Construction of Ag-TiO2 Hierarchical Micro-/Nanostructures on a Ti Plate for Photocatalysts via Femtosecond Laser Hybrid Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Process
2.3. Characterization
3. Results and Discussion
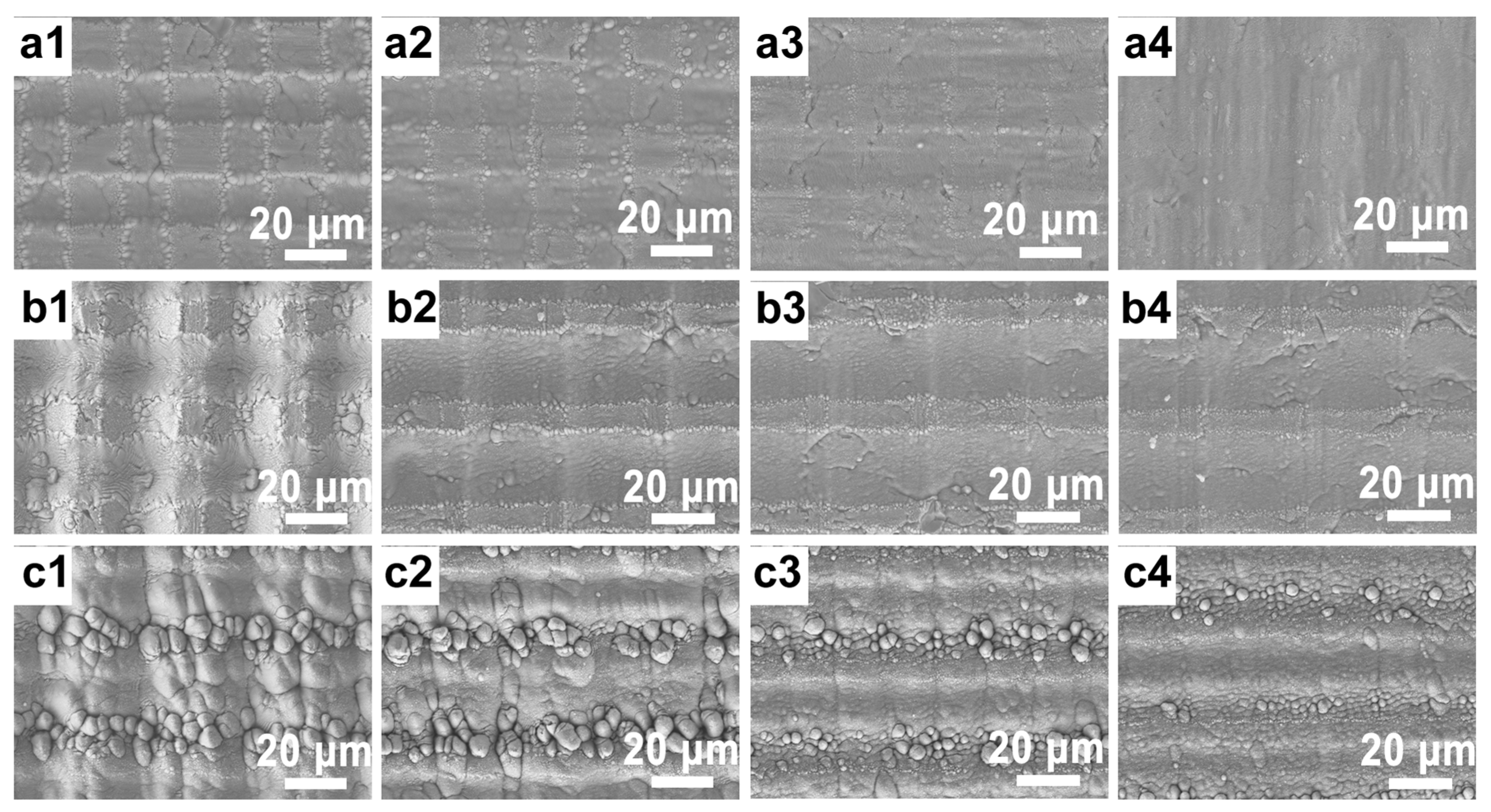
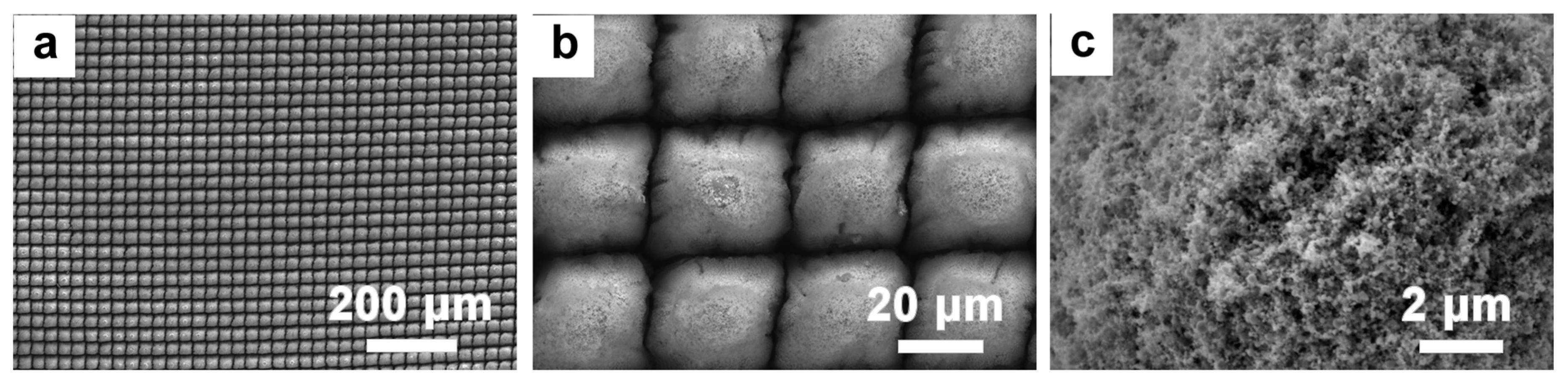
3.1. Effect of Femtosecond Laser Processing Parameters on Surface Morphology
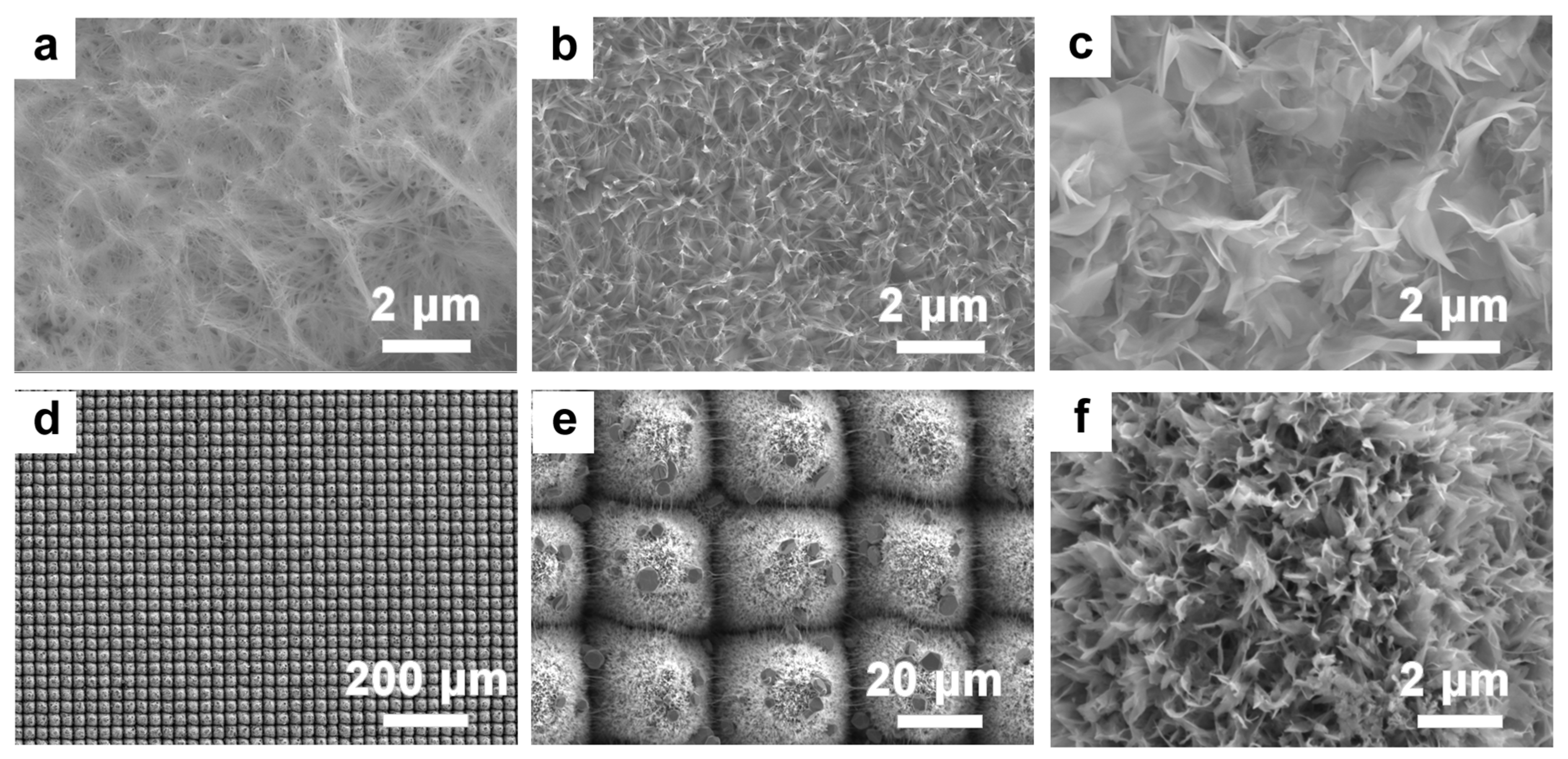
3.2. Preparation of TiO2 Nanosheets on Micro-/Nanosurfaces via Laser Machining
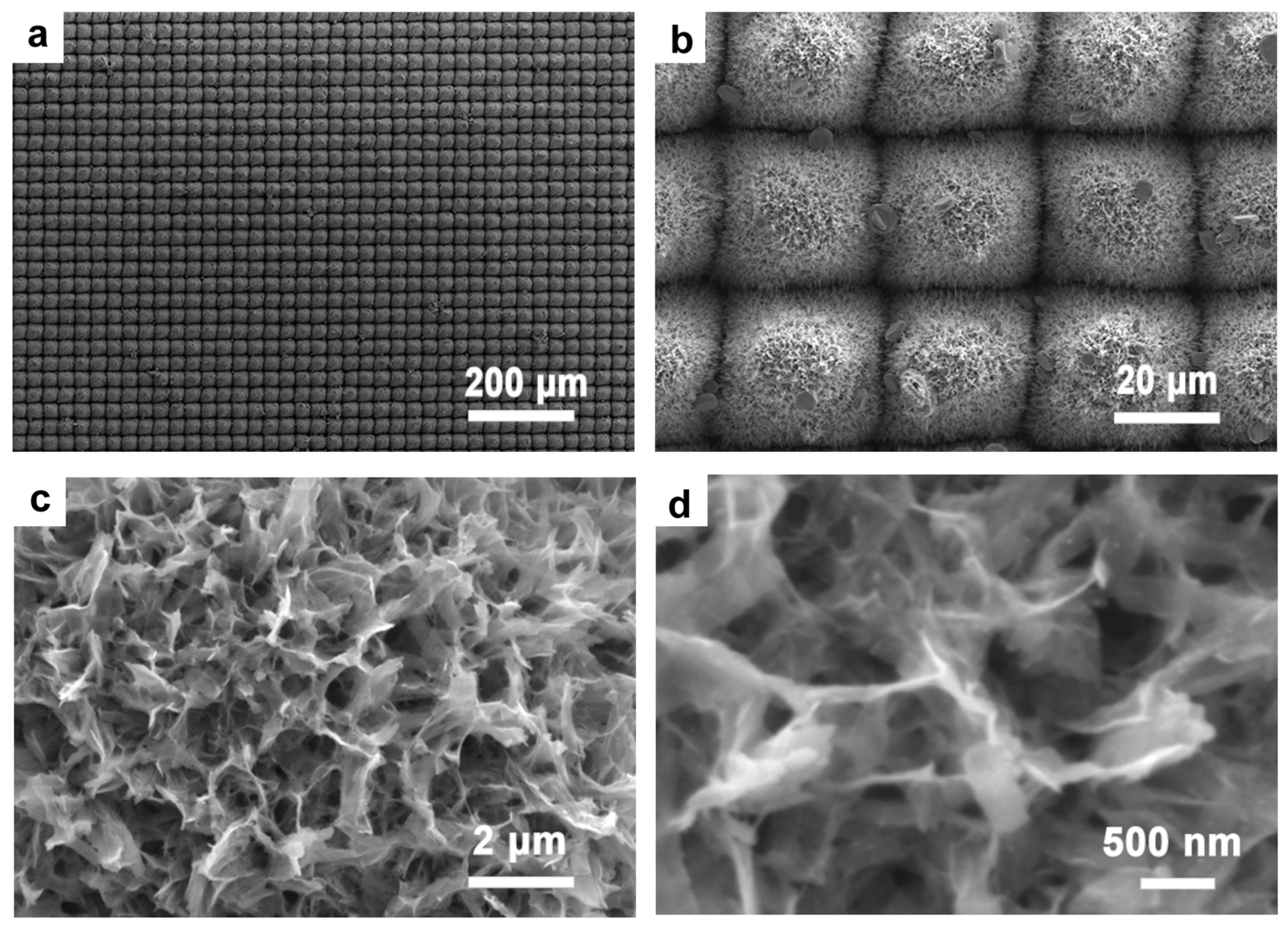
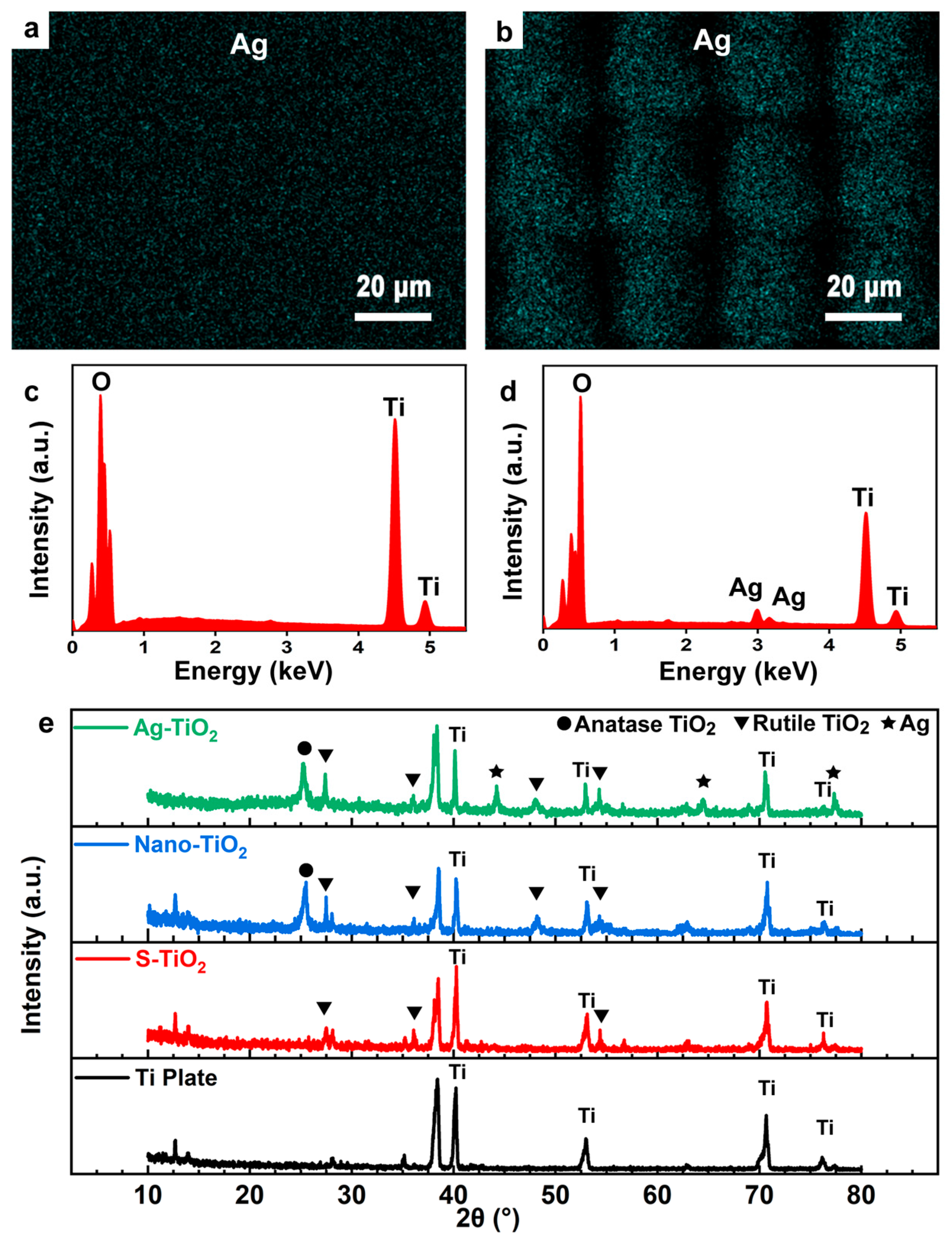
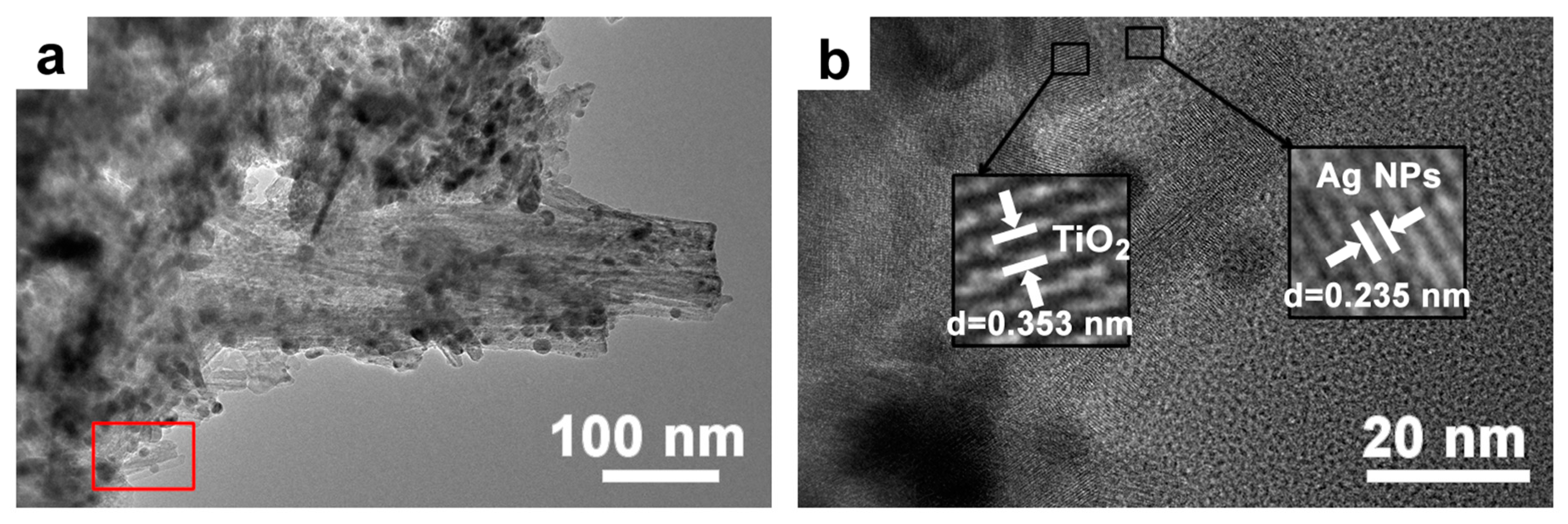
3.3. Synthesis of Silver Nanoparticles with Chemical Methods
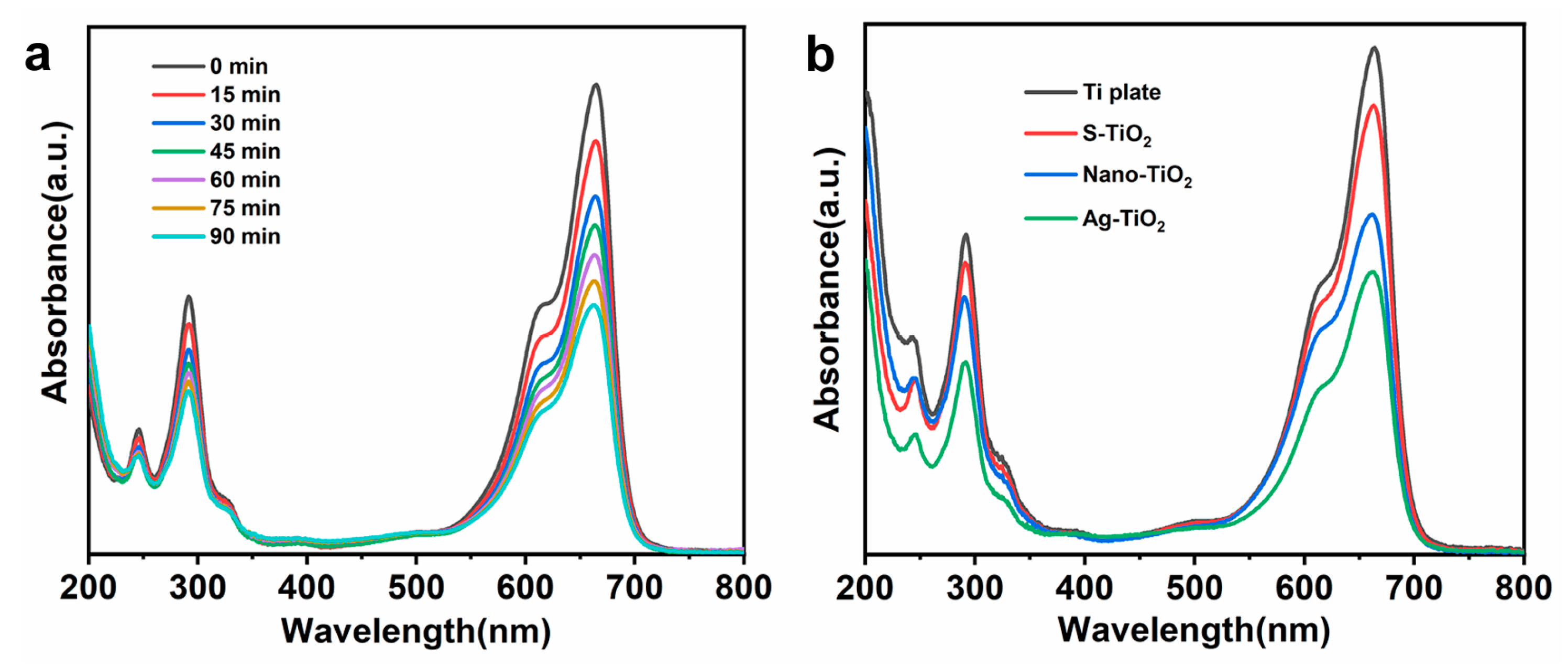
3.4. Characterization of Photocatalytic Properties of Ag-TiO2 Micro-/Nanocomposite Structures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Xu, M.; Zhang, T.; Chu, X.; Shi, K.; Li, J. Al/TiO2 composite as a photocatalyst for the degradation of organic pollutants. Environ. Sci. Pollut. Res. 2022, 30, 9738–9748. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, S.; Deng, T.; Ding, H.; Chen, W.; Chen, Y. The Preparation of TiO2 Film by the Sol-Gel Method and Evaluation of Its Self-Cleaning Property. Materials 2018, 11, 450. [Google Scholar] [CrossRef]
- Cosaert, E.; Wolfs, C.; Lambert, S.D.; Heynderickx, G.J.; Poelman, D. Deposition of Hybrid Photocatalytic Layers for Air Purification Using Commercial TiO2 Powders. Molecules 2021, 26, 6584. [Google Scholar] [CrossRef] [PubMed]
- Marinho, B.A.; de Souza, S.M.A.G.U.; de Souza, A.A.U.; Hotza, D. Electrospun TiO2 nanofibers for water and wastewater treatment: A review. J. Mater. Sci. 2020, 56, 5428–5448. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of hollow TiO2 microspheres composed of anatase polyhedra with exposed {001} facets. J. Am. Chem. Soc. 2010, 132, 11914–11916. [Google Scholar] [CrossRef]
- Macak, J.M.; Zlamal, M.; Krysa, J.; Schmuki, P. Self-Organized TiO2 Nanotube Layers as Highly Efficient Photocatalysts. Small 2007, 3, 300–304. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Y.; Lu, N.; Zhao, Y.; Yuan, X.; Zhang, H.; Teng, L.; Li, F. Design of H3PW12O40/TiO2 nano-photocatalyst for efficient photocatalysis under simulated sunlight irradiation. Appl. Surf. Sci. 2013, 285, 616–624. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, G.; Yu, J.; Fan, W. Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light. J. Mater. Chem. 2012, 22, 21337–21354. [Google Scholar] [CrossRef]
- Du, X.; Hu, J.; Liu, A.; Cao, Y. Interfacial modification and band modulation for dramatically boosted photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 588, 670–679. [Google Scholar] [CrossRef]
- Modwi, A.; Ghanem, M.A.; Al-Mayouf, A.M.; Houas, A. Lowering energy band gap and enhancing photocatalytic properties of Cu/ZnO composite decorated by transition metals. J. Mol. Struct. 2018, 1173, 1–6. [Google Scholar] [CrossRef]
- Shi, H.; He, R.; Sun, L.; Cao, G.; Yuan, X.; Xia, D. Band gap tuning of g-C3N4 via decoration with AgCl to expedite the photocatalytic degradation and mineralization of oxalic acid. J. Environ. Sci. 2019, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, X.; Bi, Y.; Han, X. ZnO/Ag–Ag2O microstructures for high-performance photocatalytic degradation of organic pollutants. Clean Technol. Environ. Policy 2018, 21, 367–378. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Zu, M.; Tang, Y.; Liu, C.; Li, W.; Chen, F. Synthesis of Fibrous Micro-nano Hierarchical Porous Cerium Dioxide Materials by the Impregnation and Thermal Decomposition Method and Its Enhanced Photocatalytic Activity. Front. Mater. 2022, 8, 775027. [Google Scholar] [CrossRef]
- Deng, A.; Zhu, Y.; Guo, X.; Zhou, L.; Jiang, Q. Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance. Materials 2018, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajji, L.; Ismail, A.A.; Bumajdad, A.; Alsaidi, M.; Ahmed, S.; Almutawa, F.; Al-Hazza, A. Construction of Au/TiO2 Heterojunction with high photocatalytic performances under UVA illumination. Ceram. Int. 2020, 46, 20155–20162. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Guo, P. Effects of Ag on the photocatalytic activity of multiple layer TiO2 films. Mater. Technol. 2016, 32, 46–51. [Google Scholar] [CrossRef]
- Kruanetr, S.; Wanchanthuek, R. Studies on preparation and characterization of Fe/TiO2 catalyst in photocatalysis applications. Mater. Res. Express 2017, 4, 076507. [Google Scholar] [CrossRef]
- Rusinque, B.; Escobedo, S.; de Lasa, H. Photoreduction of a Pd-Doped Mesoporous TiO2 Photocatalyst for Hydrogen Production under Visible Light. Catalysts 2020, 10, 74. [Google Scholar] [CrossRef]
- Bakre, P.V.; Tilve, S. Shirsat Influence of N sources on the photocatalytic activity of N-doped TiO2. Arab. J. Chem. 2020, 13, 7637–7651. [Google Scholar] [CrossRef]
- Liu, J.; Wang, P.; Fan, J.; Yu, H.; Yu, J. Hetero-phase MoC-Mo2C nanoparticles for enhanced photocatalytic H2-production activity of TiO2. Nano Res. 2020, 14, 1095–1102. [Google Scholar] [CrossRef]
- Wan, P.; Hood, Z.D.; Adhikari, S.P.; Xu, Y.; Yang, S.; Wu, S. Enhancing the photoresponse and photocatalytic properties of TiO2 by controllably tuning defects across {101} facets. Appl. Surf. Sci. 2018, 434, 711–716. [Google Scholar] [CrossRef]
- Ri, C.-H.; Han, H.-U.; Kim, Y.-S.; Jong, U.-G.; Kye, Y.-H.; Yu, C.-J. Enhancing the Photocatalytic Hydrogen Evolution Performance of the CsPbI3/MoS2 Heterostructure with Interfacial Defect Engineering. J. Phys. Chem. Lett. 2022, 13, 4007–4014. [Google Scholar] [CrossRef]
- Qiu, J.-Y.; Feng, H.-Z.; Chen, Z.-H.; Ruan, S.-H.; Chen, Y.-P.; Xu, T.-T.; Su, J.-Y.; Ha, E.-N.; Wang, L.-Y. Selective introduction of surface defects in anatase TiO2 nanosheets for highly efficient photocatalytic hydrogen generation. Rare Met. 2022, 41, 2074–2083. [Google Scholar] [CrossRef]
- Martínez-Vargas, B.L.; Durón-Torres, S.M.; Bahena, D.; Rodríguez-López, J.L.; Peralta-Hernández, J.M.; Picos, A. One-pot synthesis of ZnO–Ag and ZnO–Co nanohybrid materials for photocatalytic applications. J. Phys. Chem. Solids 2019, 135, 109120. [Google Scholar] [CrossRef]
- Barad, H.-N.; Ginsburg, A.; Cohen, H.; Rietwyk, K.J.; Keller, D.A.; Tirosh, S.; Bouhadana, Y.; Anderson, A.Y.; Zaban, A. Hot Electron-Based Solid State TiO2|Ag Solar Cells. Adv. Mater. Interfaces 2016, 3, 1500789. [Google Scholar] [CrossRef]
- Moakhar, R.S.; Jalali, M.; Kushwaha, A.; Goh, G.K.L.; Riahi-Noori, N.; Dolati, A.; Ghorbani, M. AuPd bimetallic nanoparticle decorated TiO2 rutile nanorod arrays for enhanced photoelectrochemical water splitting. J. Appl. Electrochem. 2018, 48, 995–1007. [Google Scholar] [CrossRef]
- Nie, J.; Schneider, J.; Sieland, F.; Zhou, L.; Xia, S.; Bahnemann, D.W. New insights into the surface plasmon resonance (SPR) driven photocatalytic H2 production of Au–TiO2. RSC Adv. 2018, 8, 25881–25887. [Google Scholar] [CrossRef]
- Gu, W.; Teng, F. SPR-promoted visible-light photocatalytic activity of Bi/ZIF hybrids. J. Photochem. Photobiol. A Chem. 2020, 400, 112679. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Zhang, Y.-L.; Li, Q.-K.; Zheng, J.-X.; Lu, Y.-M.; Juodkazis, S.; Chen, Q.-D.; Sun, H.-B. Biomimetic sapphire windows enabled by inside-out femtosecond laser deep-scribing. PhotoniX 2022, 3, 1. [Google Scholar] [CrossRef]
- Spellauge, M.; Doñate-Buendía, C.; Barcikowski, S.; Gökce, B.; Huber, H.P. Comparison of ultrashort pulse ablation of gold in air and water by time-resolved experiments. Light Sci. Appl. 2022, 11, 68. [Google Scholar] [CrossRef]
- Geng, J.; Yan, W.; Shi, L.; Qiu, M. Surface plasmons interference nanogratings: Wafer-scale laser direct structuring in seconds. Light Sci. Appl. 2022, 11, 189. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.-L.; Han, D.-D.; Wang, W.; Sun, H.-B. Laser fabrication of modular superhydrophobic chips for reconfigurable assembly and self-propelled droplet manipulation. PhotoniX 2021, 2, 17. [Google Scholar] [CrossRef]
- Xu, L.; Geng, J.; Shi, L.; Cui, W.; Qiu, M. Impact of film thickness in laser-induced periodic structures on amorphous Si films. Front. Optoelectron. 2023, 16, 16. [Google Scholar] [CrossRef]
- Ni, T.; Zhang, Z.; Wu, Y.; Yang, S. Effects of laser energy on the surface quality and properties of electrodeposited zinc-nickel-molybdenum coating. Nanotech. Precis. Eng. 2023, 6, 3. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Farooq, U.; Hou, X. Photoinduced switchable underwater superoleophobicity–superoleophilicity on laser modified titanium surfaces. J. Mater. Chem. A 2015, 3, 10703. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Hou, X.; Chen, F. Nature-Inspired Superwettability Achieved by Femtosecond Lasers. Ultrafast Sci. 2022, 2022, 9895418. [Google Scholar] [CrossRef]
- Hao, L.; Yan, J.; Guan, S.; Cheng, L.; Zhao, Q.; Zhu, Z.; Wang, Y.; Lu, Y.; Liu, J. Oxygen vacancies in TiO2/SnO coatings prepared by ball milling followed by calcination and their influence on the photocatalytic activity. Appl. Surf. Sci. 2019, 466, 490–497. [Google Scholar] [CrossRef]
- Liang, M.; Li, X.; Jiang, L.; Ran, P.; Wang, H.; Chen, X.; Xu, C.; Tian, M.; Wang, S.; Zhang, J.; et al. Femtosecond laser mediated fabrication of micro/nanostructured TiO2- photoelectrodes: Hierarchical nanotubes array with oxygen vacancies and their photocatalysis properties. Appl. Catal. B Environ. 2020, 277, 119231. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Huang, F.; Quan, Y.; Liu, G.; Zhang, X.; Xiong, F.; Huang, C.; Ji, M.; Li, H.; et al. Porous edge confinement: High carrier potential and low activation energy barrier synergistically boosting the efficiency of selective photocatalytic CO2 conversion. Appl. Catal. B Environ. 2023, 325, 122304. [Google Scholar] [CrossRef]
- Shao, Y.; de Groot, H.J.M.; Buda, F. Proton Acceptor near the Active Site Lowers Dramatically the O–O Bond Formation Energy Barrier in Photocatalytic Water Splitting. J. Phys. Chem. Lett. 2019, 10, 7690–7697. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Sun, X.; Xiao, Y.; Liu, F. Rational fabrication of plasmonic responsive N-Ag-TiO2-ZnO nanocages for photocatalysis under visible light. J. Alloys Compd. 2019, 772, 885–899. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.-K.; Li, Y.; Wang, Y.-J.; Qi, J.-Y.; Wang, Y.; Liu, Y.-D.; Liu, X.-Q. Construction of Ag-TiO2 Hierarchical Micro-/Nanostructures on a Ti Plate for Photocatalysts via Femtosecond Laser Hybrid Technology. Micromachines 2023, 14, 1815. https://doi.org/10.3390/mi14101815
Li Q-K, Li Y, Wang Y-J, Qi J-Y, Wang Y, Liu Y-D, Liu X-Q. Construction of Ag-TiO2 Hierarchical Micro-/Nanostructures on a Ti Plate for Photocatalysts via Femtosecond Laser Hybrid Technology. Micromachines. 2023; 14(10):1815. https://doi.org/10.3390/mi14101815
Chicago/Turabian StyleLi, Qian-Kun, Yue Li, Yan-Jun Wang, Jin-Yong Qi, Yan Wang, Yao-Dong Liu, and Xue-Qing Liu. 2023. "Construction of Ag-TiO2 Hierarchical Micro-/Nanostructures on a Ti Plate for Photocatalysts via Femtosecond Laser Hybrid Technology" Micromachines 14, no. 10: 1815. https://doi.org/10.3390/mi14101815
APA StyleLi, Q.-K., Li, Y., Wang, Y.-J., Qi, J.-Y., Wang, Y., Liu, Y.-D., & Liu, X.-Q. (2023). Construction of Ag-TiO2 Hierarchical Micro-/Nanostructures on a Ti Plate for Photocatalysts via Femtosecond Laser Hybrid Technology. Micromachines, 14(10), 1815. https://doi.org/10.3390/mi14101815






