Numerical Study on the Evaporation of a Non-Spherical Sessile Droplet
Abstract
1. Introduction
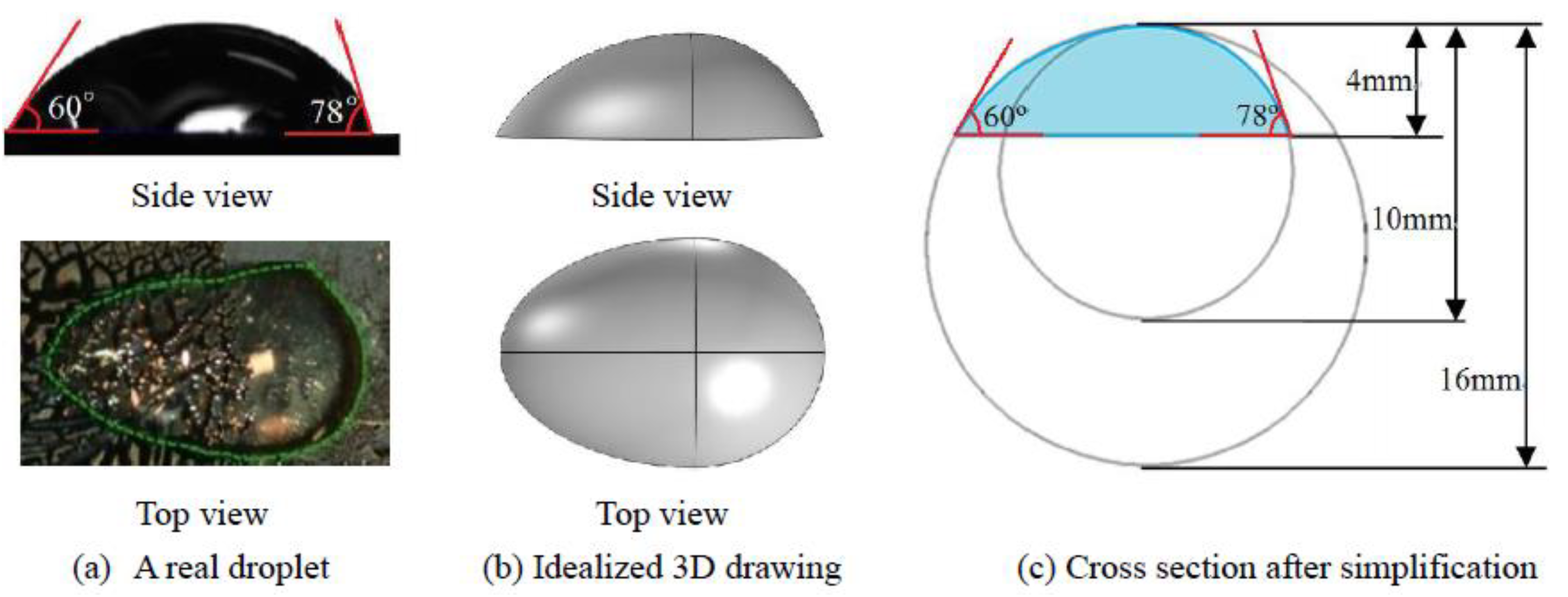
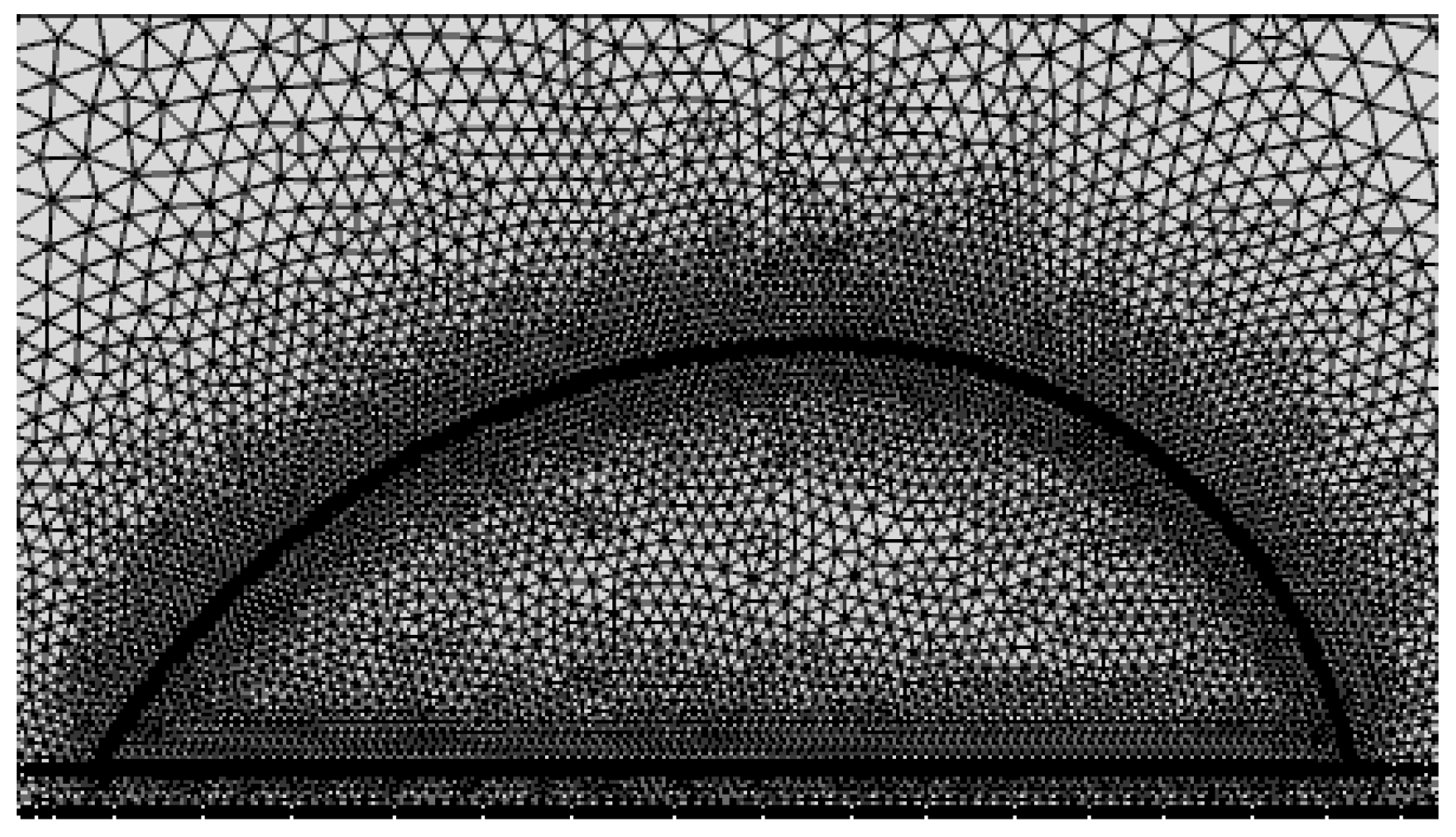
2. Numerical Simulation
2.1. Governing Equations
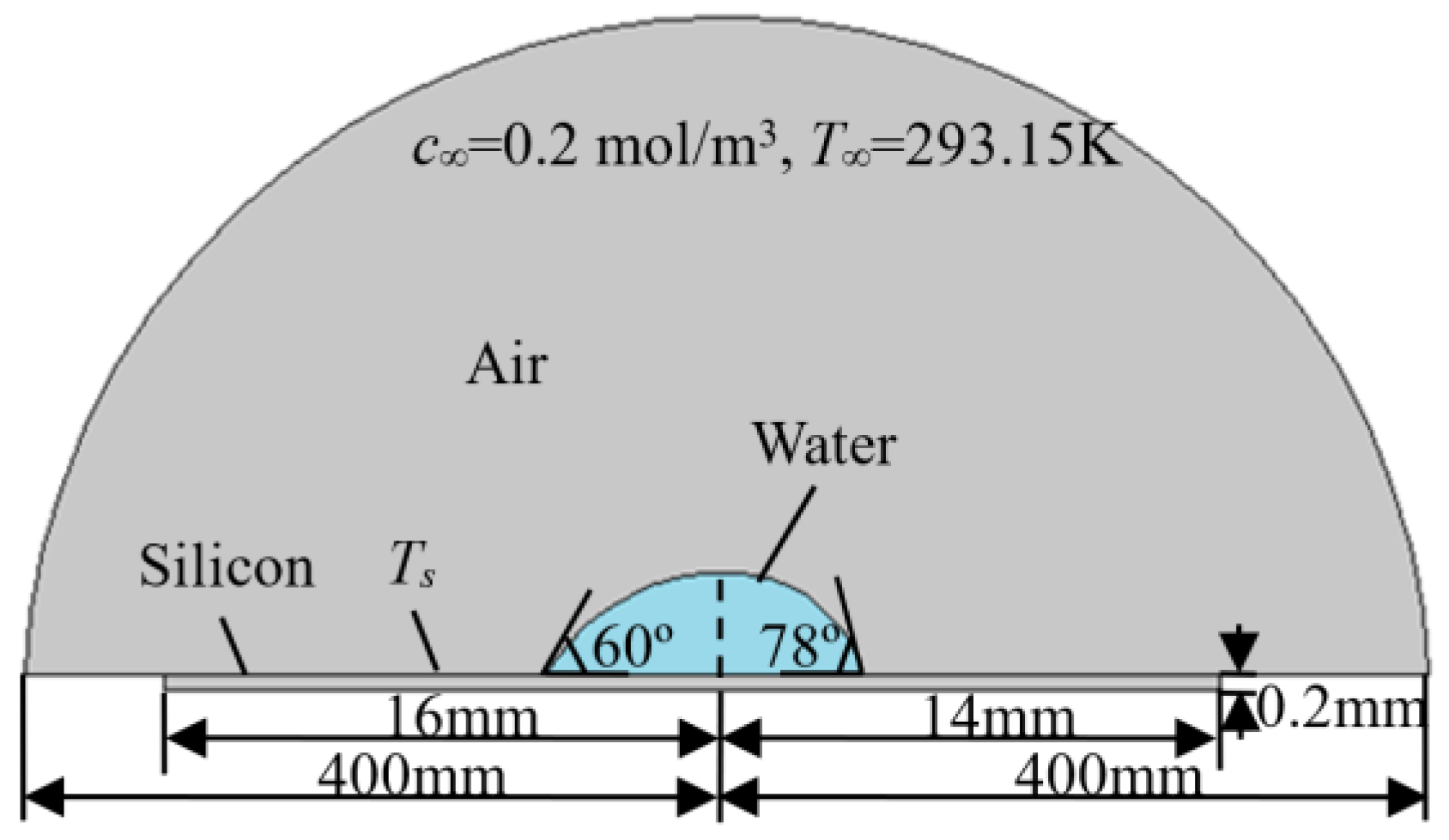
2.2. Boundary Conditions
3. Results and Discussion
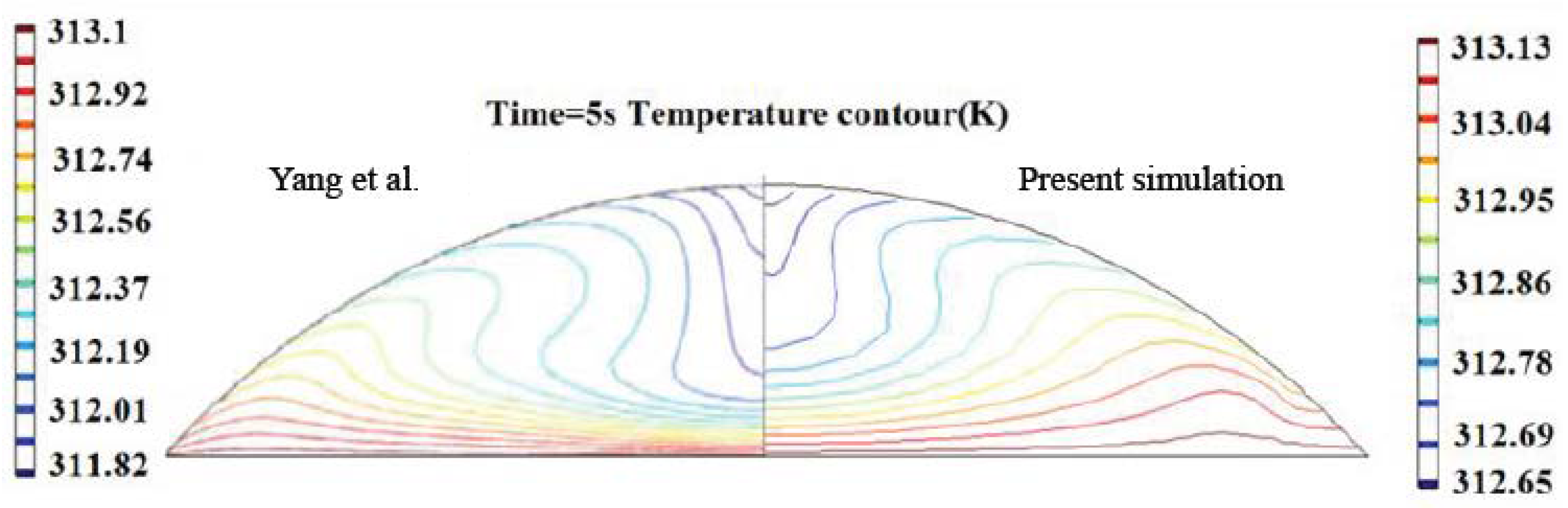
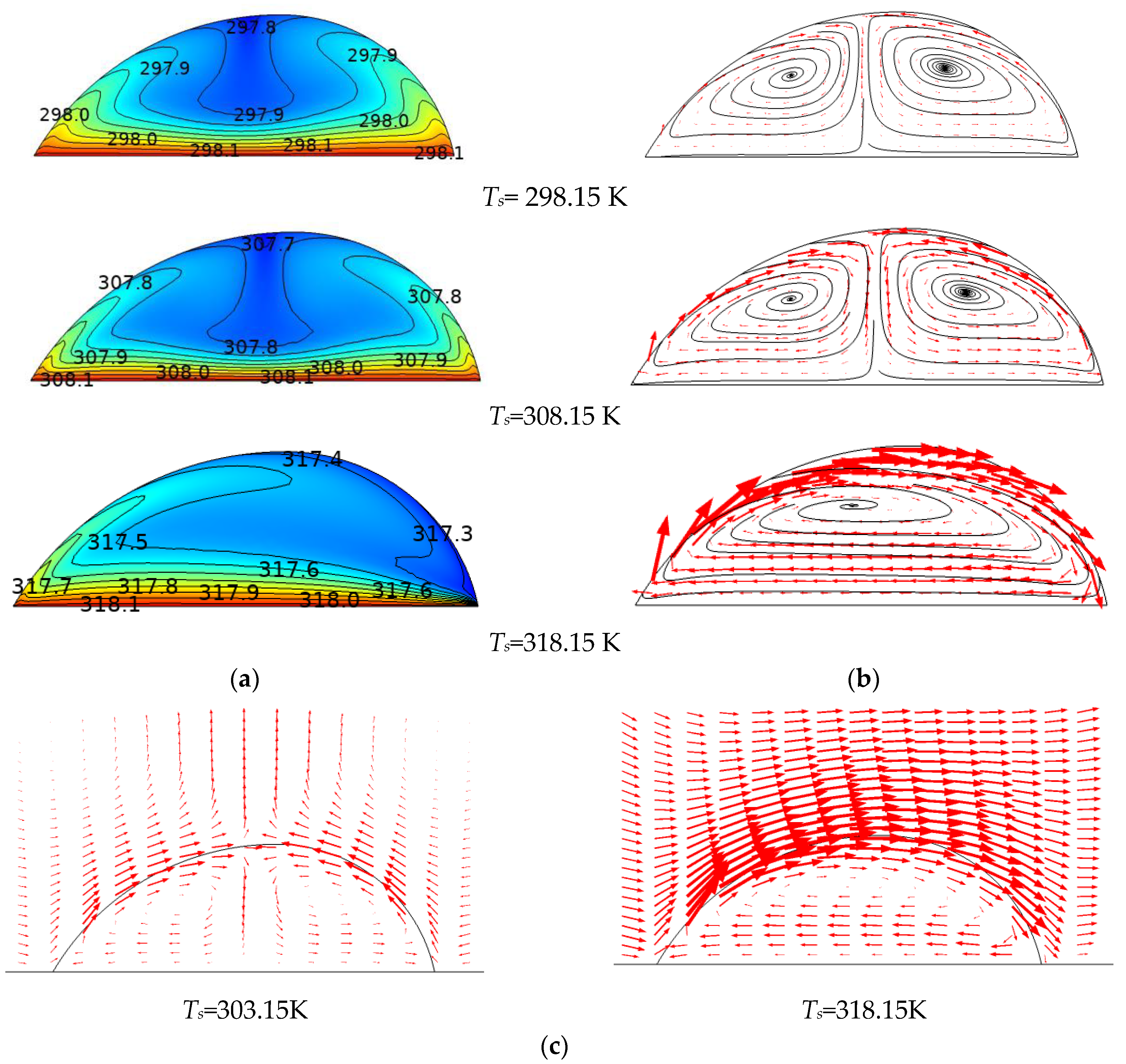
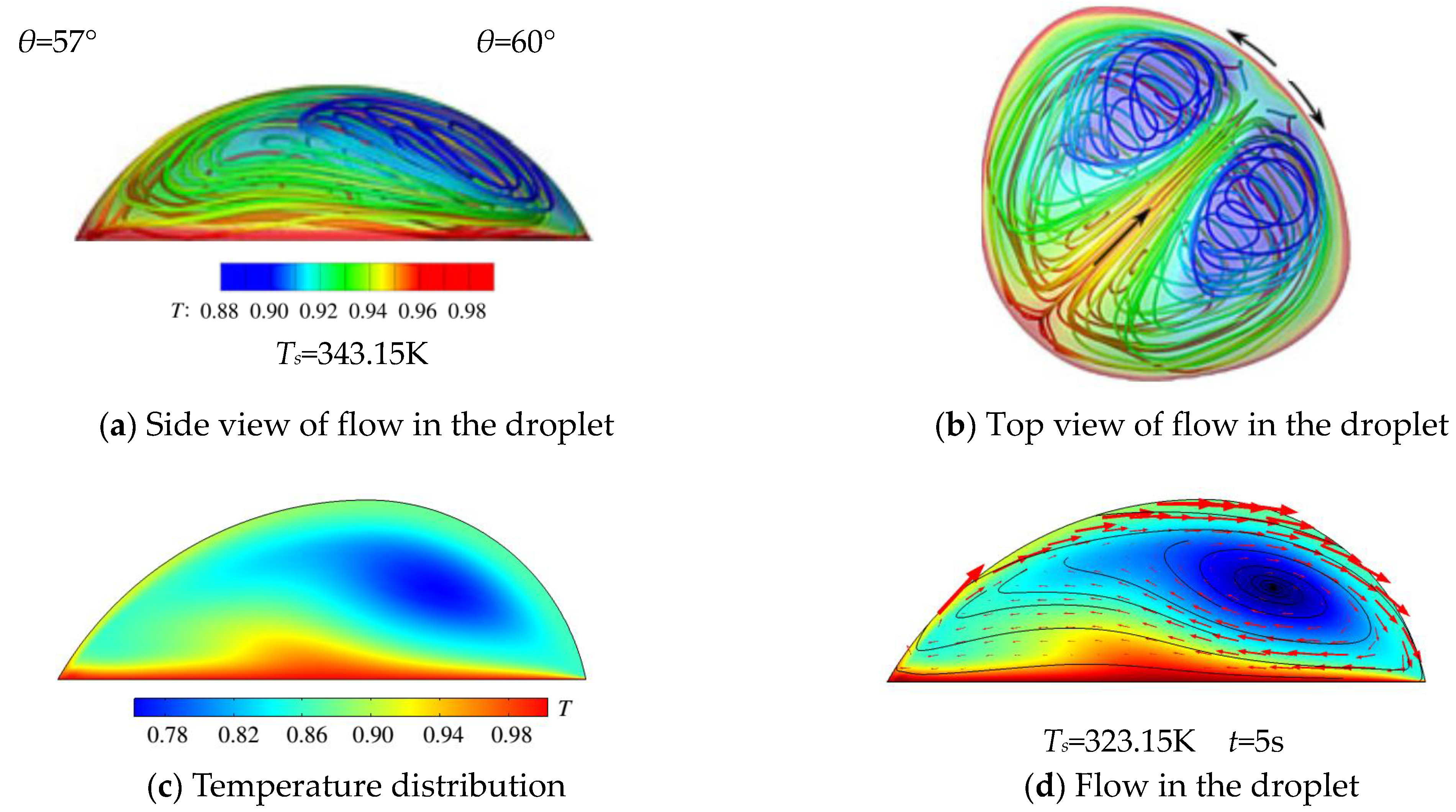
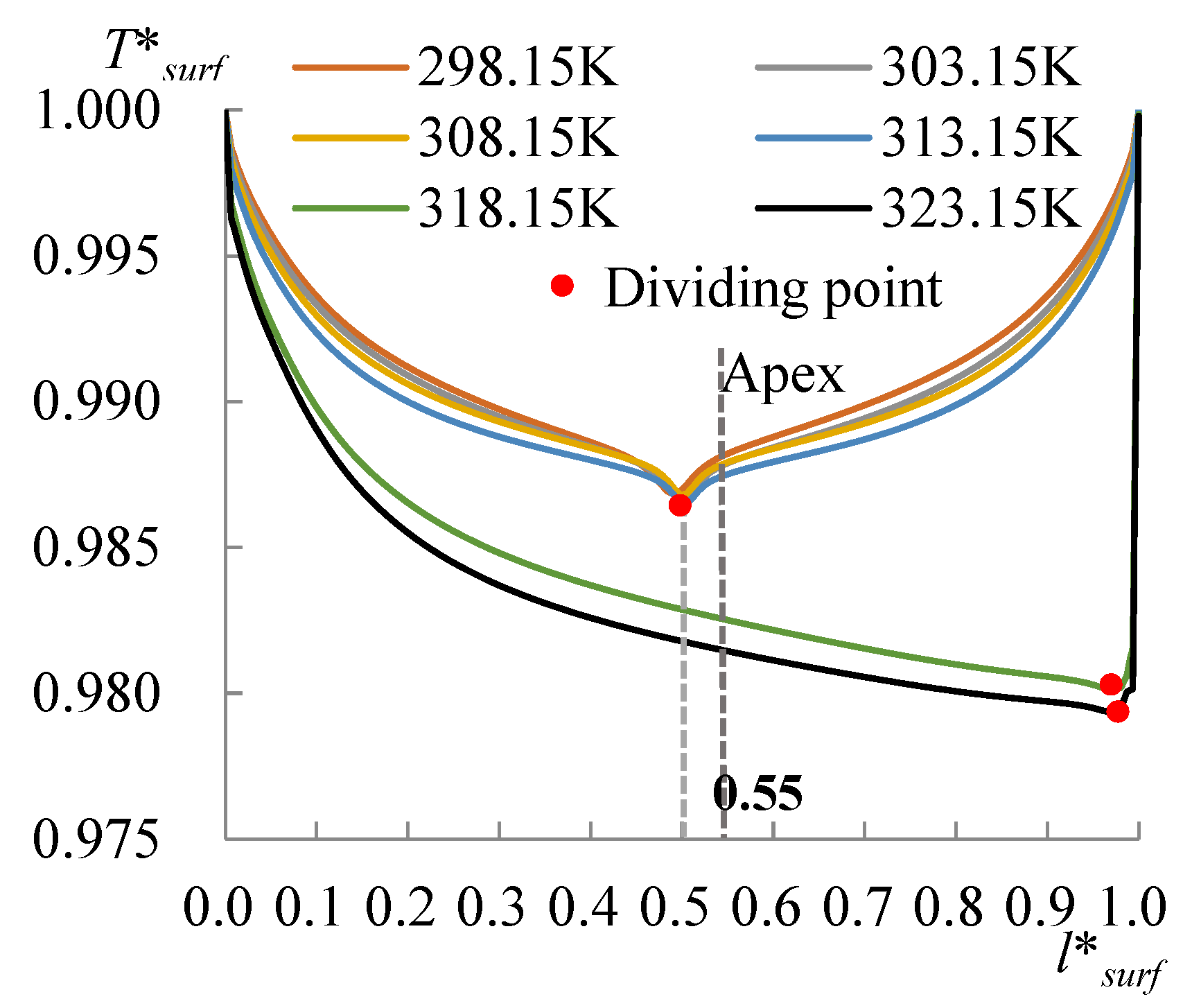
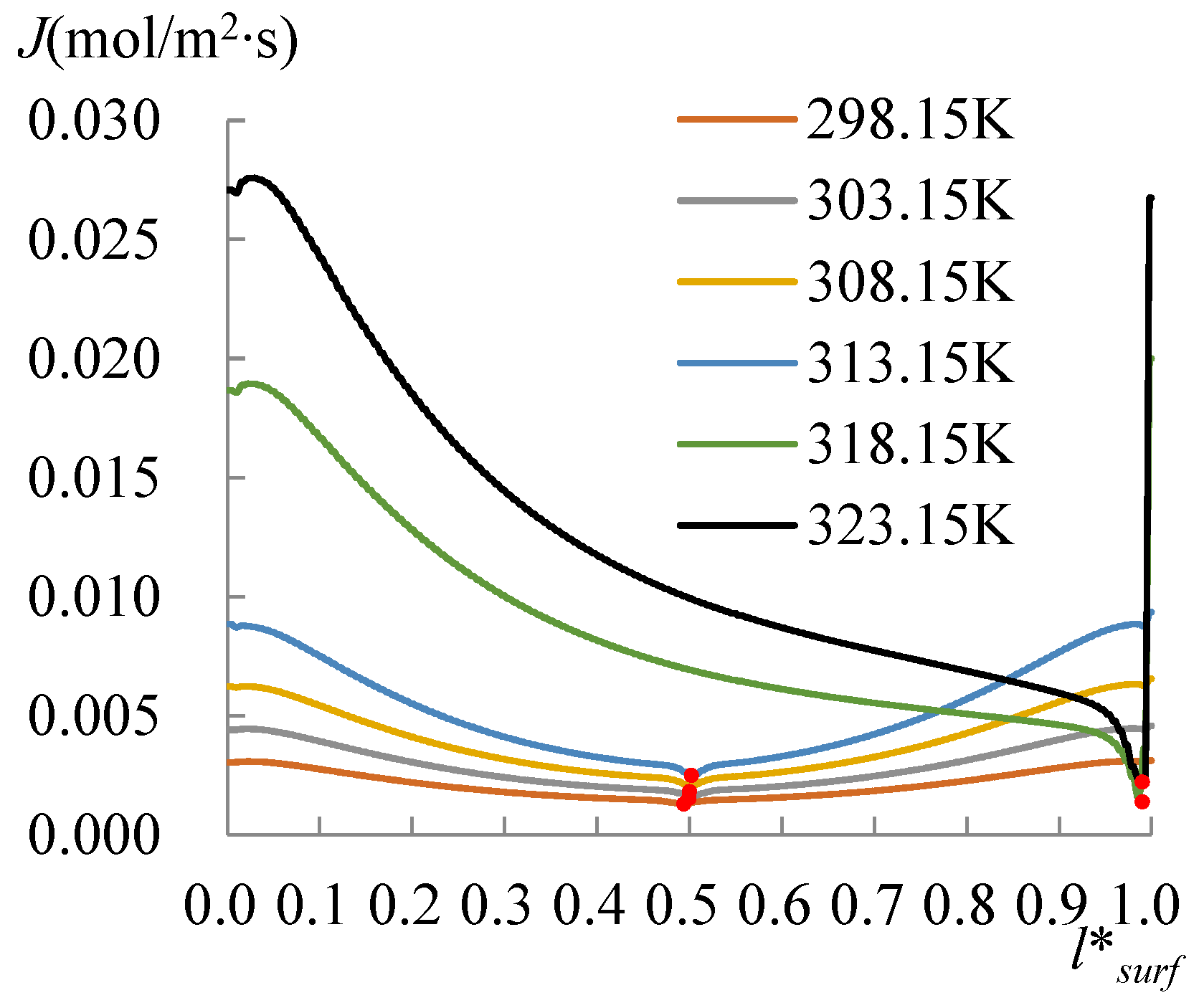
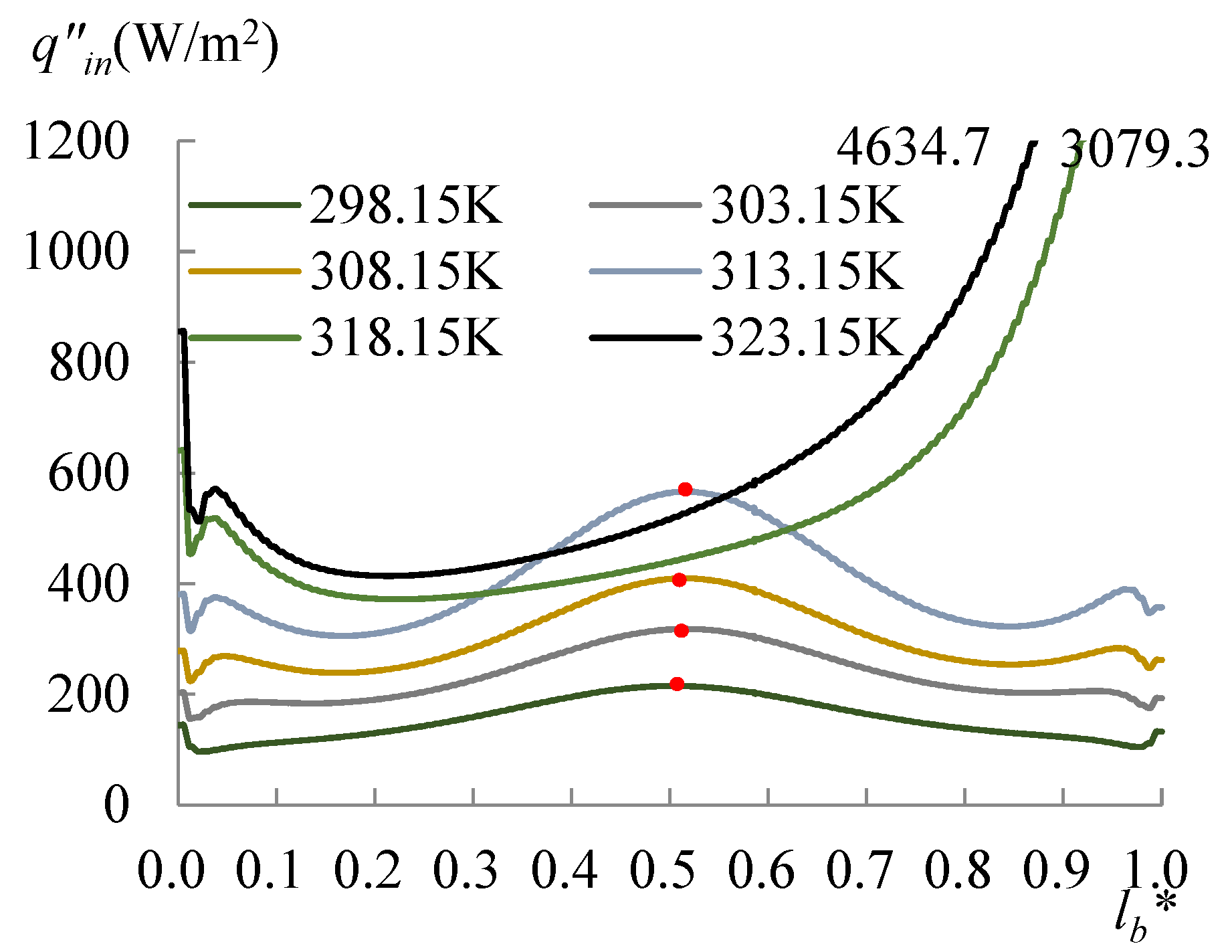
3.1. The Heating Effect
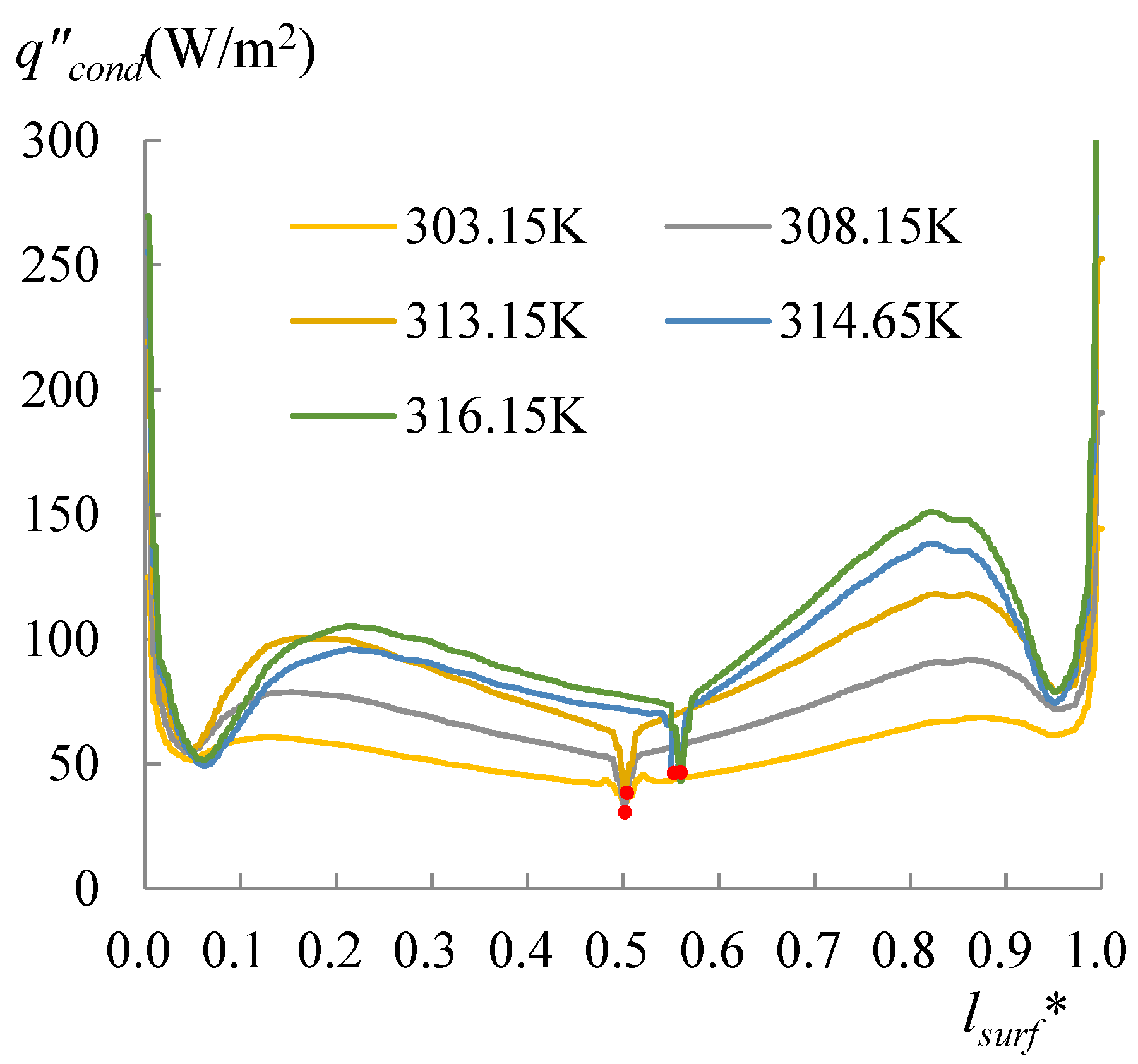
3.2. Comparison with Spherical Droplets of 60° and 78°
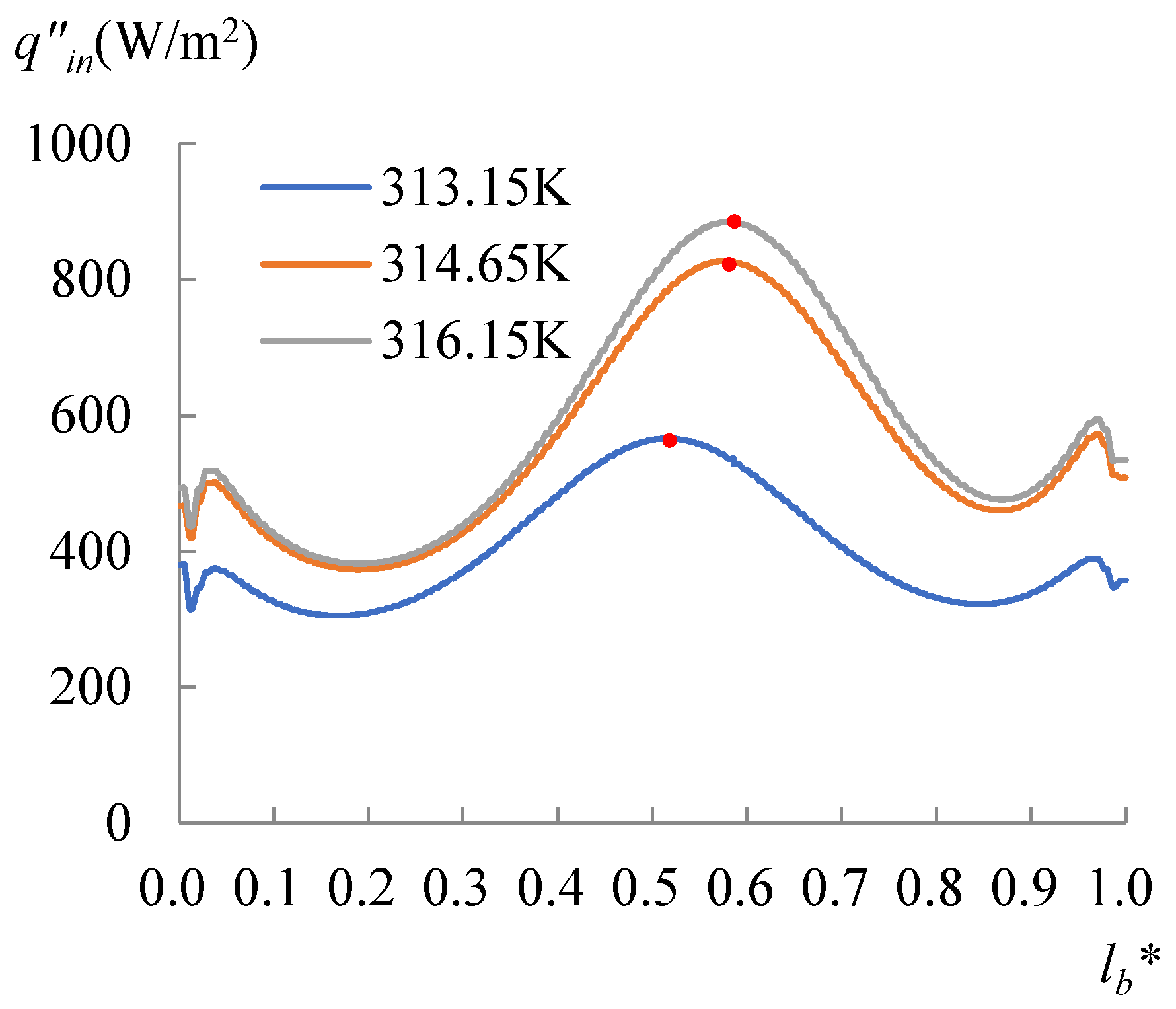
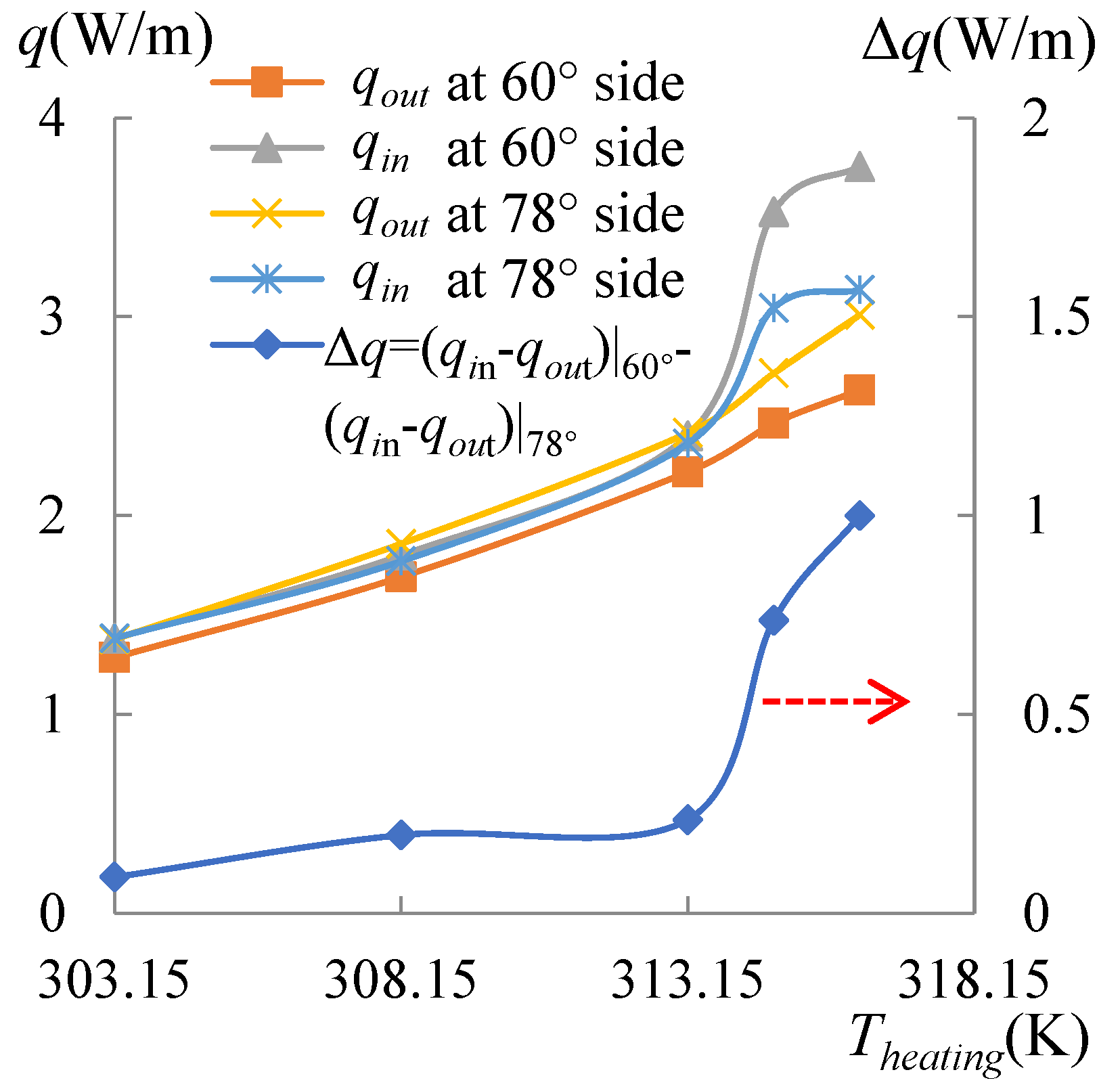
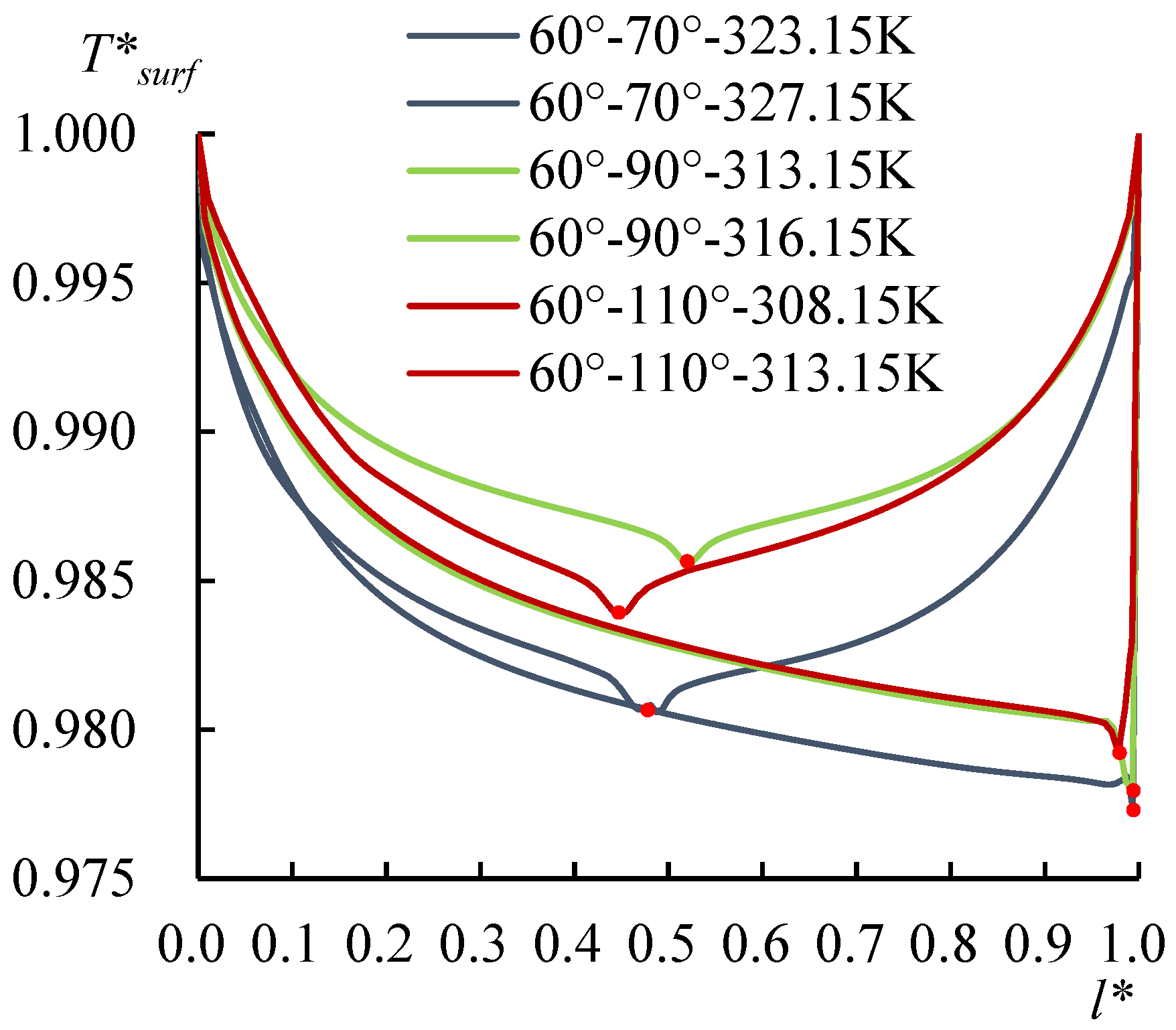
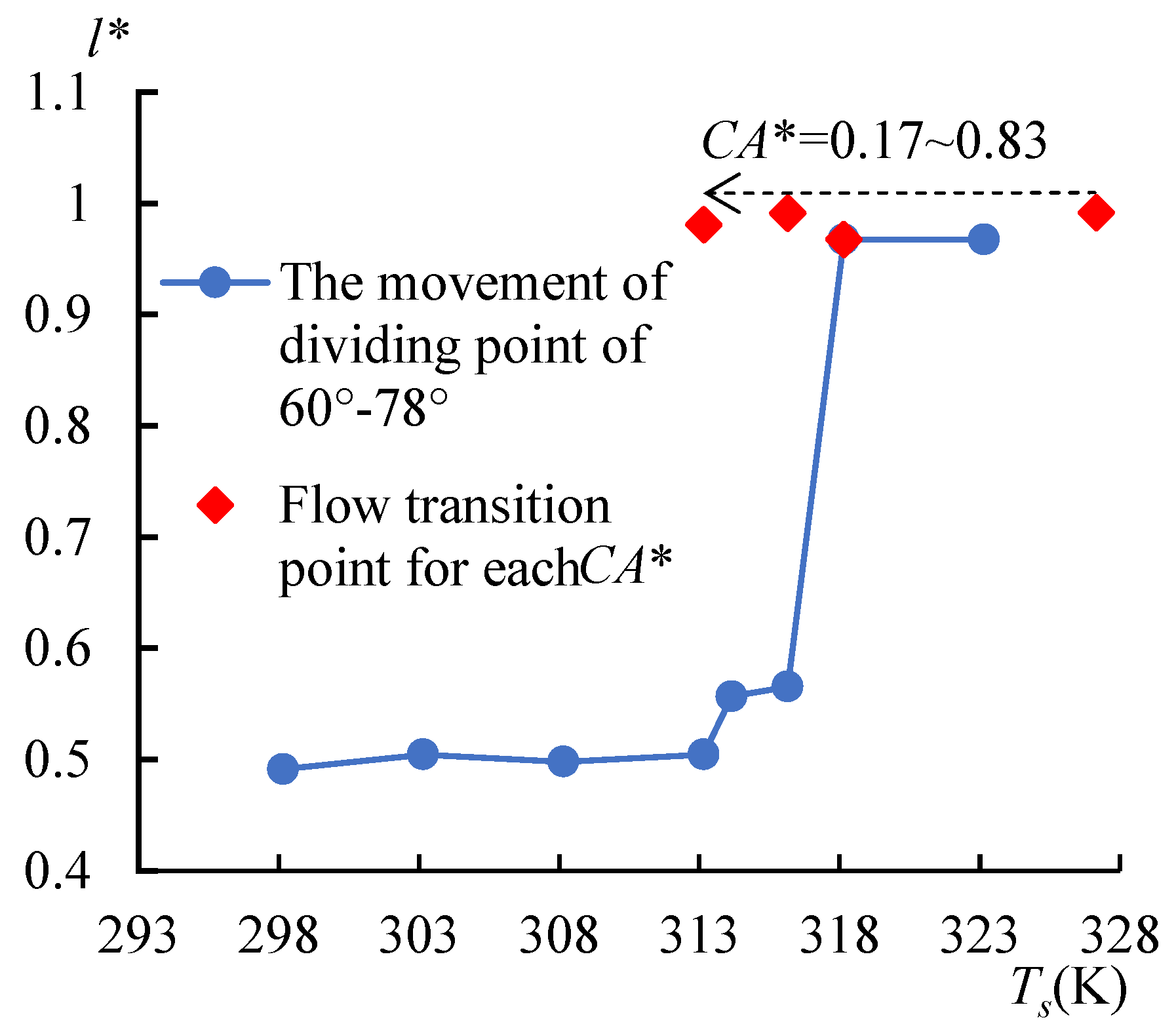
3.3. Contact Angle Discrepancy (CAD) Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| c | molar concentration, mol/m3 |
| CA | contact angle |
| Cp | specific heat at constant pressure, J/(kg·K) |
| D | diffusion coefficient, m2/s |
| fst | force per unit area due to surface tension, N/m2 |
| h | height of a droplet, m |
| hlv | latent heat of evaporation, J/kg |
| J | evaporation flux, mol/(m2·s) |
| k | thermal conductivity, W/(m·K) |
| l | length of liquid–air interface |
| m | local evaporation flux, kg/(m2·s) |
| n | normal vector |
| Mr | molar mass, kg/mol |
| p | pressure, Pa |
| q | heat flow, W/m |
| q″ | heat flux, W/m2 |
| r | radius, m |
| R | universal gas constant, J/(mol·K) |
| t | tangential vector |
| u | velocity in x direction, m/s |
| v | velocity in y direction, m/s |
Greek Symbols
| μ | dynamic viscosity, Pa·s |
| θ | contact angle, ° |
| ρ | density, kg/m3 |
| σ | surface tension, N/m |
| τ | stress tensor, N/m2 |
Subscripts
| av | average |
| b | base |
| c | curvature |
| cond | conduction |
| evap | evaporation |
| l | liquid |
| s | substrate |
| sat | saturated condition |
| surf | surface of droplet |
| v | vapor |
| ∞ | environmental condition |
References
- Liu, L.; Zhang, K.; Liu, H.; Zhang, S.; Mi, M. Experimental study on the interfacial heat transfer of sessile droplet evaporation using temperature-sensitive paint. Exp. Therm. Fluid Sci. 2021, 128, 110436. [Google Scholar] [CrossRef]
- Lohse, D. Fundamental Fluid Dynamics Challenges in Inkjet Printing. Annu. Rev. Fluid Mech. 2022, 54, 349–382. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Yarin, A.L. Drop impact cooling enhancement on nano-textured surfaces. Part I: Theory and results of the ground (1g) experiments. Int. J. Heat Mass Transf. 2014, 70, 1095–1106. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Sinha-Ray, S.; Yarin, A.L.; Weickgenannt, C.M.; Emmert, J.; Tropea, C. Drop impact cooling enhancement on nano-textured surfaces. Part II: Results of the parabolic flight experiments [zero gravity (0g) and supergravity (1.8g)]. Int. J. Heat Mass Transf. 2014, 70, 1107–1114. [Google Scholar] [CrossRef]
- Sobac, B.; Brutin, D. Structural and evaporative evolutions in desiccating sessile drops of blood. Phys. Rev. E 2011, 84, 011603. [Google Scholar] [CrossRef]
- Van Tongeren, S.P.; Degener, J.E.; Harmsen, H.J.M. Comparison of three rapid and easy bacterial DNA extraction methods for use with quantitative real-time PCR. Eur. J. Clin. Microbiol. 2011, 30, 1053–1061. [Google Scholar] [CrossRef]
- Cachile, M.; Bénichou, O.; Poulard, C.; Cazabat, A.M. Evaporating Droplets. Langmuir 2002, 18, 8070–8078. [Google Scholar] [CrossRef]
- Wang, F.-C.; Wu, H.-A. Pinning and depinning mechanism of the contact line during evaporation of nano-droplets sessile on textured surfaces. Soft Matter 2013, 9, 5703–5709. [Google Scholar] [CrossRef]
- Zhang, J.; Müller-Plathe, F.; Leroy, F. Pinning of the Contact Line during Evaporation on Heterogeneous Surfaces: Slowdown or Temporary Immobilization? Insights from a Nanoscale Study. Langmuir 2015, 31, 7544–7552. [Google Scholar] [CrossRef]
- Picknett, R.; Bexon, R. The evaporation of sessile or pendant drops in still air. J. Colloid Interface Sci. 1977, 61, 336–350. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Evaporation of a Sessile Droplet on a Substrate. J. Phys. Chem. B 2002, 106, 1334–1344. [Google Scholar] [CrossRef]
- Rowan, S.M.; Newton, M.I.; McHale, G. Evaporation of Microdroplets and the Wetting of Solid Surfaces. J. Phys. Chem. 1995, 99, 13268–13271. [Google Scholar] [CrossRef]
- Cazabat, A.-M.; Guéna, G. Evaporation of macroscopic sessile droplets. Soft Matter 2010, 6, 2591–2612. [Google Scholar] [CrossRef]
- Deegan, R.D. Pattern formation in drying drops. Phys. Rev. E 2000, 61, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhang, X.; Nguyen, T.-B.; Tran, T. Direct numerical simulation of evaporating droplets based on a sharp-interface algebraic VOF approach. Int. J. Heat Mass Transf. 2022, 184, 122282. [Google Scholar] [CrossRef]
- Tayeb, R.; Zhang, Y. Evaporation induced self-assembly of rough colloids: A multiscale simulation study. Int. J. Heat Mass Transf. 2021, 179, 121681. [Google Scholar] [CrossRef]
- Yang, K.; Hong, F.; Cheng, P. A fully coupled numerical simulation of sessile droplet evaporation using Arbitrary Lagrangian–Eulerian formulation. Int. J. Heat Mass Transf. 2014, 70, 409–420. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, P. Direct numerical simulations of sessile droplet evaporation on a heated horizontal surface surrounded by moist air. Int. J. Heat Mass Transf. 2019, 134, 828–841. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Malla, L.K.; Bhardwaj, R.; Neild, A. Colloidal deposit of an evaporating sessile droplet on a non-uniformly heated substrate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124009. [Google Scholar] [CrossRef]
- De Dier, R.; Sempels, W.; Hofkens, J.; Vermant, J. Thermocapillary Fingering in Surfactant-Laden Water Droplets. Langmuir 2014, 30, 13338–13344. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, P.J.; Sefiane, K.; Kim, J.; Matar, O.K.; Valluri, P. Evaporation of sessile drops: A three-dimensional approach. J. Fluid Mech. 2015, 772, 705–739. [Google Scholar] [CrossRef]
- Wang, T.-S.; Shi, W.-Y. Marangoni instability induced by evaporation in well-defined non-spherical sessile droplet. Int. J. Heat Mass Transf. 2019, 141, 168–179. [Google Scholar] [CrossRef]
- Cui, W.; Dong, J.; Zhao, N.; Liang, D.; Ma, H. Sessile Droplet Evaporation on a Structurally Heterogeneous Multiwalled Carbon Nanotube Membrane. J. Thermophys. Heat Transf. 2020, 34, 150–153. [Google Scholar] [CrossRef]
- Jasper, J.J. The Surface Tension of Pure Liquid Compounds. J. Phys. Chem. Ref. Data 1971, 1, 841–1010. [Google Scholar] [CrossRef]
- Irvine, T.F.; Liley, P.E. Steam and Gas Tables with Computer Equations; Academic Press: New York, NY, USA, 1984. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, W.; Cao, Y.; Wang, S.; Zhang, T.; Ma, H.; Chang, C.; Liang, D.; Dong, J. Numerical Study on the Evaporation of a Non-Spherical Sessile Droplet. Micromachines 2023, 14, 76. https://doi.org/10.3390/mi14010076
Cui W, Cao Y, Wang S, Zhang T, Ma H, Chang C, Liang D, Dong J. Numerical Study on the Evaporation of a Non-Spherical Sessile Droplet. Micromachines. 2023; 14(1):76. https://doi.org/10.3390/mi14010076
Chicago/Turabian StyleCui, Wenbin, Yang Cao, Shoupei Wang, Tianci Zhang, Hongbin Ma, Chao Chang, Dalong Liang, and Jingming Dong. 2023. "Numerical Study on the Evaporation of a Non-Spherical Sessile Droplet" Micromachines 14, no. 1: 76. https://doi.org/10.3390/mi14010076
APA StyleCui, W., Cao, Y., Wang, S., Zhang, T., Ma, H., Chang, C., Liang, D., & Dong, J. (2023). Numerical Study on the Evaporation of a Non-Spherical Sessile Droplet. Micromachines, 14(1), 76. https://doi.org/10.3390/mi14010076







