Optimal Design of a Multipole-Electromagnet Robotic Platform for Ophthalmic Surgery
Abstract
1. Introduction
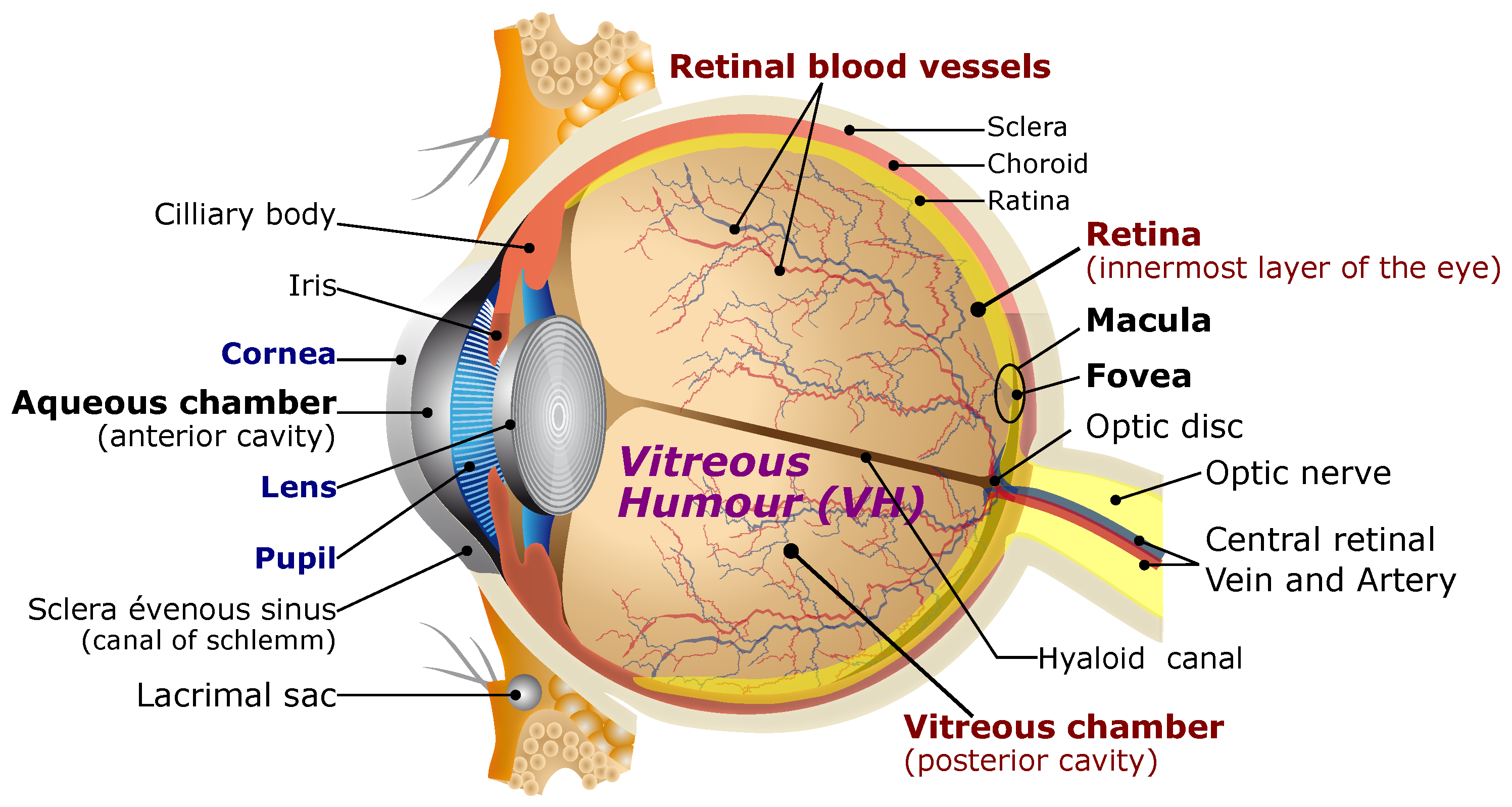
2. Ophthalmic Surgery Specifications
2.1. Ophthalmic Microrobotic MIS
2.2. Intraocular MIS Operations Requirements
3. OctoRob Platform Design
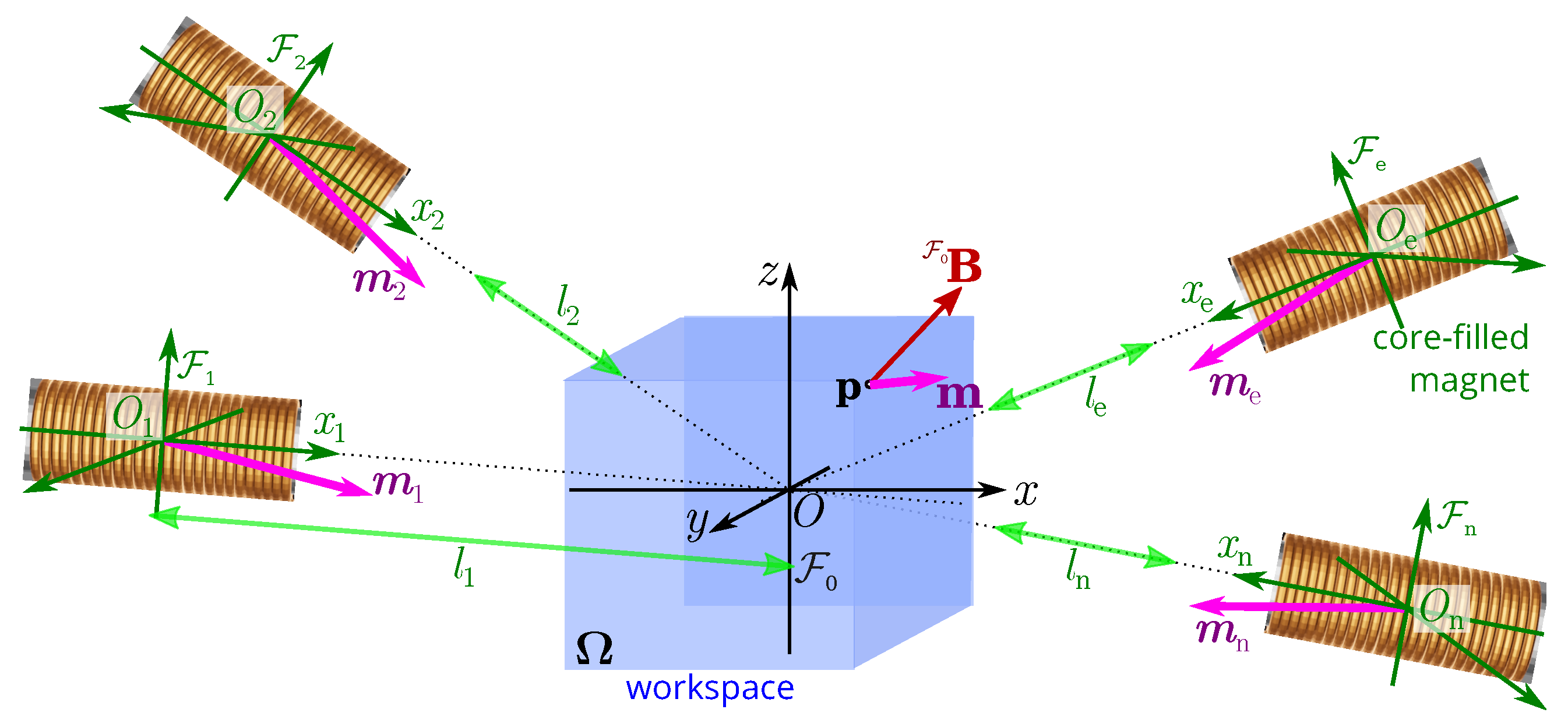
3.1. Modeling of Multipole Electromagnetic Coils System
3.2. Electromagnetic Coil Design
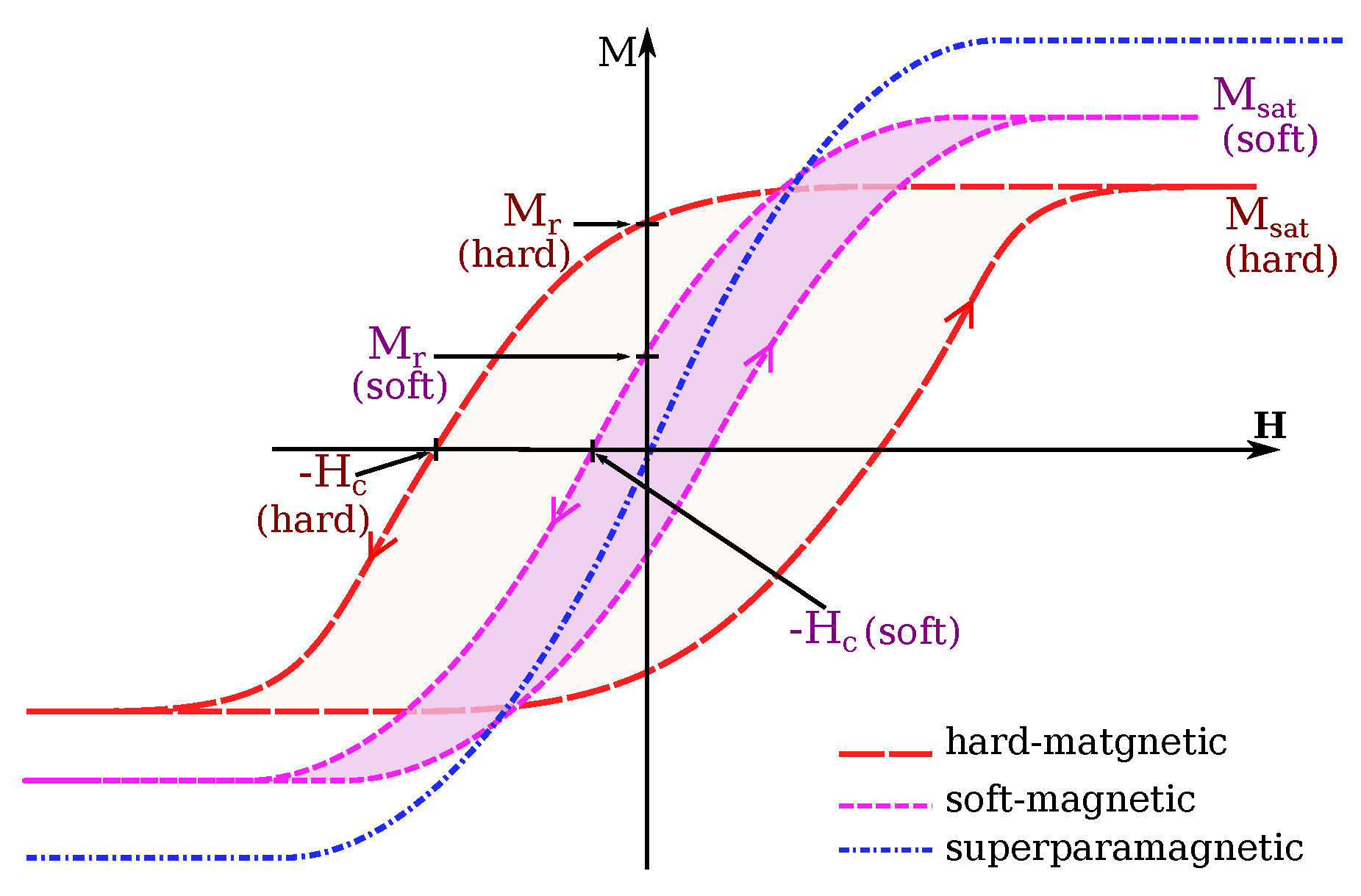
3.2.1. Magnetic Core
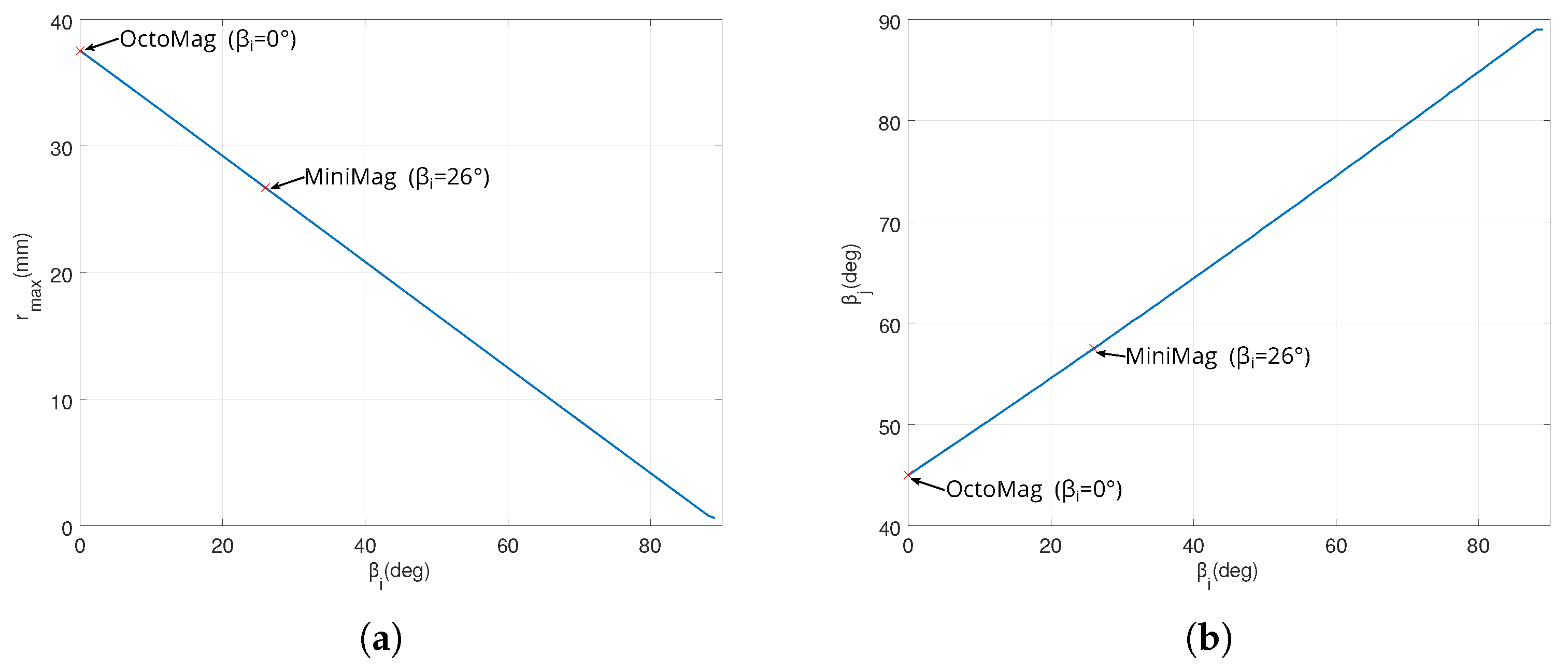
3.2.2. Optimal Electromagnets Sizing
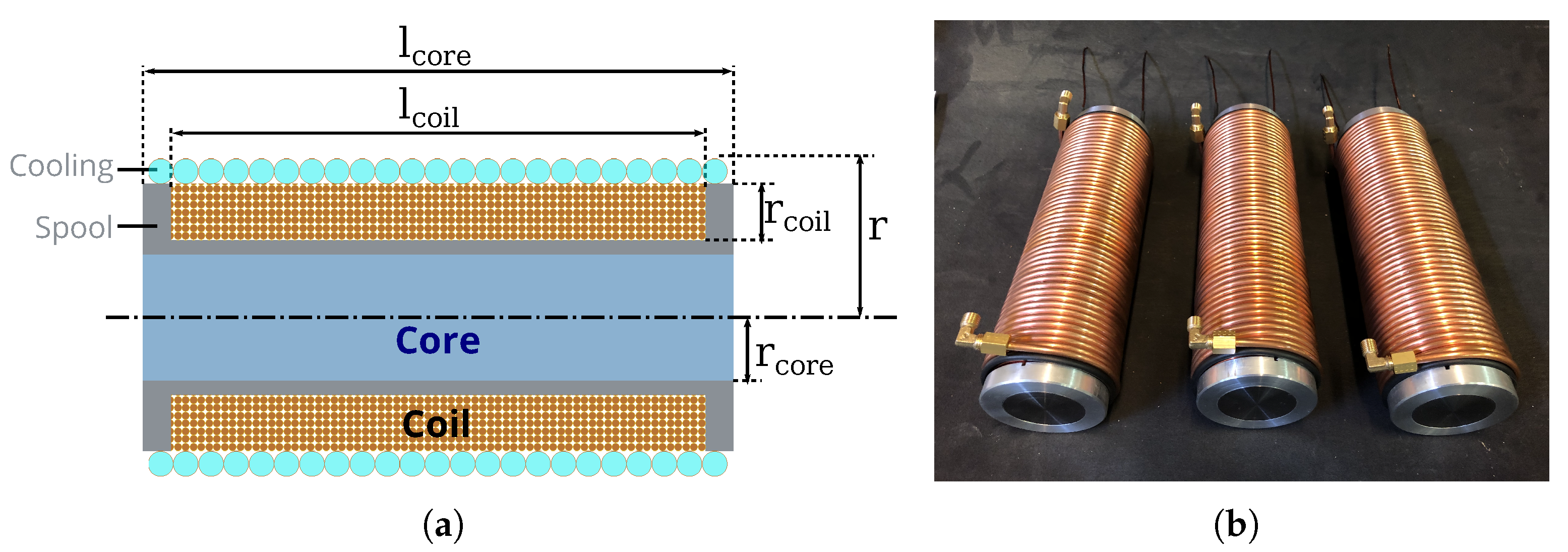
3.2.3. Coil Implementation
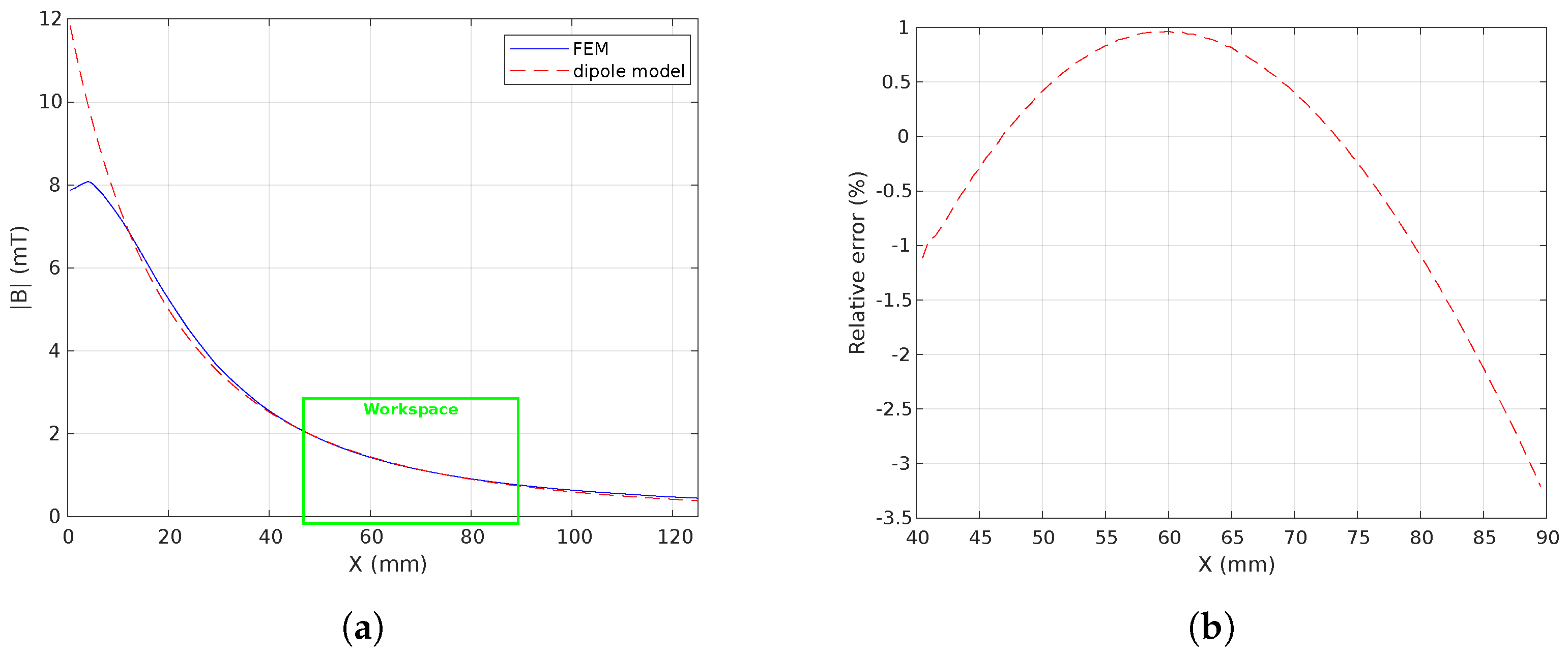
3.2.4. Coil Performance Evaluation
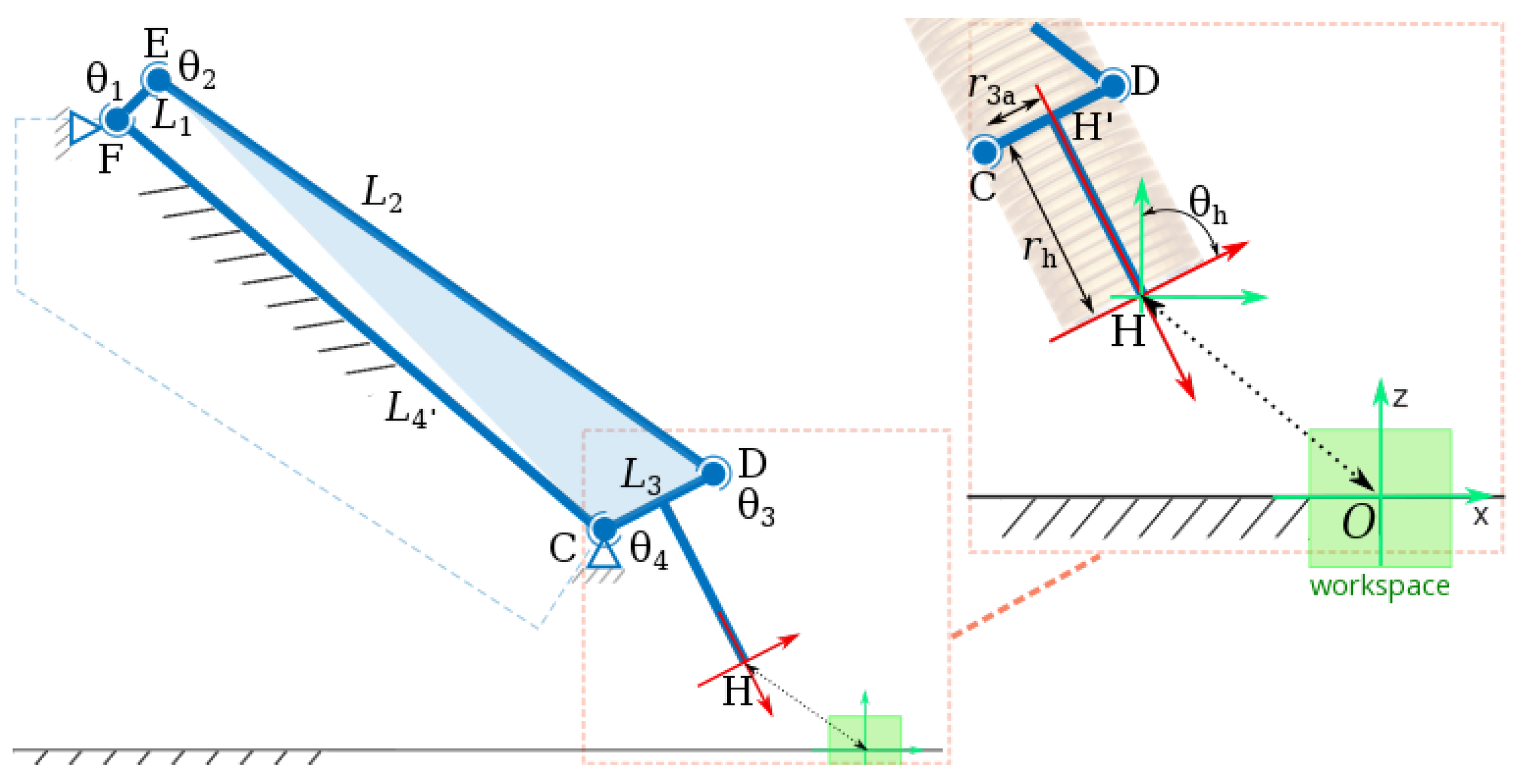
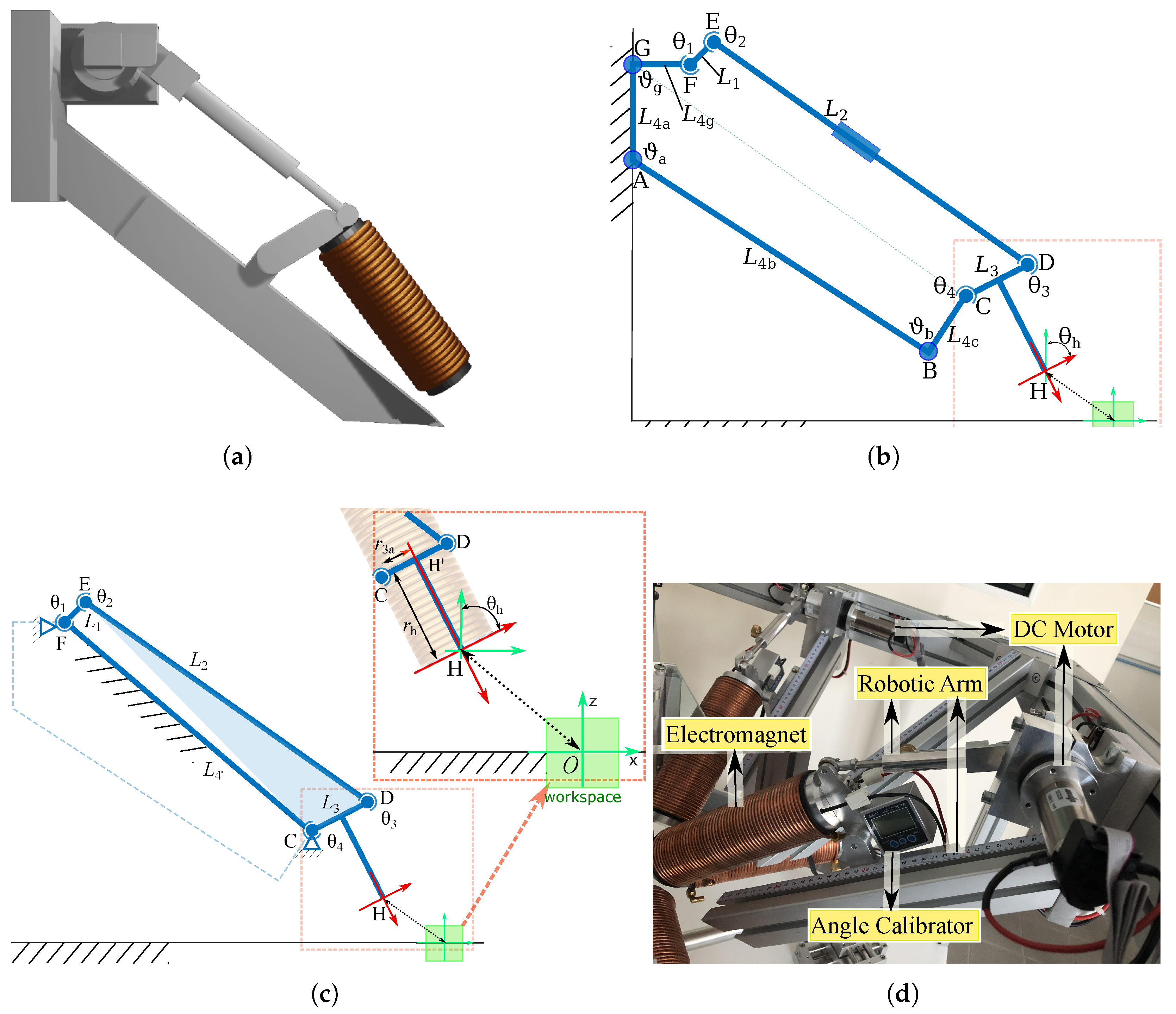
3.3. Design of the Robotic Arm
3.3.1. Robotic Arm Mechanism Description
3.3.2. Kinematic Analysis
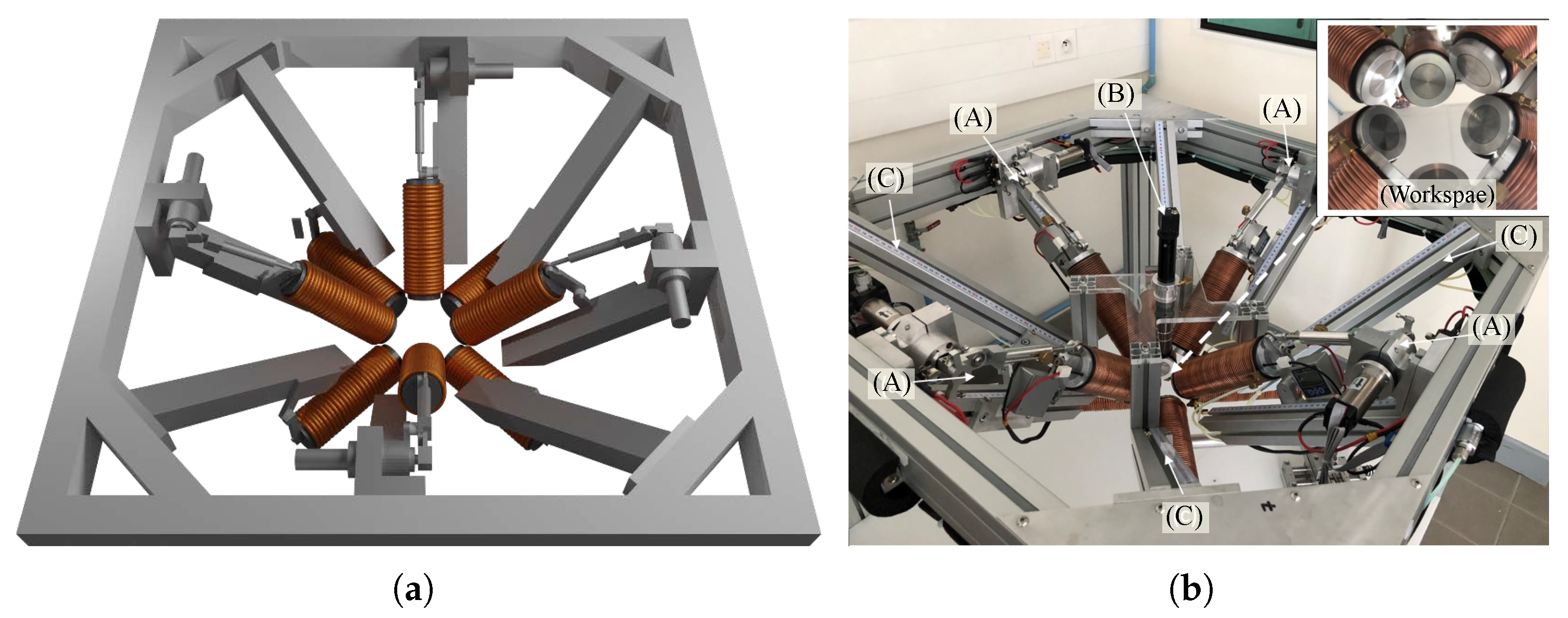
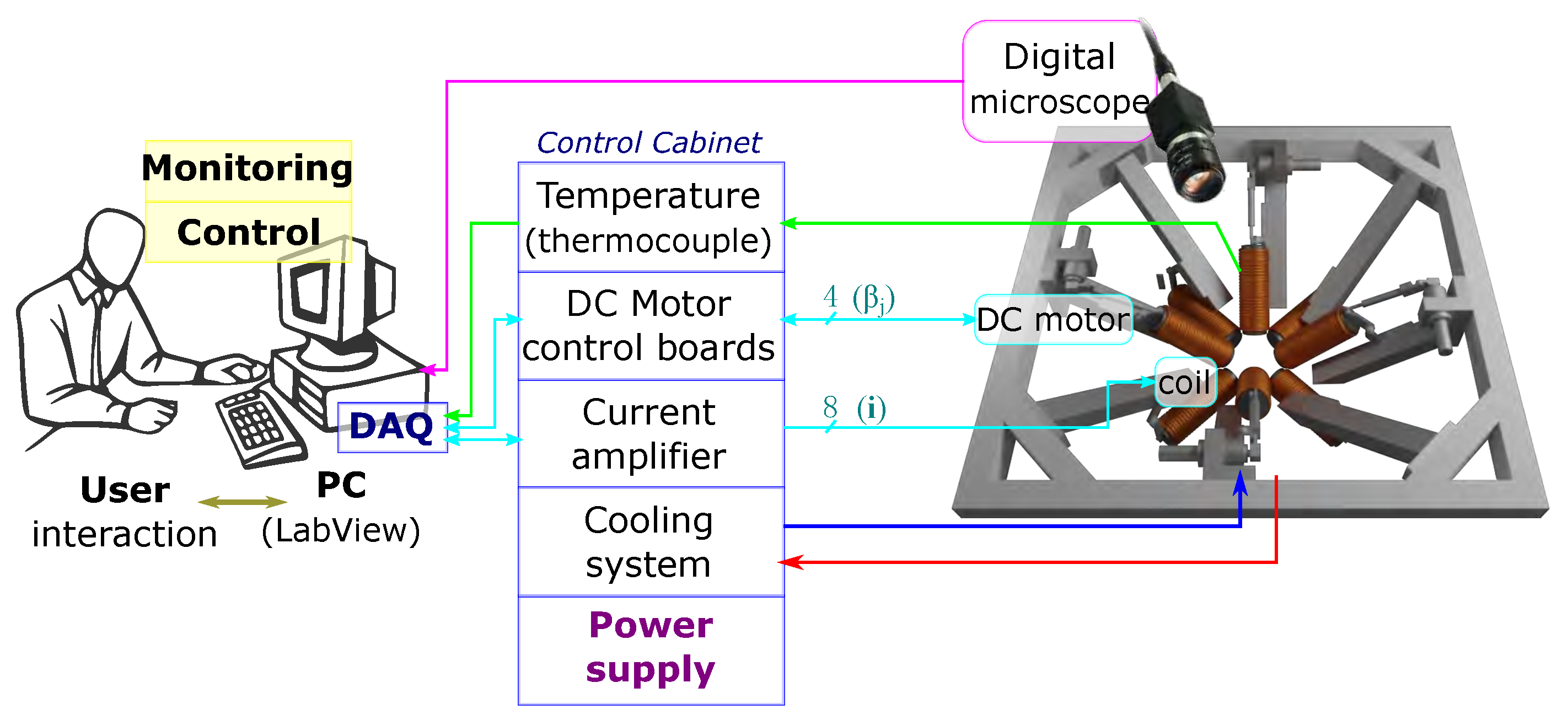
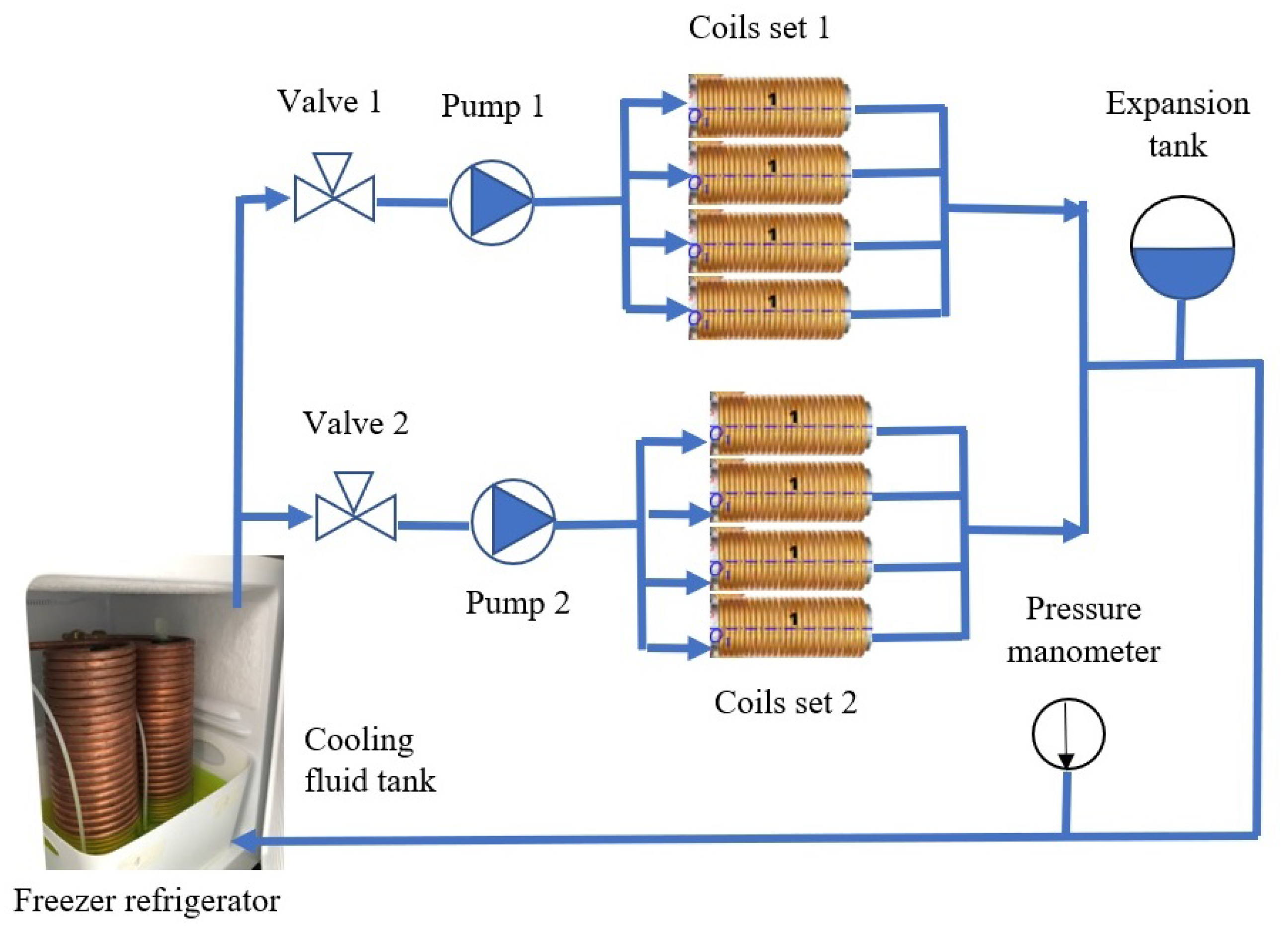
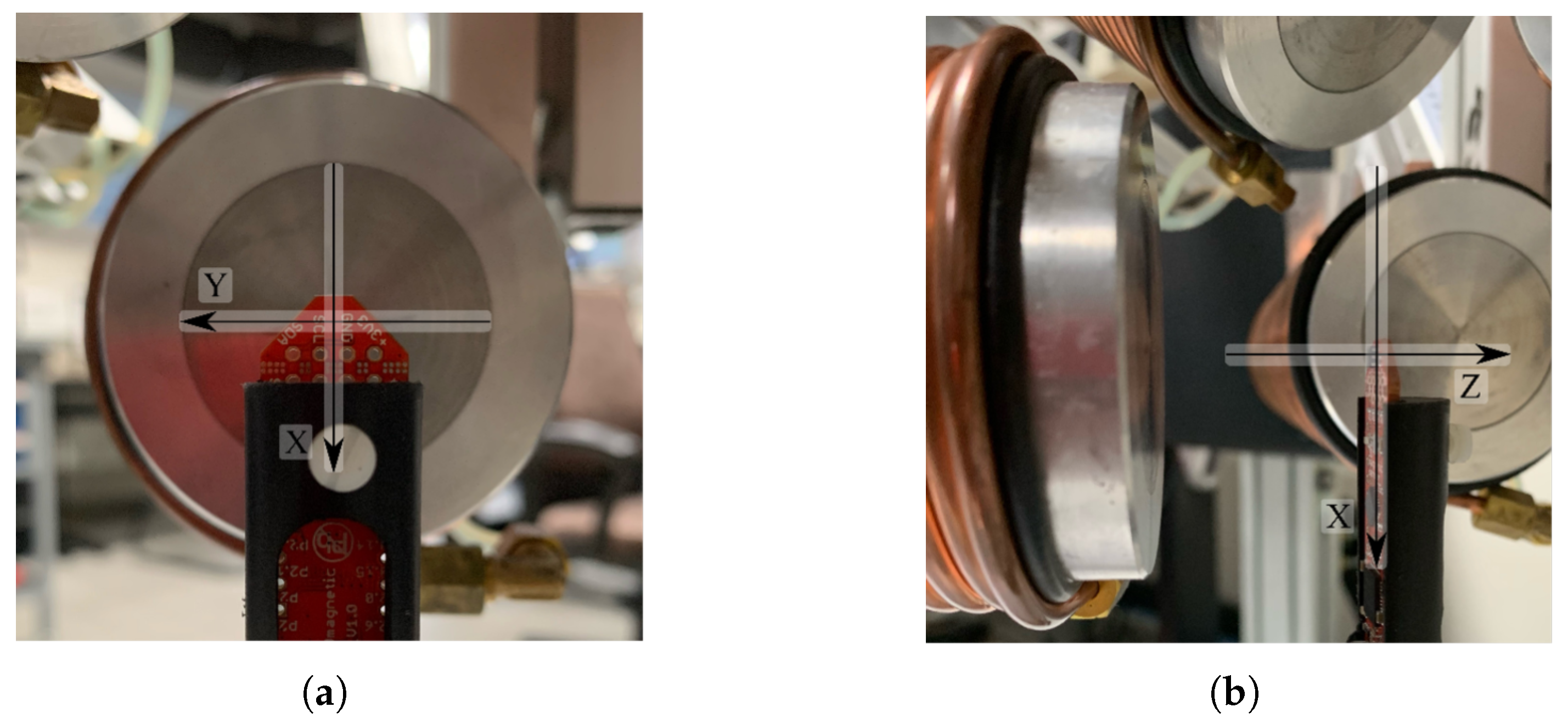
4. Implementation of the OctoRob Prototype
5. Evaluation of OctoRob
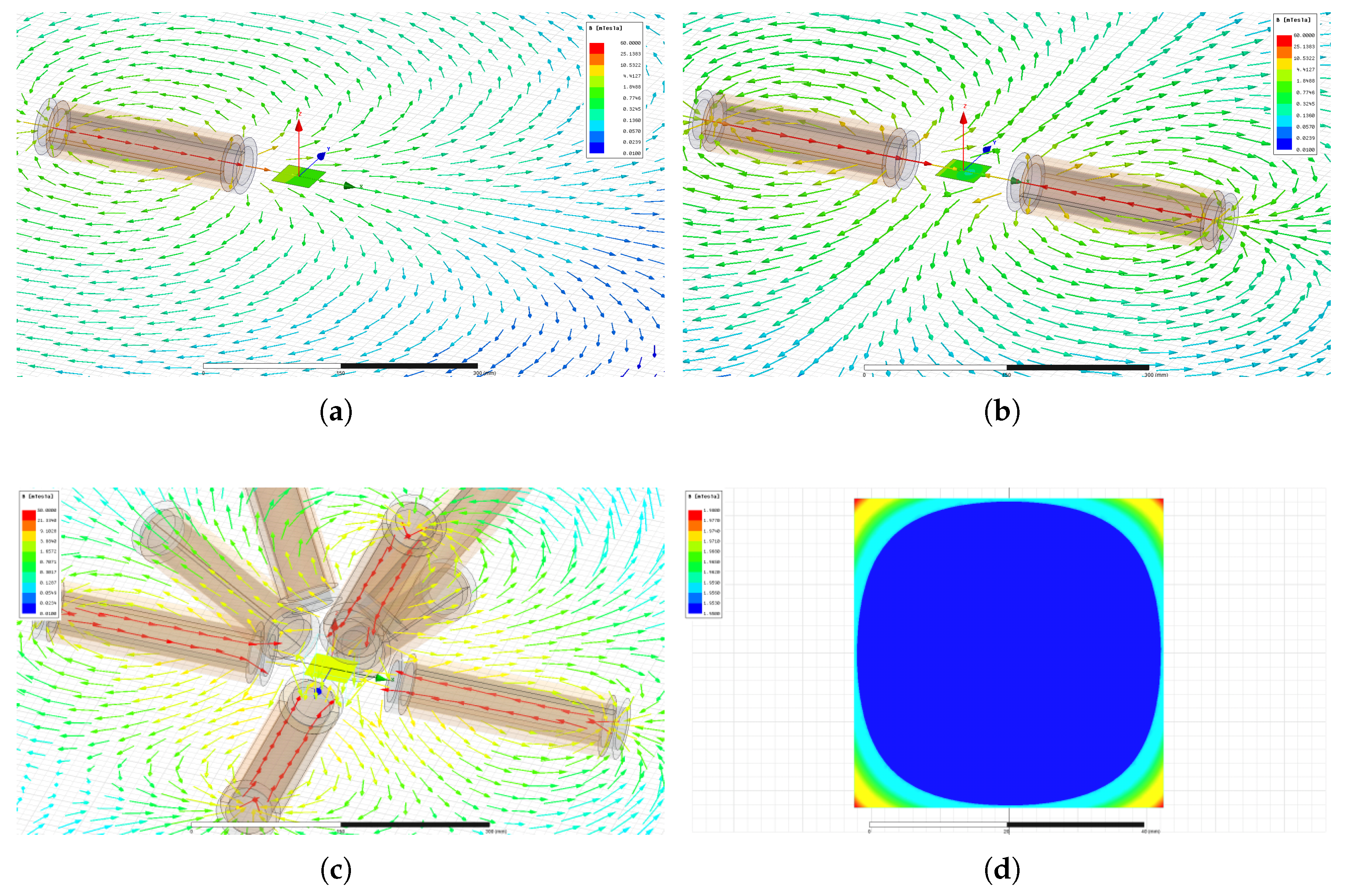
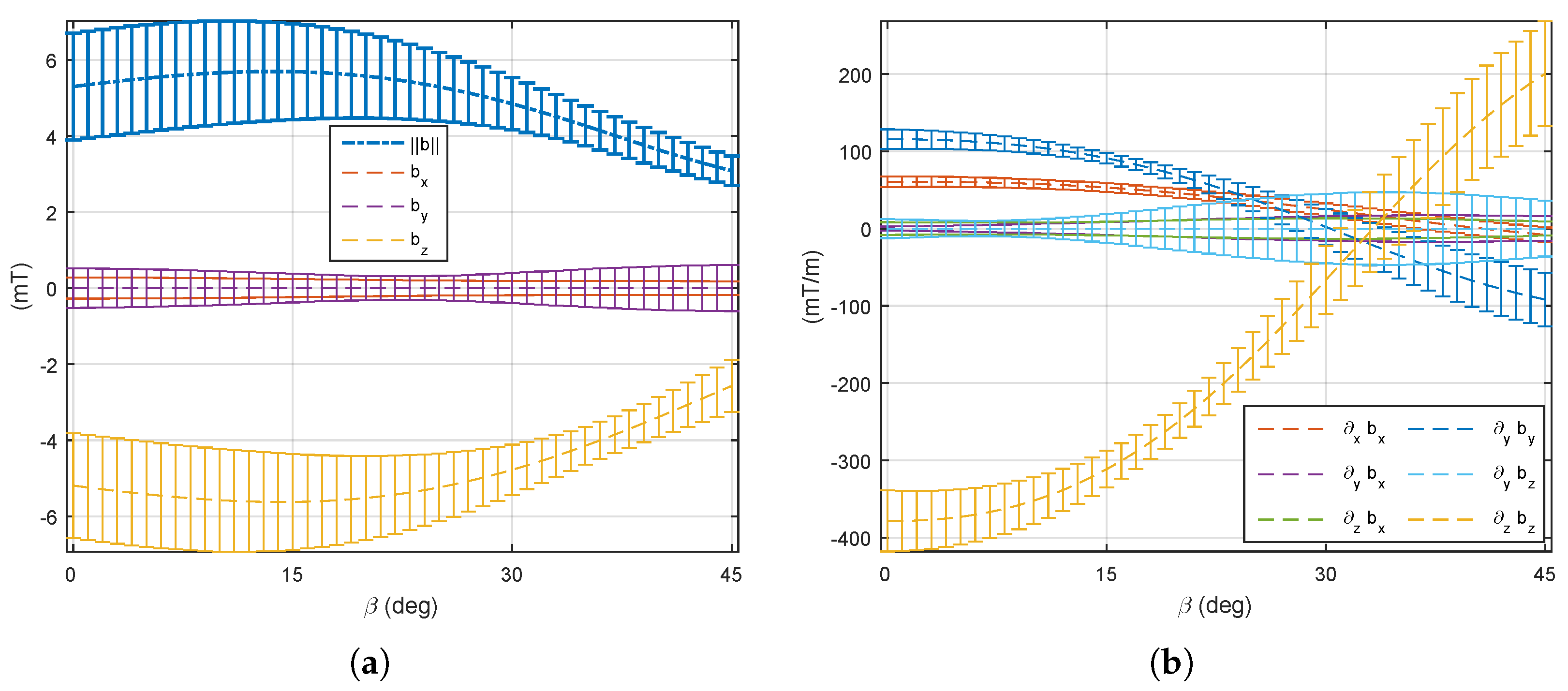
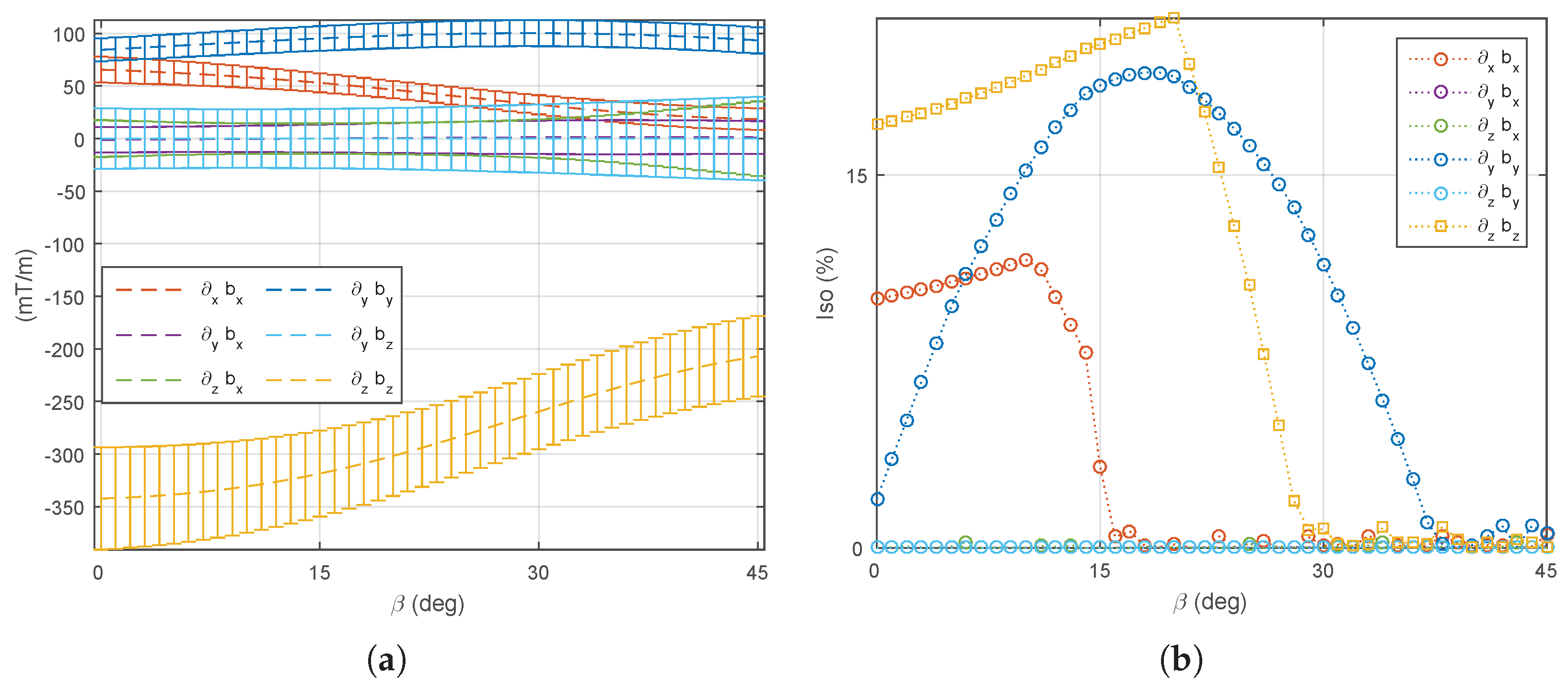
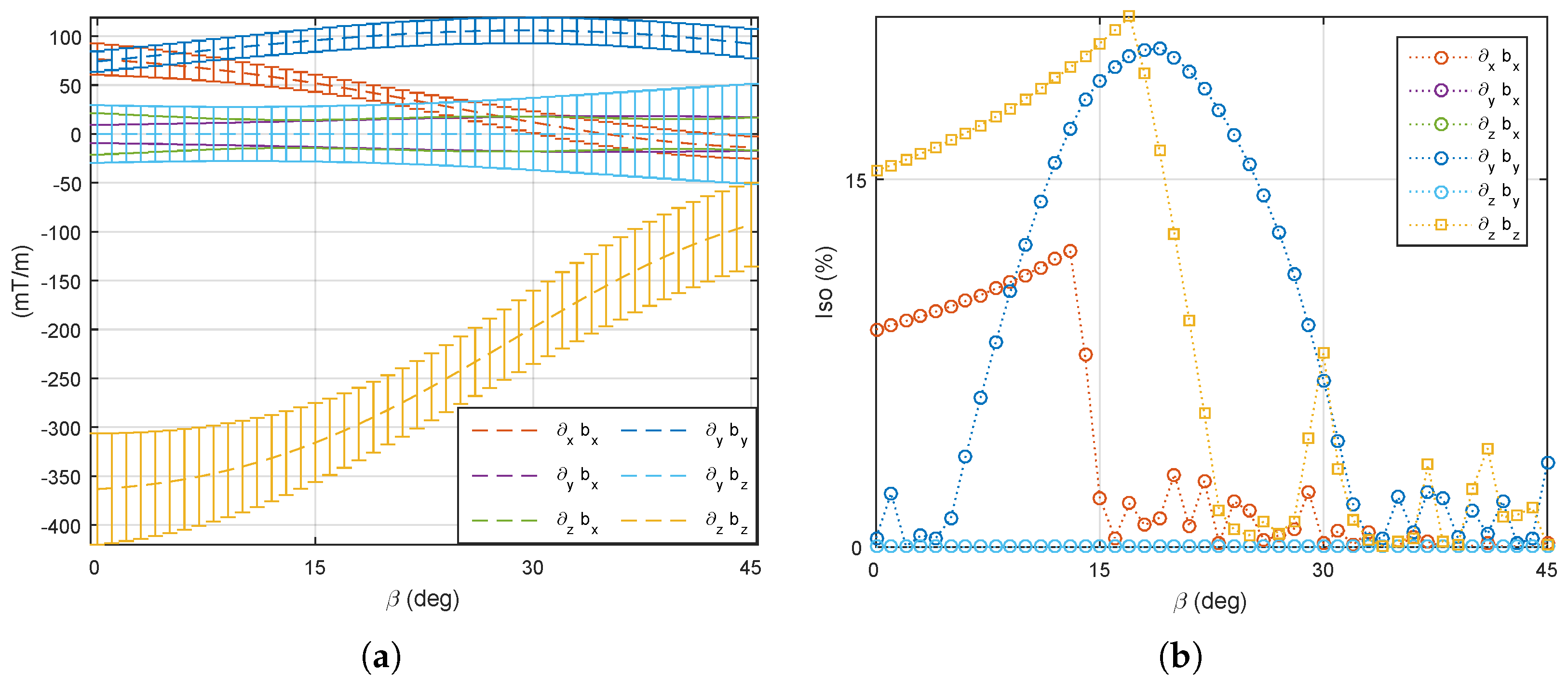
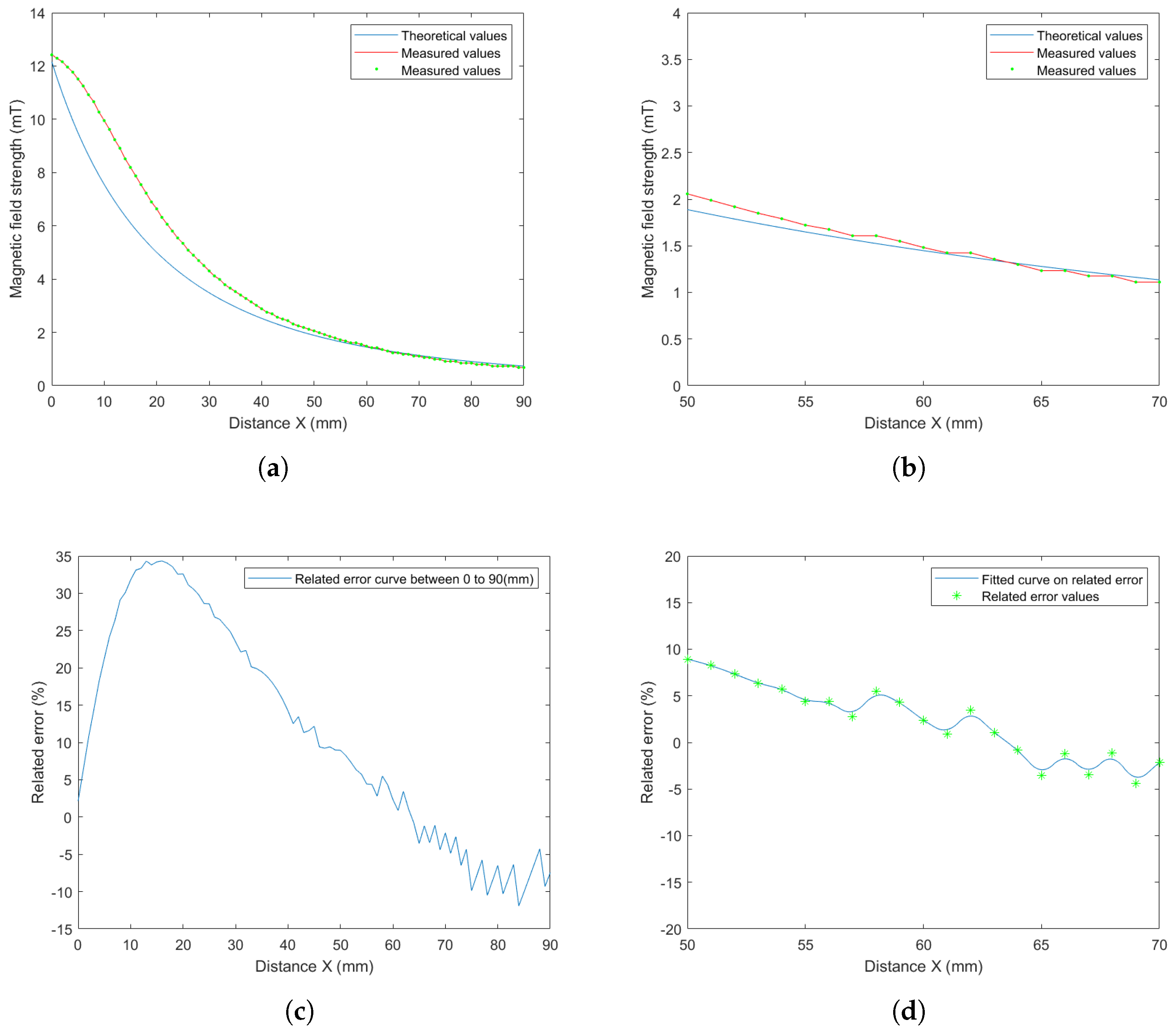
5.1. Magnetic Field and Gradient of the OctoRob Platform
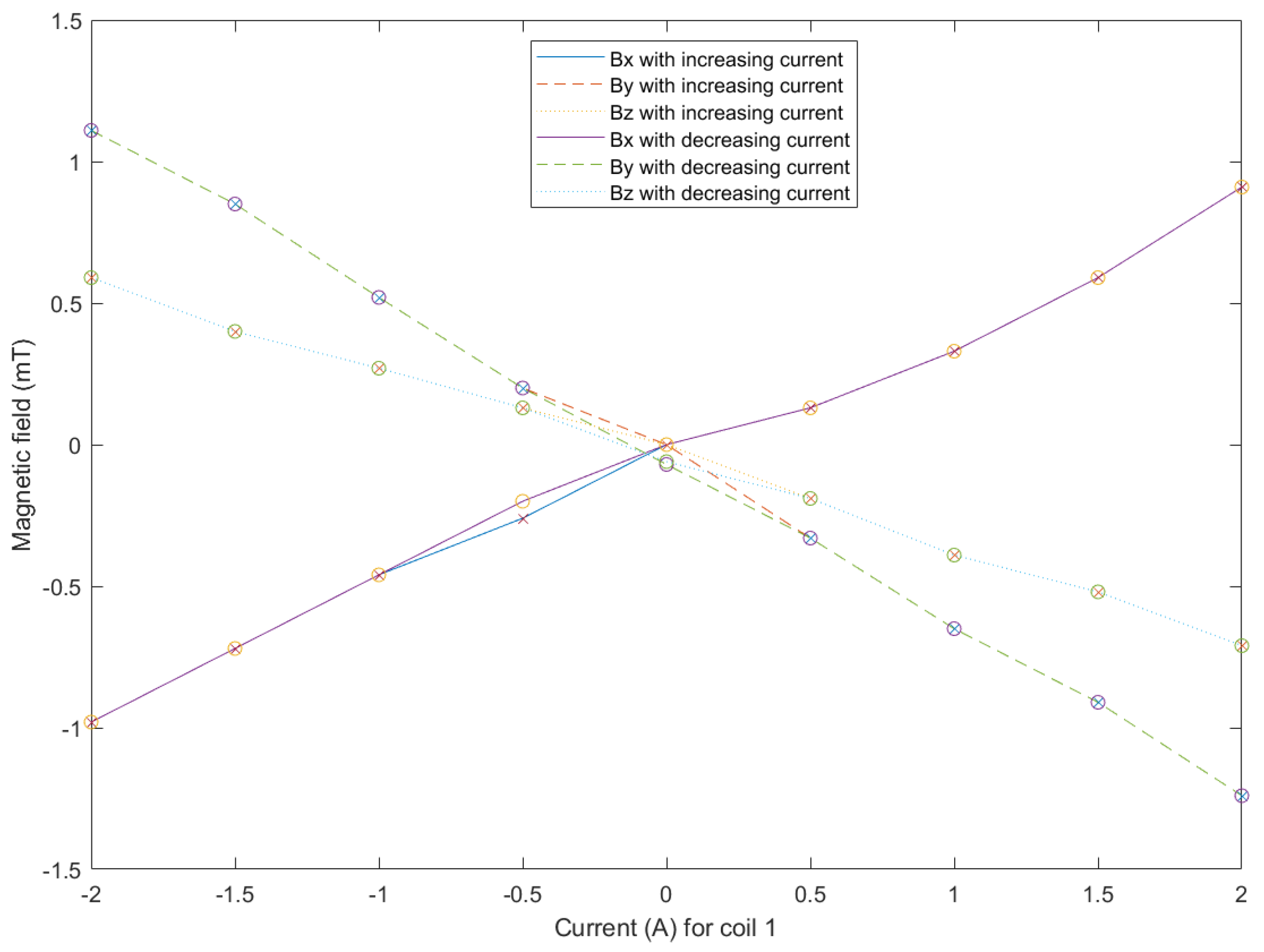
5.2. System Characterization
5.3. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOF | Degree of Freedom |
| EMA | Electromagnetic Actuation |
| MIS | Minimally Invasive Surgery |
| VH | Vitreous Humor |
| LD | Linear Dichroism |
| FEM | Finite Element Method |
| FEA | Finite Element Analysis |
| PID | Proportional Integral Derivative |
| DAQ | Data Acquisition |
| DC | Direct Current |
Appendix A
Appendix A.1. Coordinate Transformation
Appendix A.2. Definition of Transformation Matrices
Appendix A.3. Rotation Matrix
Appendix A.4. Transformation Matrix of a Pure Translation
Appendix A.5. Homogeneous Transformation Matrices

References
- Chautems, C.; Zeydan, B.; Charreyron, S.; Chatzipirpiridis, G.; Pané, S.; Nelson, B.J. Magnetically powered microrobots: A medical revolution underway? Eur. J. Cardio-Thorac. Surg. 2017, 51, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Folio, D.; Ferreira, A. Mathematical approach for the design configuration of magnetic system with multiple electromagnets. Robot. Auton. Syst. 2021, 135, 103674. [Google Scholar] [CrossRef]
- Chen, R.; Folio, D.; Ferreira, A. Analysis and Comparison of Electromagnetic Microrobotic Platforms for Biomedical Applications. Appl. Sci. 2022, 12, 456. [Google Scholar] [CrossRef]
- Kummer, M.P.; Abbott, J.J.; Kratochvil, B.E.; Borer, R.; Sengul, A.; Nelson, B.J. OctoMag: An electromagnetic system for 5-DOF wireless micromanipulation. IEEE Trans. Robot. 2010, 26, 1006–1017. [Google Scholar] [CrossRef]
- Gupta, P.K.; Jensen, P.S.; de Juan, E. Surgical forces and tactile perception during retinal microsurgery. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Cambridge, UK, 19–22 September 1999; Springer International Publishing: Berlin/Heidelberg, Germany, 1999; pp. 1218–1225. [Google Scholar]
- Jagtap, A.D.; Riviere, C.N. Applied force during vitreoretinal microsurgery with handheld instruments. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; Volume 1, pp. 2771–2773. [Google Scholar]
- Singhy, S.; Riviere, C. Physiological tremor amplitude during retinal microsurgery. In Proceedings of the IEEE Annual Northeast Bioengineering Conference, Philadelphia, PA, USA, 21 April 2002; pp. 171–172. [Google Scholar]
- Fine, H.F.; Wei, W.; Goldman, R.E.; Simaan, N. Robot-assisted ophthalmic surgery. Can. J. Ophthalmol. 2010, 45, 581–584. [Google Scholar] [CrossRef]
- Pitcher, J.D.; Wilson, J.T.; Tsao, T.C.; Schwartz, S.D.; Hubschman, J.P. Robotic eye surgery: Past, present, and future. J. Comput. Sci. Syst. Biol. 2012, 5, 1. [Google Scholar] [CrossRef]
- Spandau, U.; Scharioth, G. Cutting Edge of Ophthalmic Surgery: From Refractive SMILE to Robotic Vitrectomy; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Channa, R.; Iordachita, I.; Handa, J.T. Robotic Eye Surgery. Retina 2017, 37, 1220. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B.J. Artificial bacterial flagella: Fabrication and magnetic control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef]
- Zhang, L.; Abbott, J.J.; Dong, L.; Peyer, K.E.; Kratochvil, B.E.; Zhang, H.; Bergeles, C.; Nelson, B.J. Characterizing the swimming properties of artificial bacterial flagella. Nano Lett. 2009, 9, 3663–3667. [Google Scholar] [CrossRef]
- Zhang, L.; Peyer, K.E.; Nelson, B.J. Artificial bacterial flagella for micromanipulation. Lab Chip 2010, 10, 2203–2215. [Google Scholar] [CrossRef]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Tottori, S.; Qiu, F.; Zhang, L.; Nelson, B.J. Magnetic helical micromachines. Chem. Eur. J. 2013, 19, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.; Peyer, K.E. Micro-and nanorobots swimming in heterogeneous liquids. ACS Nano 2014, 8, 8718–8724. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef]
- Snell, R.S.; Lemp, M.A. Clinical Anatomy of the Eye; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tang, W.M.; Han, D.P. A study of surgical approaches to retinal vascular occlusions. Arch. Ophthalmol. 2000, 118, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Shahid, H.; Hossain, P.; Amoaku, W. The management of retinal vein occlusion: Is interventional ophthalmology the way forward? Br. J. Ophthalmol. 2006, 90, 627–639. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meredith, T.A. Atlas of Retinal and Vitreous Surgery. Retina 2000, 20, 321. [Google Scholar] [CrossRef]
- Bonfiglio, A.; Repetto, R.; Siggers, J.H.; Stocchino, A. Investigation of the motion of a viscous fluid in the vitreous cavity induced by eye rotations and implications for drug delivery. Phys. Med. Biol. 2013, 58, 1969. [Google Scholar] [CrossRef]
- Bonfiglio, A.; Lagazzo, A.; Repetto, R.; Stocchino, A. An experimental model of vitreous motion induced by eye rotations. Eye Vis. 2015, 2, 10. [Google Scholar] [CrossRef]
- Silva, A.F.; Alves, M.A.; Oliveira, M.S.N. Rheological Behaviour of Vitreous Humour. Rheol. Acta 2017, 56, 377–386. [Google Scholar] [CrossRef]
- Purcell, E.M. Life at low Reynolds number. Am. J. Phys. 1977, 45, 3–11. [Google Scholar] [CrossRef]
- Bekerman, I.; Gottlieb, P.; Vaiman, M. Variations in eyeball diameters of the healthy adults. J. Ophthalmol. 2014, 2014, 503645. [Google Scholar] [CrossRef] [PubMed]
- Amblard, F.; Yurke, B.; Pargellis, A.; Leibler, S. A magnetic manipulator for studying local rheology and micromechanical properties of biological systems. Rev. Sci. Instrum. 1996, 67, 818–827. [Google Scholar] [CrossRef]
- Dogangil, G.; Ergeneman, O.; Abbott, J.J.; Pané, S.; Hall, H.; Muntwyler, S.; Nelson, B.J. Toward targeted retinal drug delivery with wireless magnetic microrobots. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Prague, Czech Republic, 27 September–1 October 2008; pp. 1921–1926. [Google Scholar]
- Abolhassani, N.; Patel, R.; Moallem, M. Needle Insertion into Soft Tissue: A Survey. Med. Eng. Phys. 2007, 29, 413–431. [Google Scholar] [CrossRef]
- Chen, R.; Folio, D.; Ferreira, A. Performance metrics for a robotic actuation system using static and mobile electromagnets. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; pp. 2474–2480. [Google Scholar]
- Petruska, A.J.; Nelson, B.J. Minimum bounds on the number of electromagnets required for remote magnetic manipulation. IEEE Trans. Robot. 2015, 31, 714–722. [Google Scholar] [CrossRef]
- Wong, D.; Steager, E.B.; Kumar, V. Independent control of identical magnetic robots in a plane. IEEE Robot. Autom. Lett. 2016, 1, 554–561. [Google Scholar] [CrossRef]
- Etiévant, M.; Bolopion, A.; Régnier, S.; Andreff, N. An Improved Control-Oriented Modeling of the Magnetic Field. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; pp. 6178–6184. [Google Scholar]
- Jing, X.; Guo, W. Modeling and Configuration Design of Electromagnetic Actuation Coil for a Magnetically Controlled Microrobot. Chin. J. Mech. Eng. 2019, 32, 63. [Google Scholar] [CrossRef]
- Furlani, E.P. Permanent Magnet and Electromechanical Devices: Materials, Analysis, and Applications; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar] [CrossRef]
- McCaig, M. Permanent Magnets in Theory and Practice; Pentech Press: London, UK, 1977. [Google Scholar]
- Woolman, J.; Mottram, R. The Mechanical and Physical Properties of the British Standard En Steels; EN 40 TO EN 363; Pergamon Press: New York, NY, USA, 1964; Volume 2. [Google Scholar]
- Kummer, M.P. 5-DOF Wireless Micromanipulation Using Soft-Magnetic Core Electromagnets. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2010. [Google Scholar]
- Zhang, Z.; Menq, C.H. Design and Modeling of a 3-D Magnetic Actuator for Magnetic Microbead Manipulation. IEEE/ASME Trans. Mechatron. 2011, 16, 421–430. [Google Scholar] [CrossRef]
- Griffiths, D.J. Introduction to Electrodynamics; Cambridge University Press: Cambridge, UK, 2017; Volume 2. [Google Scholar]
- Khalil, W. Modeling and Control of Manipulators—Part I: Geometric and Kinematic Models. Ph.D. Thesis, GdR Robotics Winter School, Robotica Principia, Centre de Recherche Inria Sophia Antipolis, Méditérranée, France, 2019. [Google Scholar]





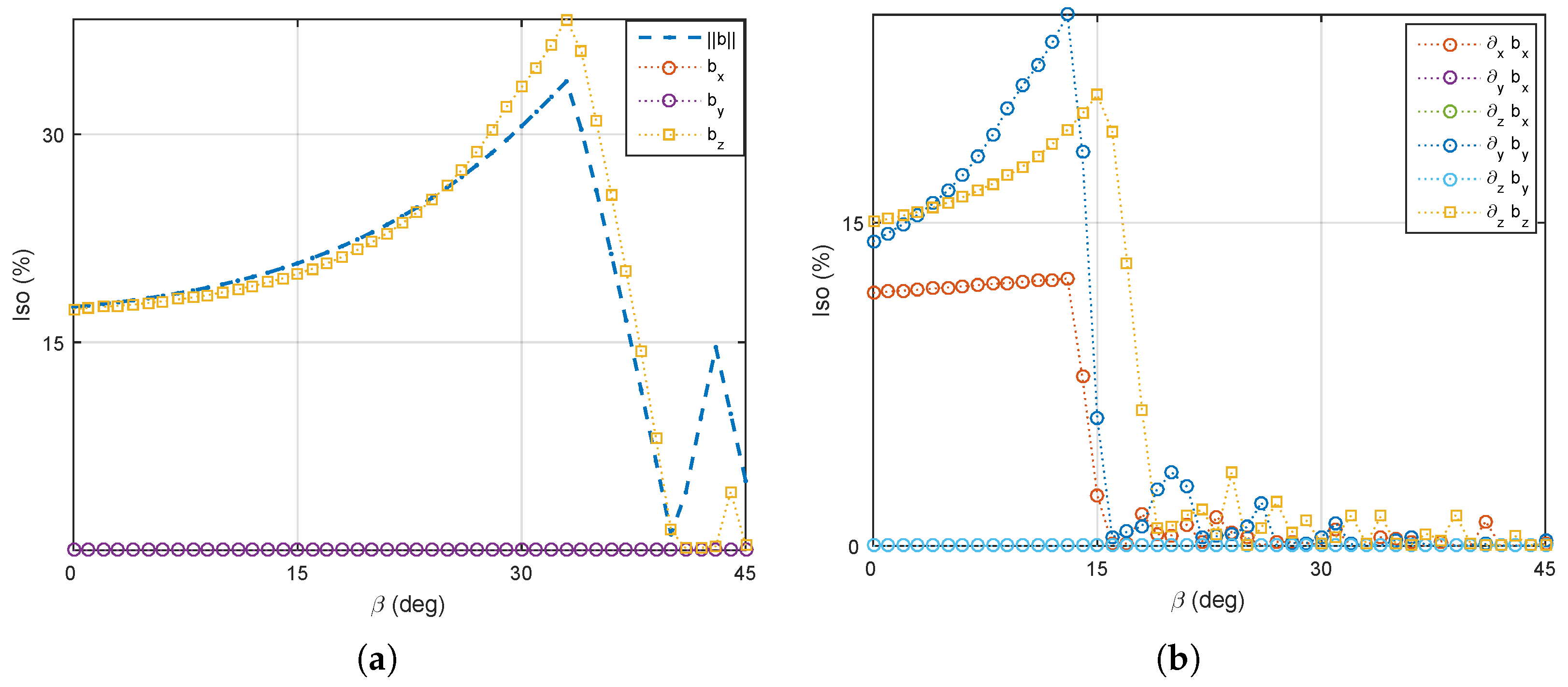

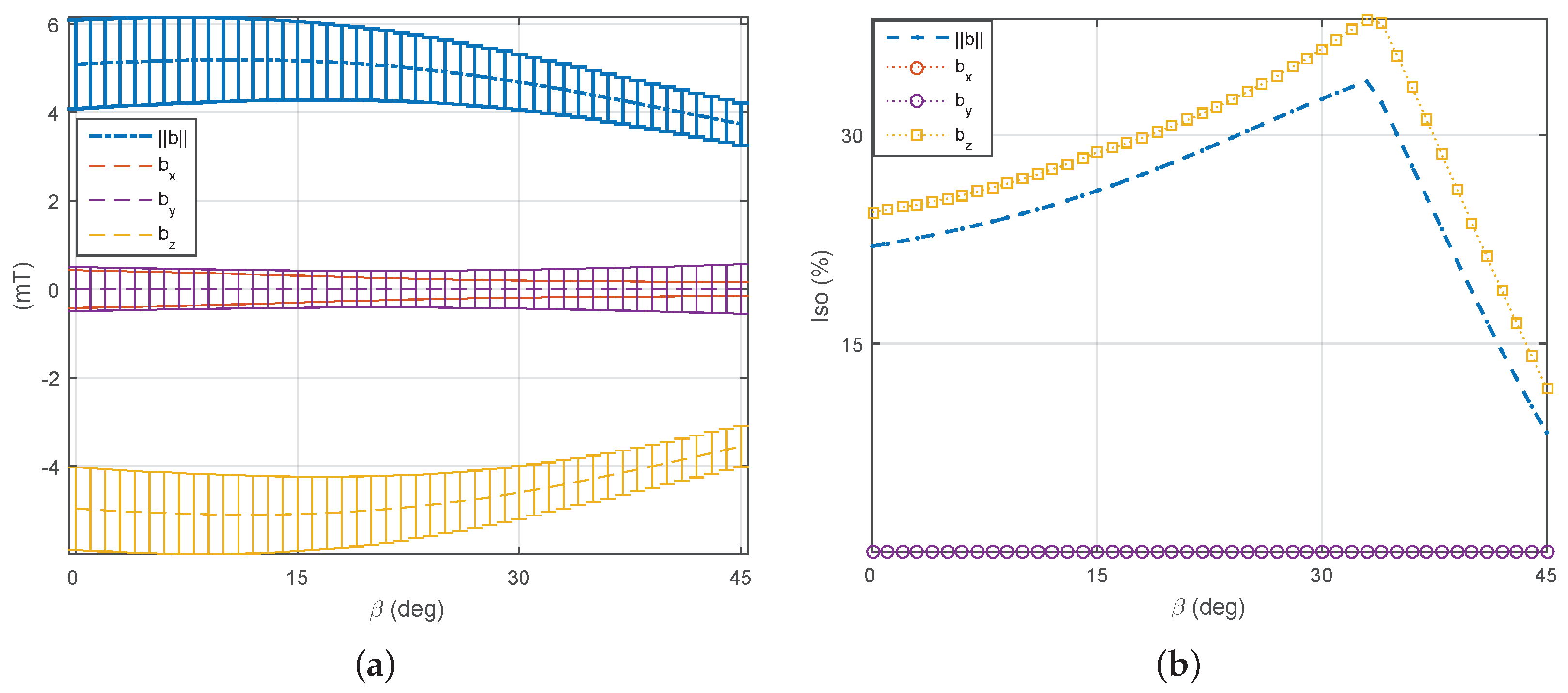

| Material | Saturation () | Remanence () | Coercivity () | Relative Permeability () | Density () |
|---|---|---|---|---|---|
| Cobalt (Co) | 250 | 890 | |||
| Iron (Fe) | 5000 | 7874 | |||
| Nickel (Ni) | 600 | 8908 | |||
| Lowcarbon steel a | 397 | 1560 | 7850 | ||
| FeCo alloy b | 100 | 7000 | 8120 | ||
| FeNi alloy c | 190,000 | 8200 |
| (a) | ||||
|---|---|---|---|---|
| Link | ||||
| () | 33 | 444 | 75 | 372 |
| (b) | ||||
| Part | ||||
| () | 100 | 365 | 68 | 60 |
| 123° | 90° | - | 90° | |
| Coil1 | Coil2 | Coil3 | Coil4 | Coil5 | Coil6 | Coil7 | Coil8 | |
|---|---|---|---|---|---|---|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Folio, D.; Ferreira, A. Optimal Design of a Multipole-Electromagnet Robotic Platform for Ophthalmic Surgery. Micromachines 2023, 14, 91. https://doi.org/10.3390/mi14010091
Chen R, Folio D, Ferreira A. Optimal Design of a Multipole-Electromagnet Robotic Platform for Ophthalmic Surgery. Micromachines. 2023; 14(1):91. https://doi.org/10.3390/mi14010091
Chicago/Turabian StyleChen, Ruipeng, David Folio, and Antoine Ferreira. 2023. "Optimal Design of a Multipole-Electromagnet Robotic Platform for Ophthalmic Surgery" Micromachines 14, no. 1: 91. https://doi.org/10.3390/mi14010091
APA StyleChen, R., Folio, D., & Ferreira, A. (2023). Optimal Design of a Multipole-Electromagnet Robotic Platform for Ophthalmic Surgery. Micromachines, 14(1), 91. https://doi.org/10.3390/mi14010091






