467. [Google Scholar] [CrossRef]
- Musiani, M.; Orazem, M.; Tribollet, B.; Vivier, V. Impedance of blocking electrodes having parallel cylindrical pores with distributed radii. Electrochim. Acta 2011, 56, 8014–8022. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, X.; Xie, Y.; Wang, Z.; Huang, J.; Qu, Y.; Mu, D.; Zhang, X.; Yang, W.; Zhang, H. Additive Engineering Enables Ionic-Liquid Electrolyte-Based Supercapacitors To Deliver Simultaneously High Energy and Power Density. Acs Sustain. Chem. Eng. 2023, 11, 5685–5695. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Huang, H.; Chu, X.; Xie, Y.; Xiong, D.; Yan, C.; Zhao, H.; Zhang, H.; Yang, W. Unraveling and Regulating Self-Discharge Behavior of Ti3C2Tx MXene-Based Supercapacitors. Acs Nano 2020, 14, 4916–4924. [Google Scholar] [CrossRef]
- Pu, S.; Wang, Z.; Xie, Y.; Fan, J.; Xu, Z.; Wang, Y.; He, H.; Zhang, X.; Yang, W.; Zhang, H. Origin and Regulation of Self-Discharge in MXene Supercapacitors. Adv. Funct. Mater. 2023, 33, 2208715. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Lust, E. Optimized structure of nanoporous carbon-based double-layer capacitors. J. Electrochem. Soc. 2005, 152, E24–E33. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, C.; Fu, X.; Shen, M.; Li, K.; Liu, X.; Yao, H.-C.; Zhang, Y.; Yao, K.X. Synthesis of porous NiCoS nanosheets with Al leaching on ordered mesoporous carbon for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123367. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhang, P.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.; Deng, S. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem. Eng. J. 2019, 355, 309–319. [Google Scholar] [CrossRef]
- Xu, S.; Xia, F.; Wang, X.; IEEE. Beyond Electrolytic Capacitor: High Frequency On-chip Micro Supercapacitor with Large Capacitance Density. In Proceedings of the 65th IEEE Annual International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 9–11 December 2019. [Google Scholar]
- Mohammadi, A.V.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, 1165. [Google Scholar] [CrossRef]
- Brousse, K.; Martin, C.; Brisse, A.L.; Lethien, C.; Simon, P.; Taberna, P.L.; Brousse, T. Anthraquinone modification of microporous carbide derived carbon films for on-chip micro-supercapacitors applications. Electrochim. Acta 2017, 246, 391–398. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.; Zhao, X.; Chen, K.; Gu, B.; Yang, T.; Zhang, H.; Yang, W.; Chen, J. Tailoring carbon nanomaterials via a molecular scissor. Nano Today 2021, 36, 101033. [Google Scholar] [CrossRef]
- Song, H.K.; Jung, Y.H.; Lee, K.H.; Dao, L.H. Electrochemical impedance spectroscopy of porous electrodes: The effect of pore size distribution. Electrochim. Acta 1999, 44, 3513–3519. [Google Scholar] [CrossRef]
- Raistrick, I.D. Impedance studies of porous electrodes. Electrochim. Acta 1990, 35, 1579–1586. [Google Scholar] [CrossRef]
- Yuh, C.Y.; Selman, J.R. Abschatzung der Porenstruktur Poroser Elektroden aus Impedanzmessunchen. AIChE J. 1976, 21, 539. [Google Scholar]
- De Levie, R. On porous electrodes in electrolyte solutions: I. Capacitance effects. Electrochim. Acta 1963, 8, 751–780. [Google Scholar] [CrossRef]
- Murbach, M.D.; Schwartz, D.T. Extending Newman’s Pseudo-Two-Dimensional Lithium-Ion Battery Impedance Simulation Approach to Include the Nonlinear Harmonic Response. J. Electrochem. Soc. 2017, 164, E3311–E3320. [Google Scholar] [CrossRef]
- Huang, J. Generalization of Porous Electrode Theory for Noninteger Dimensional Space. J. Phys. Chem. C 2018, 122, 557–565. [Google Scholar] [CrossRef]
- Sharma, T.; Holm, T.; Diaz-Real, J.A.; Mérida, W. Experimental verification of pore impedance theory: Drilled graphite electrodes with gradually more complex pore size distribution. Electrochim. Acta 2019, 317, 528–541. [Google Scholar] [CrossRef]
- Han, F.; Qian, O.; Meng, G.; Lin, D.; Chen, G.; Zhang, S.; Pan, Q.; Zhang, X.; Zhu, X.; Wei, B. Structurally integrated 3D carbon tube grid-based high-performance filter capacitor. Science 2022, 377, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.H.; Choi, S.-H.; Lee, W. Curved Silicon Nanowires with Ribbon-like Cross Sections by Metal-Assisted Chemical Etching. Acs Nano 2011, 5, 5242–5248. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lin, Y.; Chen, J.; Lin, Q.; Wu, Y.; Su, W.; Wang, W.; Fan, Z. Three-dimensional nanotube electrode arrays for hierarchical tubular structured high-performance pseudocapacitors. Nanoscale 2016, 8, 13280–13287. [Google Scholar] [CrossRef]
- Grigoras, K.; Keskinen, J.; Gronberg, L.; Yli-Rantala, E.; Laakso, S.; Valimaki, H.; Kauranen, P.; Ahopelto, J.; Prunnila, M. Conformal titanium nitride in a porous silicon matrix: A nanomaterial for in-chip supercapacitors. Nano Energy 2016, 26, 340–345. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. Stabilized TiN Nanowire Arrays for High-Performance and Flexible Supercapacitors. Nano Lett. 2012, 12, 5376–5381. [Google Scholar] [CrossRef]
- Yang, P.; Chao, D.; Zhu, C.; Xia, X.; Zhang, Y.; Wang, X.; Sun, P.; Tay, B.K.; Shen, Z.X.; Mai, W.; et al. Ultrafast-Charging Supercapacitors Based on Corn-Like Titanium Nitride Nanostructures. Adv. Sci. 2016, 3, 1500299. [Google Scholar] [CrossRef]
- Bhosale, K.S.; Zope, A.A.; Pillai, G.; Li, S.-S. Tiny-Gap Titanium-Nitride-Composite MEMS Resonator Designs With High Power Handling Capability. J. Microelectromech. Syst. 2023, 32, 173–183. [Google Scholar] [CrossRef]
- Taberna, P.L.; Simon, P.; Fauvarque, J.F. Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J. Electrochem. Soc. 2003, 150, A292–A300. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef]
| Sample Parameter | Pore Diameter (nm) | Pore Depth (μm) |
|---|---|---|
| Varied pore depths (1M TEMABF4/AN) | 170 | 1/2/5/30 |
| Varied pore diameters (1M TEMABF4/AN) | 70/170/270/370 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Xu, M.; Li, Z.; Liu, S.; Wang, Y.; Shan, L.; Wang, X.; Xu, S. Experimental Investigation of Electrochemical Capacitive Responses versus Pore Geometries through Artificial Nanotubes. Micromachines 2023, 14, 1909. https://doi.org/10.3390/mi14101909
Dai J, Xu M, Li Z, Liu S, Wang Y, Shan L, Wang X, Xu S. Experimental Investigation of Electrochemical Capacitive Responses versus Pore Geometries through Artificial Nanotubes. Micromachines. 2023; 14(10):1909. https://doi.org/10.3390/mi14101909
Chicago/Turabian StyleDai, Jianyou, Minghao Xu, Zhangshanhao Li, Shuoxiang Liu, Yuyao Wang, Lei Shan, Xiaohong Wang, and Sixing Xu. 2023. "Experimental Investigation of Electrochemical Capacitive Responses versus Pore Geometries through Artificial Nanotubes" Micromachines 14, no. 10: 1909. https://doi.org/10.3390/mi14101909
APA StyleDai, J., Xu, M., Li, Z., Liu, S., Wang, Y., Shan, L., Wang, X., & Xu, S. (2023). Experimental Investigation of Electrochemical Capacitive Responses versus Pore Geometries through Artificial Nanotubes. Micromachines, 14(10), 1909. https://doi.org/10.3390/mi14101909





