Hybrid Systems of Nanofibers and Polymeric Nanoparticles for Biological Application and Delivery Systems
Abstract
:1. Introduction
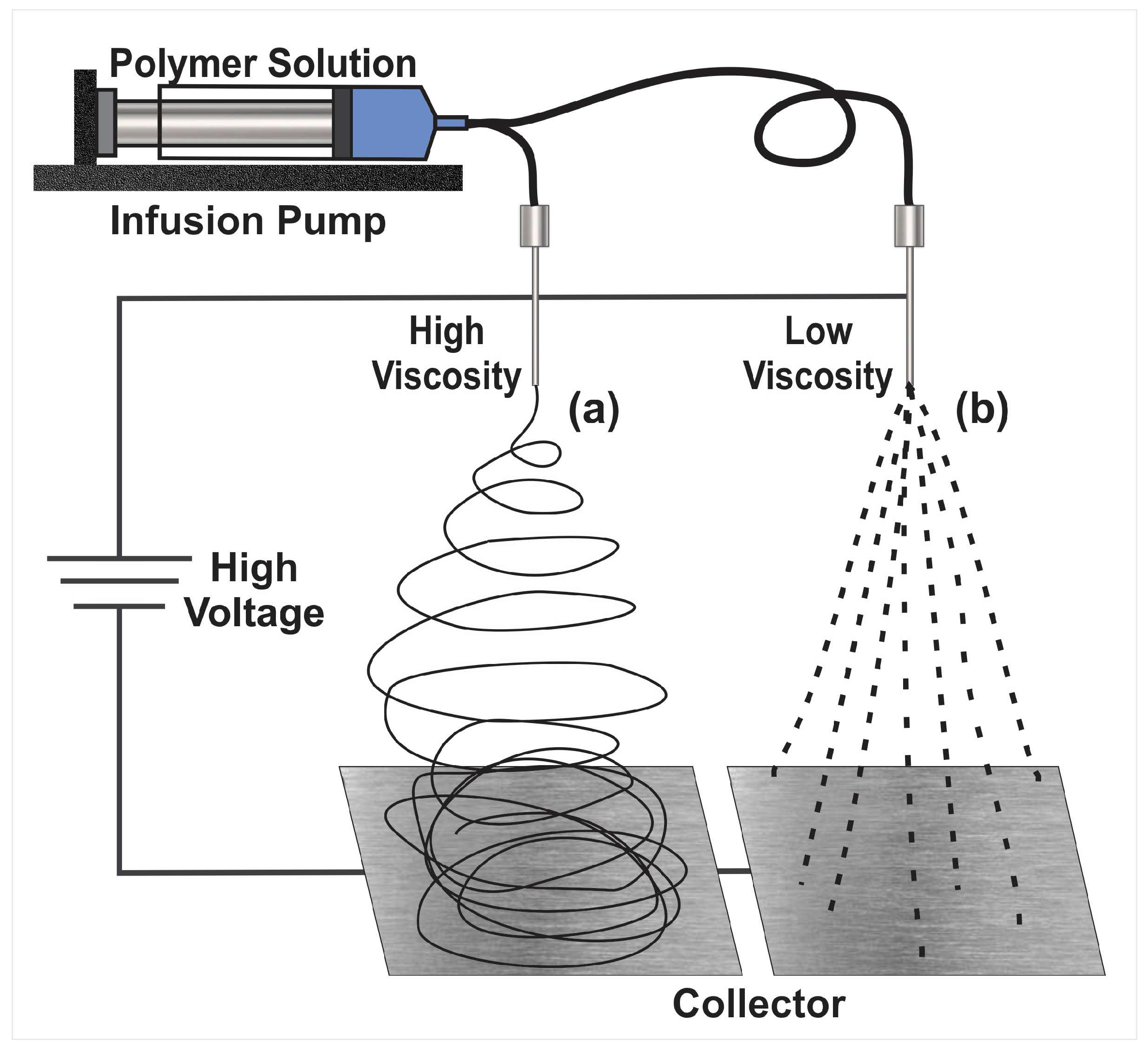
2. The Electrohydrodynamic Method
2.1. Electrospinning
2.2. Limitations in the Electrohydrodynamic Processes
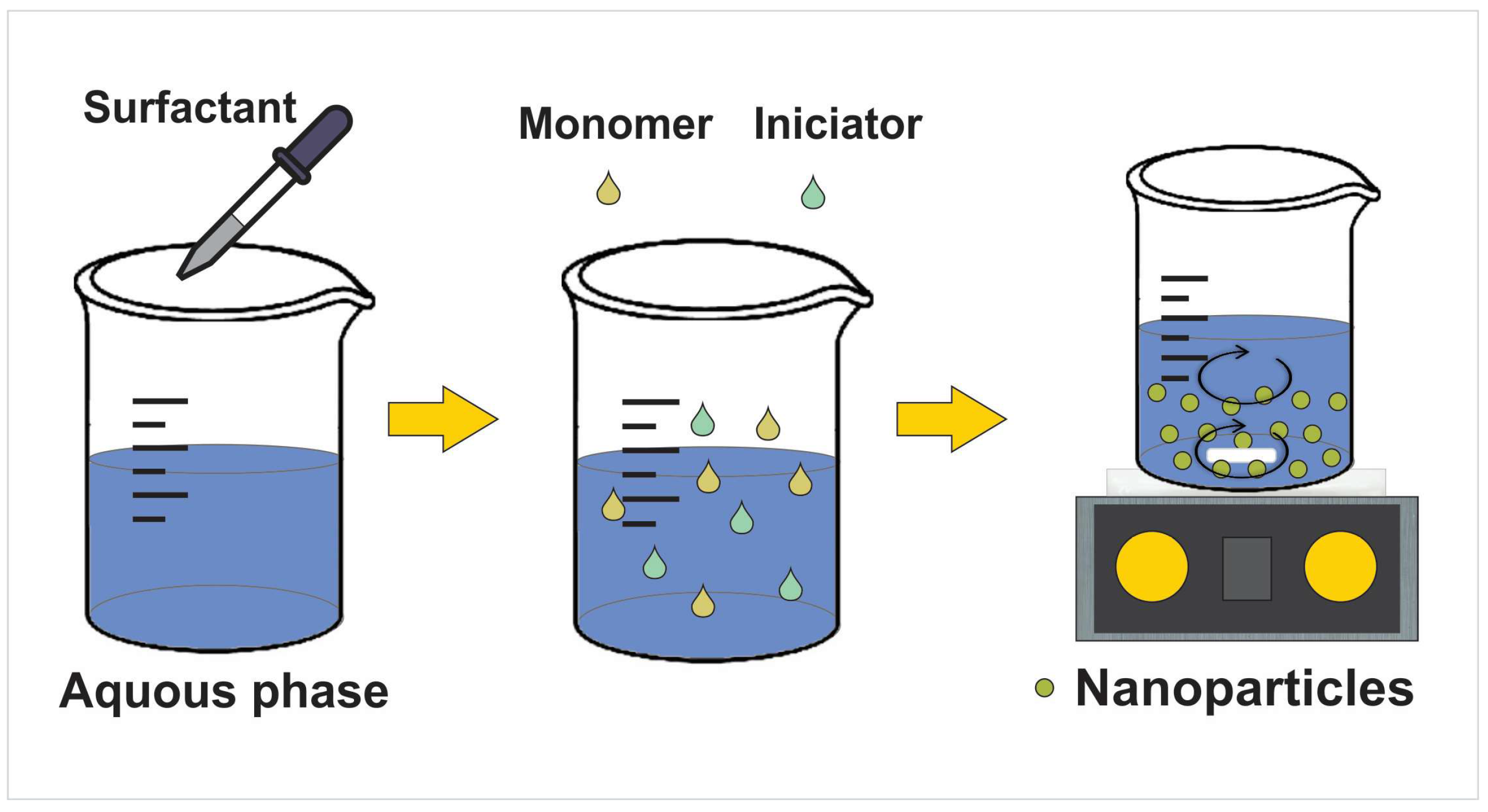
3. Nanoparticles’ Preparation and Drug Loading
| Nanoparticles | Application (Advantage) | Synthesis Method | Size Diameter Nanoparticles | Loading Method and Drug Loaded | References |
|---|---|---|---|---|---|
| Bacterial cellulose whisker (BCW NP) | Increase the filler density in composite nanofibers | Plasma treatment using a microwave oven | Nanoparticles of an average size of 49.1 ± 13 nm | Without drug | [59] |
| functionalized bacterial cellulose whisker (f-BCW NP) | Increase the filler density in composite nanofibers. Increased the glass transition temperature | Plasma treatment using a microwave oven | Nanoparticles of an average size of 49.1 ± 13 nm | Without drug | [59] |
| L-lysine based nanogel | Increased in varying degrees. The response to ammonia | In vitro enzymatic biodegradation of 4-Lys-macrogel | Nanogels size was about 60 nm with a narrow size distribution | Without drug | [33] |
| Chitosan (CS) | Antibacterial activity | Oil-in-water (o/w) type emulsion, ionic gelation | Average particle size of the five nanoparticles ranged from 94.3 ± 2.1 to 246.1 ± 6.3 nm with the in- crease of chitosan concentration. | Oil-in-water (o/w) type emulsion. Moringa oil | [60] |
| Chitosan | Improvement in the morphology, thermal, mechanical, antibacterial properties and cytobiocompatibiliy Biocidal agents to potentiate the wound healing process | Nanoparticles thermally synthesized with chitosan acting as both a capping and reducing agent | Average particle size of 53.6 ± 20.5 nm | Thermally synthesized Silver | [56] |
| Chitosan | Wound dressing, wound healing with antibacterial, antioxidant, and cell proliferation properties | Electrosprayed | Curcumin (CUR) loaded into chitosan nanoparticles average diameter: 32.17 nm | Nanoencapsulation of curcumin by emulsified curcumin-chitosan solution | [57] |
| Poly(N-isopropylacrylamide-acrylic acid) | Promote the wound healing process to achieve higher wound healing efficacy | Free radical nanoprecipitation polymerization | Average size ~100 nm | Without drug | [61] |
| Lignin | Cell viability and differentiation, along with neurite length extension were promoted | Commercially available | Average size ~90 nm | Without drug | [62] |
| Lignin | Bone tissue engineering | Precipitation, via a simultaneous pH and solvent shifting technology | Average size count at 100–200 nm range | Without drug | [63] |
| Keratin | Potential neural tissue applications | Electrospray deposition | Average size 250–350 nm range | Without drug | [64] |
| Carboxymethyl-hexanoyl/chitosan | Increased its cytotoxic effect in melanoma cells | Ionotropic gelation | Average size of 32.6 ± 1.2 nm | Pyrazoline. In situ | [65] |
| Chitosan-aniline nanogels | Bactericide | Complexation-reduction | Average size of 78 ± 19 nm | Silver nanoparticles In situ | [66] |
| Chitosan-tripolyphosphate | Encapsulation and release of therapeutic proteins | Ionotropic gelation | Average size 194 ± 3 nm | Transforming Growth Factor β3 (TGF-β3) In situ | [67] |
| Carboxymethyl chitosan | Exhibits antibacterial properties and promotes skin wound healing | Electrostatic droplet | Average size 164.6 ± 5 nm | Encapsulates antibacterial peptide (OH-CATH30). In situ | [23] |
| Chitosan | Vaginal controlled release of benzydamine | Ionic gelation | Average size 128–710 nm range. (Depending on the formulation) | Benzydamine. In situ | [68] |
| Chitosan | Antibacterial. Encapsulation of antibacterial agent | Ionic gelation | Average size 10–25 nm range | Antibiotics (e.g., ciprofloxacin, ofloxacin, levoxacin, gemifloxacin). Absorption | [69] |
| Poly (methacrylic acid) | Peripheral nerve regeneration. | Suspension polymerization. Molecular imprinted | Average size of 80–115 nm range | 4-aminopyridine (4-AP). Entrapping the 4-AP molecules via hydrogen bond formation. | [54] |
| Poly (lactic-co-glycolide) | Topical vaginal drug delivery | Nanoprecipitation. Passive PEGylation with Pluronic® | Average size 172 ± 19 nm | Rhodamine, directly conjugated. Antiretroviral drug etravirine (ETR). In situ | [70] |
| Chitosan | Antibacterial, mucoadhesive, encapsulation of drug | Ionic gelation | Average size ~300–400 nm | Epinephrine. In situ | [71] |
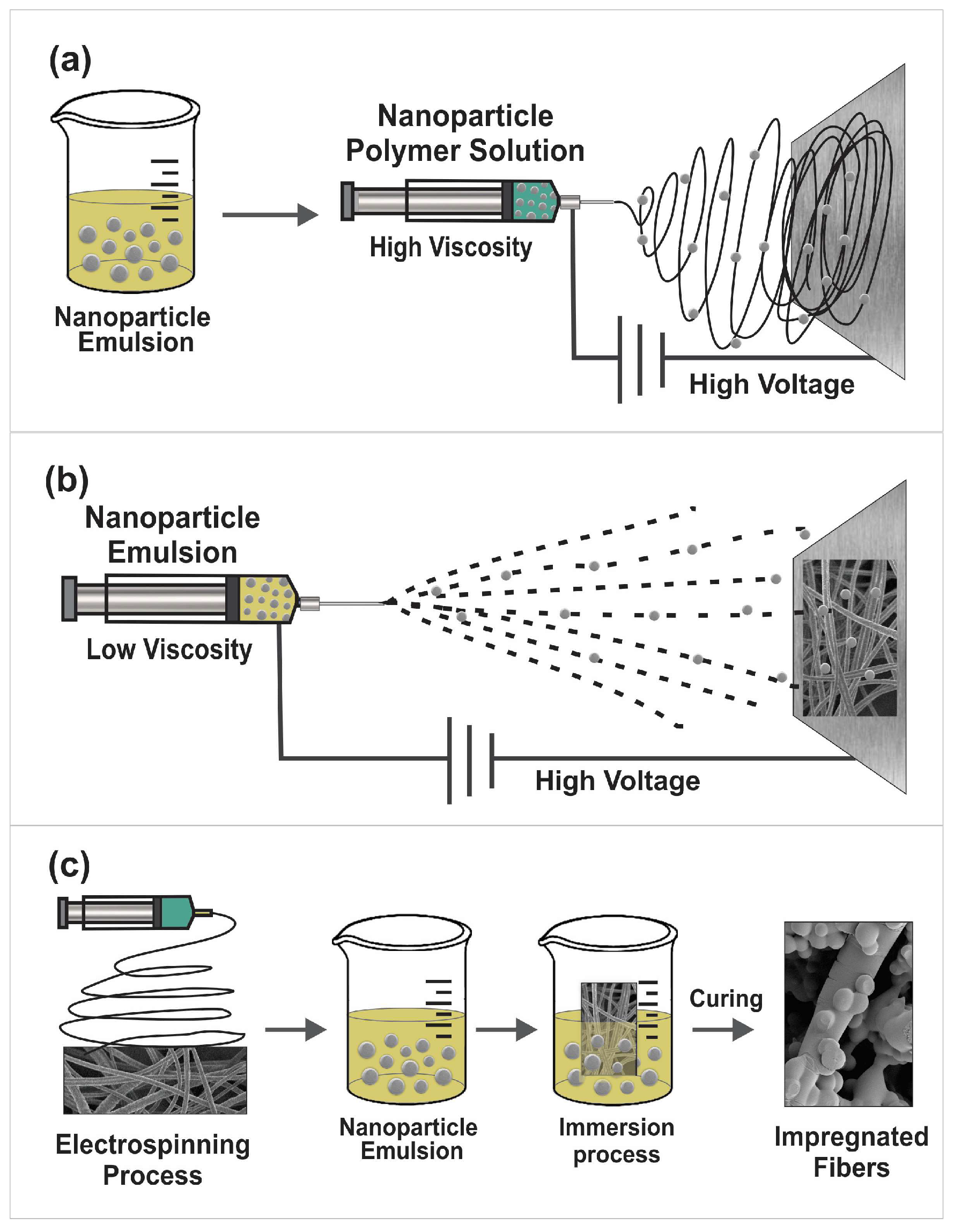
4. Incorporation of Nanoparticles into Nanofibers
5. Hybrid Systems for Control of Reactive Oxygen Species for Tissue Regeneration
6. Materials Used in Hybrid Systems
6.1. Poly(D,L-Lactide-co-Glycolide) (PLGA)
6.2. Keratin/Poly (Vinyl Alcohol) (PVA)
6.3. Chitosan
6.4. Poly(Vinyl Alcohol) (PVA)
6.5. Poly(Vinyl Pyrrolidone) (PVP)
6.6. Poly(Caprolactone) (PCL)
6.7. Poly(Vinylidene Fluoride) (PVDF)
6.8. Polymer Mixtures
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: A comprehensive review. Int. J. Nanomedicine 2020, 15, 2563–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikam, A.P.; Ratnaparkhiand, M.P.; Chaudhari, S.P. Nanoparticles—An overview. Int. J. Res. Dev. Pharm. Life Sci. 2017, 3, 1121–1127. [Google Scholar]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Bravo, J.M.; Villarreal-Gomez, L.J.; Serrano-Medina, A. Electrospinning for Drug Delivery Systems: Drug Incorporation Techniques. In Electrospinning: Material, Techniques, and Biomedical Applications; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef] [Green Version]
- Puttananjegowda, K.; Takshi, A.; Thomas, S. Silicon carbide nanoparticles electrospun nanofibrous enzymatic glucose sensor. Biosens. Bioelectron. 2021, 186, 113285. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nano fi bers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Jafarpour, M.; Aghdam, A.S.; Koşar, A.; Cebeci, F.Ç.; Ghorbani, M. Electrospinning of ternary composite of PMMA-PEG-SiO2 nanoparticles: Comprehensive process optimization and electrospun properties. Mater. Today Commun. 2021, 29, 102865. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Citation: Polymer-Based Nanofiber-Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Fang, K.; Liu, S.; Wu, J.; Luo, J.; Tyler, R.; Zhang, S. OQL036 topical gel inhibits the skin toxicity associated with 5-fluorouracil/capecitabine: Results from in vitro and in vivo preclinical studies. Eur. J. Cancer 2022, 174, S77–S78. [Google Scholar] [CrossRef]
- Liu, W.; Bi, W.; Sun, Y.; Wang, L.; Yu, X.; Cheng, R.; Yu, Y.; Cui, W. Biomimetic organic-inorganic hybrid hydrogel electrospinning periosteum for accelerating bone regeneration. Mater. Sci. Eng. C 2020, 110, 110670. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Raza, Z.A.; Nazir, A. Current applications of electrospun polymeric nanofibers in cancer therapy. Mater. Sci. Eng. C 2019, 97, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.F.; Poletto, F.; Eberhardt, M.J.; Domingues, S.C.; De Sousa, F.B.; Tebaldi, M.L. Polymer-hybrid nanosystems for antiviral applications: Current advances. Biomed. Pharmacother. 2021, 146, 112249. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Aguirre, Y.; Aguado-Castrejón, K.; González-Méndez, I. La nanomedicina y los sistemas de liberación de fármacos: ¿la (r)evolución de la terapia contra el cáncer? Educ. Quim. 2016, 27, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Sayin, S.; Tufani, A.; Emanet, M.; Genchi, G.G.; Sen, O.; Shemshad, S.; Ozdemir, E.; Ciofani, G.; Ozaydin Ince, G. Electrospun Nanofibers With pH-Responsive Coatings for Control of Release Kinetics. Front. Bioeng. Biotechnol. 2019, 7, 309. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Encapsulation of β-carotene into food-grade nanofibers via coaxial electrospinning of hydrocolloids: Enhancement of oxidative stability and photoprotection. Food Hydrocoll. 2022, 133, 107949. [Google Scholar] [CrossRef]
- Sharif, N.; Golmakani, M.T.; Niakousari, M.; Ghorani, B.; Lopez-Rubio, A. Food-grade gliadin microstructures obtained by electrohydrodynamic processing. Food Res. Int. 2019, 116, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Khoshnoudi-Nia, S.; Sharif, N.; Jafari, S.M. Loading of phenolic compounds into electrospun nanofibers and electrosprayed nanoparticles. Trends Food Sci. Technol. 2020, 95, 59–74. [Google Scholar] [CrossRef]
- Batty, C.J.; Gallovic, M.D.; Williams, J.; Ross, T.M.; Bachelder, E.M.; Ainslie, K.M. Multiplexed electrospray enables high throughput production of cGAMP microparticles to serve as an adjuvant for a broadly acting influenza vaccine. Int. J. Pharm. 2022, 622, 121839. [Google Scholar] [CrossRef]
- Chen, N.; Gan, Y.; Luo, Y.; Jiang, Z. A review on the technology development and fundamental research of electrospray combustion of liquid fuel at small-scale. Fuel Process. Technol. 2022, 234, 107342. [Google Scholar] [CrossRef]
- Zhou, H.; Modi, S.; Biswas, P. Controlled synthesis of charged lignin nanocarriers by electrospray. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129314. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, L.; Ying, D.; Jia, J.; Shao, J. Sustainable electrospray polymerization fabrication of thin-film composite polyamide nanofiltration membranes for heavy metal removal. Desalination 2022, 539, 115952. [Google Scholar] [CrossRef]
- Zou, P.; Lee, W.H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- ur Rehman Khan, A.; Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Multifunctional bioactive core-shell electrospun membrane capable to terminate inflammatory cycle and promote angiogenesis in diabetic wound. Bioact. Mater. 2021, 6, 2783–2800. [Google Scholar] [CrossRef]
- Khodadadi, M.; Alijani, S.; Montazeri, M.; Esmaeilizadeh, N.; Sadeghi-Soureh, S.; Pilehvar-Soltanahmadi, Y. Recent advances in electrospun nanofiber-mediated drug delivery strategies for localized cancer chemotherapy. J. Biomed. Mater. Res. Part A 2020, 108, 1444–1458. [Google Scholar] [CrossRef]
- Christy, P.N.; Basha, S.K.; Kumari, V.S.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K.; Arasu, M.V.; Al-Dhabi, N.A.; Ignacimuthu, S. Biopolymeric nanocomposite scaffolds for bone tissue engineering applications—A review. J. Drug Deliv. Sci. Technol. 2020, 55, 101452. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Gómez, L.J.; Serrano-Medina, A.; Torres-Martínez, E.J.; Perez-González, G.L.; Cornejo-Bravo, J.M. Polymeric advanced delivery systems for antineoplasic drugs: Doxorubicin and 5-fluorouracil. e-Polymers 2018, 18, 359–372. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, X.; Qiu, X.; Gao, T.; Yu, W.; Zhang, M.; Song, L.; Liu, D.; Dong, J.; Jiang, Z.; et al. Bioactive composite Janus nanofibrous membranes loading Ciprofloxacin and Astaxanthin for enhanced healing of full-thickness skin defect wounds. Appl. Surf. Sci. 2023, 610, 155290. [Google Scholar] [CrossRef]
- Ismail, H.M.; Ali-Adib, S.; Younes, H.M. Reactive and functionalized electrospun polymeric nanofibers for drug delivery and tissue engineering applications. Ther. Deliv. 2019, 10, 397–399. [Google Scholar] [CrossRef]
- Xu, Q.; Ensign, L.M.; Boylan, N.J.; Schön, A.; Gong, X.; Yang, J.C.; Lamb, N.W.; Cai, S.; Yu, T.; Freire, E.; et al. Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9, 9217–9227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.Q.; Wu, L.L.; Cui, H.C.; Zhang, H.N.; Yu, J.Y. A rapid ammonia sensor based on lysine nanogel-sensitized PANI/PAN nanofibers. J. Mater. Chem. B 2016, 4, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B 2016, 4, 4801–4812. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.S.; Nelson, M.T.; Xue, R.; DeJesus, J.K.; Viapiano, M.S.; Lannutti, J.J.; Sarkar, A.; Winter, J.O. Mimicking white matter tract topography using core-shell electrospun nanofibers to examine migration of malignant brain tumors. Biomaterials 2013, 34, 5181–5190. [Google Scholar] [CrossRef] [Green Version]
- Carlisle, C.R.; Coulais, C.; Namboothiry, M.; Carroll, D.L.; Hantgan, R.R.; Guthold, M. The mechanical properties of individual, electrospun fibrinogen fibers. Biomaterials 2009, 30, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Sharpe, J.M.; Lee, H.; Hall, A.R.; Bonin, K.; Guthold, M. Mechanical properties of electrospun, blended fibrinogen: PCL nanofibers. Nanomaterials 2020, 10, 1843. [Google Scholar] [CrossRef]
- Ventola, C.L.; Bharali, D.J.; Mousa, S.A. The Nanomedicine Revolution: Part 1: Emerging Concepts. Pharmacy and Therapeutics. Pharmacol. Ther. 2010, 128, 512–525. [Google Scholar]
- Sultana, F.; Manirujjaman; Imran-Ul-Haque; Arafat, M.; Sharmin, S. An overview of nanogel drug delivery system. J. Appl. Pharm. Sci. 2013, 3, 95–105. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Peres, L.B.; Peres, L.B.; Faria, T.J.; De Assis, J.V.; de Almeida, M.V.; Gonçalves, O.H.; de Araújo, P.H.; Sayer, C. PLLA/PMMA blend in polymer nanoparticles: Influence of processing methods. Colloid Polym. Sci. 2017, 295, 1621–1633. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate nanoformulation: Influence of process and selected variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef]
- Urbaniak, T.; Musiał, W. Influence of solvent evaporation technique parameters on diameter of submicron lamivudine-poly-ε-caprolactone conjugate particles. Nanomaterials 2019, 9, 1240. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.L.; Gratieri, T.; Cunha-Filho, M.; Gelfuso, G.M. Polymeric nanocapsules: A review on design and production methods for pharmaceutical purpose. Methods 2022, 199, 54–66. [Google Scholar] [CrossRef]
- Elzayat, A.; Adam-Cervera, I.; Álvarez-Bermúdez, O.; Muñoz-Espí, R. Nanoemulsions for synthesis of biomedical nanocarriers. Colloids Surf. B Biointerfaces 2021, 203, 111764. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Henriquez, G.; Narayan, M. Enhancing the delivery of chemotherapeutics: Role of biodegradable polymeric nanoparticles. Molecules 2018, 23, 2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallah-Darrehchi, M.; Zahedi, P.; Safarian, S.; Ghaffari-Bohlouli, P.; Aeinehvand, R. Conductive conduit based on electrospun poly (L-lactide-co-D, L-lactide) nanofibers containing 4-aminopyridine-loaded molecularly imprinted poly (methacrylic acid) nanoparticles used for peripheral nerve regeneration. Int. J. Biol. Macromol. 2021, 190, 499–507. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Abdelhakim, H.E. Drug Delivery Applications of Coaxial Electrospun Nanofibres in Cancer Therapy. Molecules 2022, 27, 1803. [Google Scholar] [CrossRef]
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-drug delivery of Ag-chitosan nanoparticles and phenytoin via core-shell PVA/PCL electrospun nanofibers. Carbohydr. Polym. 2021, 270, 118373. [Google Scholar] [CrossRef]
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-rad, E.; Moslehi, M.; Ganji, A. Wound healing performance of PCL / chitosan based electrospun nanofiber electrosprayed with curcumin loaded chitosan nanoparticles. Carbohydr. Polym. 2021, 259, 117640. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yoon, O.J. Thermal Characteristics of Polyethylene Oxide and Functionalized Bacterial Cellulose Whisker Nanoparticle Composite Nanofibers. Macromol. Res. 2016, 24, 973–979. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Qiu, X.; Jiao, A.; Luo, W.; Lin, X.; Zhang, X.; Zhang, Z.; Hong, J.; Cai, P.; et al. Bioactive Materials Incorporating redox-sensitive nanogels into bioabsorbable nanofibrous membrane to acquire ROS-balance capacity for skin regeneration. Bioact. Mater. 2021, 6, 3461–3472. [Google Scholar] [CrossRef]
- Amini, S.; Saudi, A.; Amirpour, N.; Jahromi, M.; Najafabadi, S.S.; Kazemi, M.; Rafienia, M.; Salehi, H. Application of electrospun polycaprolactone fibers embedding lignin nanoparticle for peripheral nerve regeneration: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 159, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.K.; Kharaghani, D.; Sun, L.; Ullah, S.; Sarwar, M.N.; Ullah, A.; Khatri, M.; Yoshiko, Y.; Gopiraman, M.; Kim, I.S. Synthesized bioactive lignin nanoparticles/polycaprolactone nanofibers: A novel nanobiocomposite for bone tissue engineering. Biomater. Adv. 2023, 144, 213203. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yang, X.; Deng, J.; Zhu, L.; Wang, B.; Hao, S. Keratin nanoparticles-coating electrospun PVA nano fi bers for potential neural tissue applications. J. Mater. Sci. Mater. Med. 2019, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Rengifo, A.F.; Stefanes, N.M.; Toigo, J.; Mendes, C.; Argenta, D.F.; Dotto, M.E.; da Silva, M.C.; Nunes, R.J.; Caon, T.; Parize, A.L.; et al. PEO-chitosan nano fi bers containing carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanoparticles loaded with pyrazoline for skin cancer treatment. Eur. Polym. J. 2019, 119, 335–343. [Google Scholar] [CrossRef]
- Ballesteros, C.A.S.; Correa, D.S.; Zucolotto, V. Polycaprolactone nanofiber mats decorated with photoresponsive nanogels and silver nanoparticles: Slow release for antibacterial control. Mater. Sci. Eng. C 2020, 107, 110334. [Google Scholar] [CrossRef]
- Sydow, S.; De Cassan, D.; Hänsch, R.; Gengenbach, T.R.; Easton, C.D.; Thissen, H.; Menzel, H. Layer-by-layer deposition of chitosan nanoparticles as drug-release coatings for PCL nanofibers. Biomater. Sci. 2019, 7, 233–246. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef]
- Pour Khalili, N.; Parsa, M.; Moradi, R. Polyvinyl alcohol nanofibers encompass Chitosan/Tripolyphosphate nanogels for controlled release of gemifloxacin antibiotic. Mater. Today Proc. 2022, 65, 2920–2925. [Google Scholar] [CrossRef]
- Krogstad, E.A.; Ramanathan, R.; Nhan, C.; Kraft, J.C.; Blakney, A.K.; Cao, S.; Ho, R.J.Y.; Woodrow, K.A. Nanoparticle-releasing nanofiber composites for enhanced in vivo vaginal retention. Biomaterials 2017, 144, 1–16. [Google Scholar] [CrossRef]
- Atashgahi, M.; Ghaemi, B.; Valizadeh, A.; Moshiri, A.; Nekoofar, M.H.; Amani, A. Epinephrine-entrapped chitosan nanoparticles covered by gelatin nanofibers: A bi-layer nano-biomaterial for rapid hemostasis. Int. J. Pharm. 2021, 608, 121074. [Google Scholar] [CrossRef]
- Lan, T.; Shao, Z.Q.; Wang, J.Q.; Gu, M.J. Fabrication of hydroxyapatite nanoparticles decorated cellulose triacetate nanofibers for protein adsorption by coaxial electrospinning. Chem. Eng. J. 2015, 260, 818–825. [Google Scholar] [CrossRef]
- Shetty, K.; Bhandari, A.; Yadav, K.S. Nanoparticles incorporated in nanofibers using electrospinning: A novel nano-in-nano delivery system. J. Control. Release 2022, 350, 421–434. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, B.; Jie, Q.; Wei, B.Y.; Fan, J.; Zhang, H.Y.; Zhang, J.K.; Li, X.J.; Shi, J.; Luo, Z.J.; et al. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone 2014, 66, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced drug delivery systems containing herbal components for wound healing. Int. J. Pharm. 2022, 617, 121617. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Yang, I.H.; Deen, G.R.; Mo, X. Engineering PCL/lignin nanofibers as an antioxidant scaffold for the growth of neuron and Schwann cell. Colloids Surf. B Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.J.; Lin, Q.; Jiang, L.; Zhang, D.; Li, Z.; Ma, B.; Zhang, C.; Li, L.; Kai, D.; et al. Lignin-Incorporated Nanogel Serving As an Antioxidant Biomaterial for Wound Healing. ACS Appl. Bio Mater. 2021, 4, 3–13. [Google Scholar] [CrossRef]
- Hérou, S.; Bailey, J.J.; Kok, M.; Schlee, P.; Jervis, R.; Brett, D.J.L.; Shearing, P.R.; Ribadeneyra, M.C.; Titirici, M. High-Density Lignin-Derived Carbon Nanofiber Supercapacitors with Enhanced Volumetric Energy Density. Adv. Sci. 2021, 8, 2100016. [Google Scholar] [CrossRef]
- He, Y.; Qin, L.; Fang, Y.; Dan, Z.; Shen, Y.; Tan, G.; Huang, Y.; Ma, C. Electrospun PLGA nanomembrane: A novel formulation of extended- release bupivacaine delivery reducing postoperative pain. Mater. Des. 2020, 193, 108768. [Google Scholar] [CrossRef]
- Malik, R.; Garg, T.; Goyal, A.K.; Rath, G. Polymeric nanofibers: Targeted gastro-retentive drug delivery systems. J. Drug Target. 2015, 23, 109–124. [Google Scholar] [CrossRef]
- Han, C.; Cai, N.; Chan, V.; Liu, M.; Feng, X.; Yu, F. Enhanced drug delivery, mechanical properties and antimicrobial activities in poly(lactic acid) nanofiber with mesoporous Fe3O4-COOH nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 559, 104–114. [Google Scholar] [CrossRef]
- Rathinamoorthy, R. Nanofiber for drug delivery system—Principle and application. Pakistan Text. J. 2012, 16, 45–48. [Google Scholar]
- de Guzman, R.C.; Merrill, M.R.; Richter, J.R.; Hamzi, R.I.; Greengauz-Roberts, O.K.; Van Dyke, M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 2011, 32, 8205–8217. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martinez, E.J.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; Pérez-González, G.L.; Villarreal-Gómez, L.J. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.; Ajji, A. Core—Shell Structured PEO-Chitosan Nanofibers by Coaxial Electrospinning. Biomacromolecules 2012, 13, 412–421. [Google Scholar] [CrossRef]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan’ s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Severyukhina, A.N.; Petrova, N.V.; Yashchenok, A.M.; Bratashov, D.N.; Smuda, K.; Mamonova, I.A.; Yurasov, N.A.; Puchinyan, D.M.; Georgieva, R.; Bäumler, H.; et al. Light-induced antibacterial activity of electrospun chitosan-based material containing photosensitizer. Mater. Sci. Eng. C 2017, 70, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, M.; Zolfagharzadeh, V.; Bagher, Z.; Soltani, H.; Ai, J. Cell encapsulation in core-shell microcapsules through coaxial electrospinning system and horseradish peroxidase-catalyzed crosslinking. Biomed. Phys. Eng. Express 2020, 6, 015022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ma, Y.; Xu, X.; Ji, Q.; Feng, M.; Cheng, C.; Feng, Y.; He, B.; Mo, R. Enzyme-instructed hybrid nanogel/nanofiber oligopeptide hydrogel for localized protein delivery. Acta Pharm. Sin. B 2021, 11, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Antiqueira-Santos, P.; Teixeira, W.K.; Flores, A.F.; Piovesan, L.A.; Nery, L.E.; de Souza Votto, A.P. Synthesis of pyrazoline fatty chain derivatives and its effects on melanoma cells. Bioorg. Med. Chem. Lett. 2021, 41, 127988. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shao, G.; Ren, F.; Yang, M.; Nie, Y.; Peng, Q.; Zhang, P. Cerium oxide nanoparticle-loaded polyvinyl alcohol nanogels delivery for wound healing care systems on surgery. Drug Deliv. 2021, 28, 390–399. [Google Scholar] [CrossRef]
- Li, R.; Cheng, Z.; Yu, X.; Wang, S.; Han, Z.; Kang, L. Preparation of antibacterial PCL/PVP-AgNP Janus nanofibers by uniaxial electrospinning. Mater. Lett. 2019, 254, 206–209. [Google Scholar] [CrossRef]
- Sundhari, D.; Dhineshbabu, N.R.; Sutha, S.; Raja Saravanan, M.E. Encapsulation of bioactive agent (Curcumin, Moringa) in electrospun nanofibers—Some insights into recent research trends. Mater. Today Proc. 2021, 46, 2682–2685. [Google Scholar] [CrossRef]
- Samadi, A.; Salati, M.A.; Safari, A.; Jouyandeh, M.; Barani, M.; Singh Chauhan, N.P.; Golab, E.G.; Zarrintaj, P.; Kar, S.; Seidi, F.; et al. Comparative review of piezoelectric biomaterials approach for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2022, 33, 1555–1594. [Google Scholar] [CrossRef]
- Ghaffari-Bohlouli, P.; Zahedi, P.; Shahrousvand, M. Enhanced osteogenesis using poly (L-lactide-co-D,L-lactide)/poly (acrylic acid) nanofibrous scaffolds in presence of dexamethasone-loaded molecularly imprinted polymer nanoparticles. Int. J. Biol. Macromol. 2020, 165, 2363–2377. [Google Scholar] [CrossRef]
- Philip, P.; Tomlal Jose, E.; Chacko, J.K.; Philip, K.C.; Thomas, P.C. Preparation and characterisation of surface roughened PMMA electrospun nanofibers from PEO-PMMA polymer blend nanofibers. Polym. Test. 2019, 74, 257–265. [Google Scholar] [CrossRef]
- Ge, G.; Wang, Q.; Zhang, Y.Z.; Alshareef, H.N.; Dong, X. 3D Printing of Hydrogels for Stretchable Ionotronic Devices. Adv. Funct. Mater. 2021, 31, 2107437. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Y.Z.; Fang, S.; Sun, Y.; Niu, J.; Lai, W.Y. Wireless Human-Machine Interface Based on Artificial Bionic Skin with Damage Reconfiguration and Multisensing Capabilities. ACS Appl. Mater. Interfaces 2022, 14, 47300–47309. [Google Scholar] [CrossRef] [PubMed]
- Haghniaz, R.; Kim, H.J.; Montazerian, H.; Baidya, A.; Tavafoghi, M.; Chen, Y.; Zhu, Y.; Karamikamkar, S.; Sheikhi, A.; Khademhosseini, A. Tissue adhesive hemostatic microneedle arrays for rapid hemorrhage treatment. Bioact. Mater. 2023, 23, 314–327. [Google Scholar] [CrossRef]
- Hong, Y.; Zou, J.; Ge, G.; Xiao, W.; Gao, L.; Shao, J.; Dong, X. Finite element modeling simulation-assisted design of integrated microfluidic chips for heavy metal ion stripping analysis. J. Phys. D. Appl. Phys. 2017, 50, 415303. [Google Scholar] [CrossRef]
- Huang, X.; Ge, G.; She, M.; Ma, Q.; Lu, Y.; Zhao, W.; Shen, Q.; Wang, Q.; Shao, J. Self-healing hydrogel with multiple dynamic interactions for multifunctional epidermal sensor. Appl. Surf. Sci. 2022, 598, 153803. [Google Scholar] [CrossRef]
- Palomino, K.; Magaña, H.; Meléndez-López, S.G.; Cornejo-Bravo, J.M.; Serrano-Medina, A.; Alatorre-Meda, M.; Rodríguez-Velázquez, E.; Rivero, I. Loading and release of a model high-molecular-weight protein from temperature-sensitive micro-structured hydrogels. MRS Commun. 2019, 9, 1041–1045. [Google Scholar] [CrossRef]
- Palomino, K.; Cornejo-Bravo, J.M.; Magaña, H.; Serrano-Medina, A. Microstructured hydrogels with modulated transition temperature for positive control release. Dig. J. Nanomater. Biostructures 2018, 13, 141–154. [Google Scholar]
- Palomino, K.; Suarez-Meraz, K.A.; Serrano-Medina, A.; Olivas, A.; Samano, E.C.; Cornejo-Bravo, J.M. Microstructured poly(N-isopropylacrylamide) hydrogels with fast temperature response for pulsatile drug delivery. J. Polym. Res. 2015, 22, 199. [Google Scholar] [CrossRef]
- Torres-Martínez, E.J.; Pérez-González, G.L.; Serrano-Medina, A.; Grande, D.; Vera-Graziano, R.; Cornejo-Bravo, J.M.; Villarreal-Gómez, L.J. Drugs Loaded into Electrospun Polymeric Nanofibers for Delivery. J. Pharm. Pharm. Sci. 2019, 22, 313–331. [Google Scholar] [CrossRef]

| Nanoparticles | Nanofibers | Applications | Method | Size Diameter Nanoparticles | Size Diameter Nanofibers | References |
|---|---|---|---|---|---|---|
| Bacterial cellulose whisker (BCW NP) and functionalized bacterial cellulose whisker (f-BCW NP) | Poly (ethylene oxide) (PEO) | Improving the thermal characteristics of bacterial cellulose whisker for biological applications. | Electrospinning. Plasma treatment using a microwave oven. | Nanoparticles of an average size of 49.1 ± 13 nm, concentrations of nanoparticles (0.05, 0.1, 0.3, and 0.5 wt%) | The diameter of PEO nanofibers was 0.37 ± 0.09 μm. The diameter of PEO/BCW NP and PEO/f-BCW NP composite nanofibers was about 0.38 ± 0.12 μm and 0.46 ± 0.08 (0.05%) 0.41 ± 0.21 and 0.57 ± 0.07 μm (0.1%). Showed heterogeneous nanostructures with various sizes and sometimes bead-like structure (0.3% y 0.5%) | [59] |
| L-lysine based nanogel | Poly (aniline)/poly (acrylonitrile) (PANI/PAN) | Ammonia sensor based | Electrospinning. Enzyme biodegradation | Nanogels size was about 60 nm with a narrow size distribution | The diameters of the fibers PANI/PAN 250–300 average | [33] |
| Chitosan (CS) | Gelatin (type B from porcine skin) | Biocontrol of Listeria monocytogenes and Staphylococcus aureus on cheese | Electrospinning. Emulsion and ionic gelation | Average particle size of the five nanoparticles ranged from 94.3 ± 2.1 to 246.1 ± 6.3 nm with the increase of chitosan concentration | The thickness of the gelatin nanofibers was 0.102 ± 0.004 mm | [60] |
| Chitosan | Core-shell PVA/PCL | Cell proliferation and antibacterial activity for tissue regeneration and wound healing. | Coaxial electrospinning. Nanoparticles Thermally synthesized with chitosan acting as both a capping and reducing agent | Average particle size of 53.6 ± 20.5 nm | The diameters of the fibers 70 nm average | [57] |
| Chitosan | (PCL)/Chitosan | Wound dressing, wound healing with antibacterial, antioxidant, and cell proliferation properties. | Electrospinning. Electrosprayed | Chitosan nanoparticles average diameter: 25.46 nm and curcumin (CUR) loaded into chitosan nanoparticles average diameter: 32.17 nm. | PCL nanofiber (mean diameter 214.9 nm) PCL/CS nanofiber (mean diameter 115.6 nm). PCL/CS/CUR nanofiber (mean diameter 100.08 nm). PCL/CS/CUR electrosprayed with CURCSNPs (mean diameter 99.84 nm). | [57] |
| PNA | PLLA | Promote the wound healing process to achieve higher wound healing efficacy | Airbrushing. Radical emulsion polymerization | Average size ~100 nm | PLLA nanofiber diameters of 100–500 nm | [61] |
| Lignin | PCL | Potential to be applied for nerve regeneration | Electrospinning. Kraft lignin nanoparticles (Mw = 9000; Sigma-Aldrich; Germany) | Average size ~90 nm | The mean diameter of 0, 5, 10 and 15% Lignin fibers was 269 ± 60, 291 ± 82, 334 ± 86, and 364 ± 97 nm, respectively | [62] |
| Lignin | PCL | Bone tissue engineering | Electrospinning. Simultaneous pH and solvent shifting | Average size count at 100–200 nm range | Diameter of the nanofibers, ranging from 400 to 2200 nm | [63] |
| Keratin | PVA | Potential neural tissue applications | electrospinning and electrospray deposition | Average size 250–350 nm range | No information | [64] |
| Carboxymethyl-hexanoyl chitosan | PEO-chitosan | Skin cancer treatment | Electrospinning. Ionotropic Gelation | Average size of 32.6 ± 1.2 nm | The average size of the PEO-CS nanofibers was 157.1 ± 5.0 nm. With nanoparticles 197.8 ± 4.1 nm) | [65] |
| Silver nanoparticles, Chitosan-aniline nanogels | PCL | Antibacterial Applications | Electrospinning complexation-reduction, ionotropic Gelation | Average size of 78 ± 19 nm | The nanofibers presented diameters of 240 ± 70 nm | [66] |
| Chitosan | PCL | Drug delivery system: encapsulation and release of therapeutic proteins (Transforming Growth Factor β3) | Electrospinning. Ionotropic Gelation | Average size 194 ± 3 nm | The layer thickness of the multilayer systems in the dry state was determined as a function of the number of layers | [67] |
| carboxymethyl-chitosan | PVA/chitosan | Encapsulates antibacterial peptide (OH-CATH30) Exhibits antibacterial properties and promotes skin wound healing | Electrospinning, electrostatic droplet | Average size 164.6 ± 5 nm | The mean diameter of the control group (mean ± SD) was 357.34 ± 110.45 nm, which de- creased from 371.80 ± 110.31 nm to 327.48 ± 114.28 nm after the drug was loaded | [23] |
| Chitosan | Poly (vinylpyrrolidone) (PVP) | Vaginal controlled release of benzydamine | Electrospinning. Ionic gelation | Average size 128–710 nm range. (Depends of the formulation) | Average size 436 ± 155 nm and 557 ± 221 nm | [68] |
| Chitosan | PVP | Potential application as antibacterial fabrics for wound dressings. Mats are especially applicable for the treatment of diabetic wounds | Electrospinning. Ionic gelation | Average size 10–25 nm range | Average size 150 nm to 250 nm range | [69] |
| Methacrylic acid | Poly (L-lactide-co-D, L- lactide) containing multi-walled carbon nanotubes | Peripheral nerve regeneration | Electrospinning, Emulsion polymerization. Molecular imprinted | Average size of 80–115 nm range | Average size 92 nm | [54] |
| Poly lactic-co-glycolide | PVP, PVA | Topical Vaginal Delivery Platform for nanoparticles | Electrospinnning nanoprecipitation | Average size 172 ± 19 nm | The diameter of the composite fiber and the diameter of the nanoparticles were around 200–300 nm, with PVA fibers having a mean diameter of 248 ± 88 nm, PVP fibers of 297 ± 125 nm | [70] |
| Chitosan | Gelatin | For rapid hemostasis | Electrospinning. Ionic gelation | Size of ~300–400 nm | The diameter of the nanofibers is about 305 ± 50 nm | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Molinero, H.Y.; Serrano-Medina, A.; Palomino-Vizcaino, K.; López-Maldonado, E.A.; Villarreal-Gómez, L.J.; Pérez-González, G.L.; Cornejo-Bravo, J.M. Hybrid Systems of Nanofibers and Polymeric Nanoparticles for Biological Application and Delivery Systems. Micromachines 2023, 14, 208. https://doi.org/10.3390/mi14010208
Vargas-Molinero HY, Serrano-Medina A, Palomino-Vizcaino K, López-Maldonado EA, Villarreal-Gómez LJ, Pérez-González GL, Cornejo-Bravo JM. Hybrid Systems of Nanofibers and Polymeric Nanoparticles for Biological Application and Delivery Systems. Micromachines. 2023; 14(1):208. https://doi.org/10.3390/mi14010208
Chicago/Turabian StyleVargas-Molinero, Hever Yuritzy, Aracely Serrano-Medina, Kenia Palomino-Vizcaino, Eduardo Alberto López-Maldonado, Luis Jesús Villarreal-Gómez, Graciela Lizeth Pérez-González, and José Manuel Cornejo-Bravo. 2023. "Hybrid Systems of Nanofibers and Polymeric Nanoparticles for Biological Application and Delivery Systems" Micromachines 14, no. 1: 208. https://doi.org/10.3390/mi14010208
APA StyleVargas-Molinero, H. Y., Serrano-Medina, A., Palomino-Vizcaino, K., López-Maldonado, E. A., Villarreal-Gómez, L. J., Pérez-González, G. L., & Cornejo-Bravo, J. M. (2023). Hybrid Systems of Nanofibers and Polymeric Nanoparticles for Biological Application and Delivery Systems. Micromachines, 14(1), 208. https://doi.org/10.3390/mi14010208










