Fabrication of a Biomass-Derived Activated Carbon-Based Anode for High-Performance Li-Ion Batteries
Abstract
1. Introduction
2. Experimental Details
2.1. Fabrication of Activated Carbon
2.2. Materials Characterization
2.3. Cell Fabrication and Electrochemical Characterization
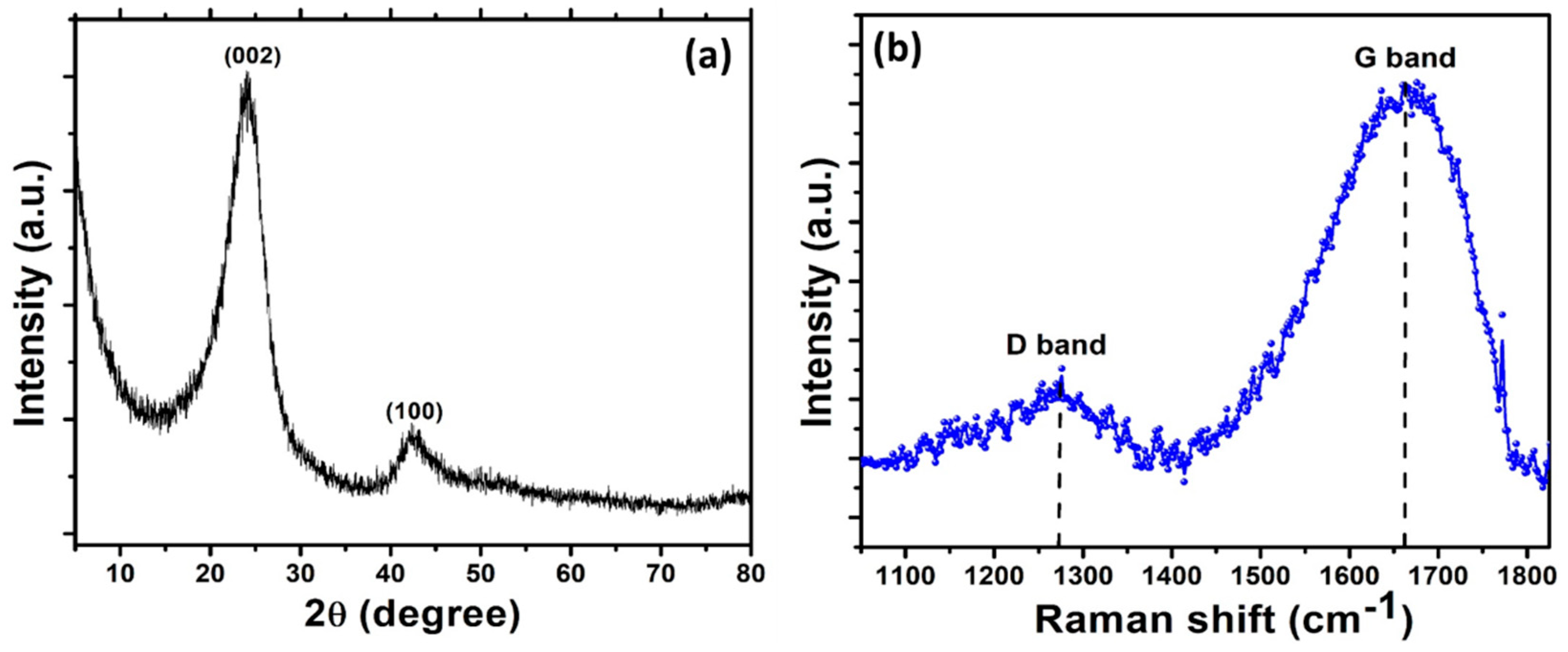
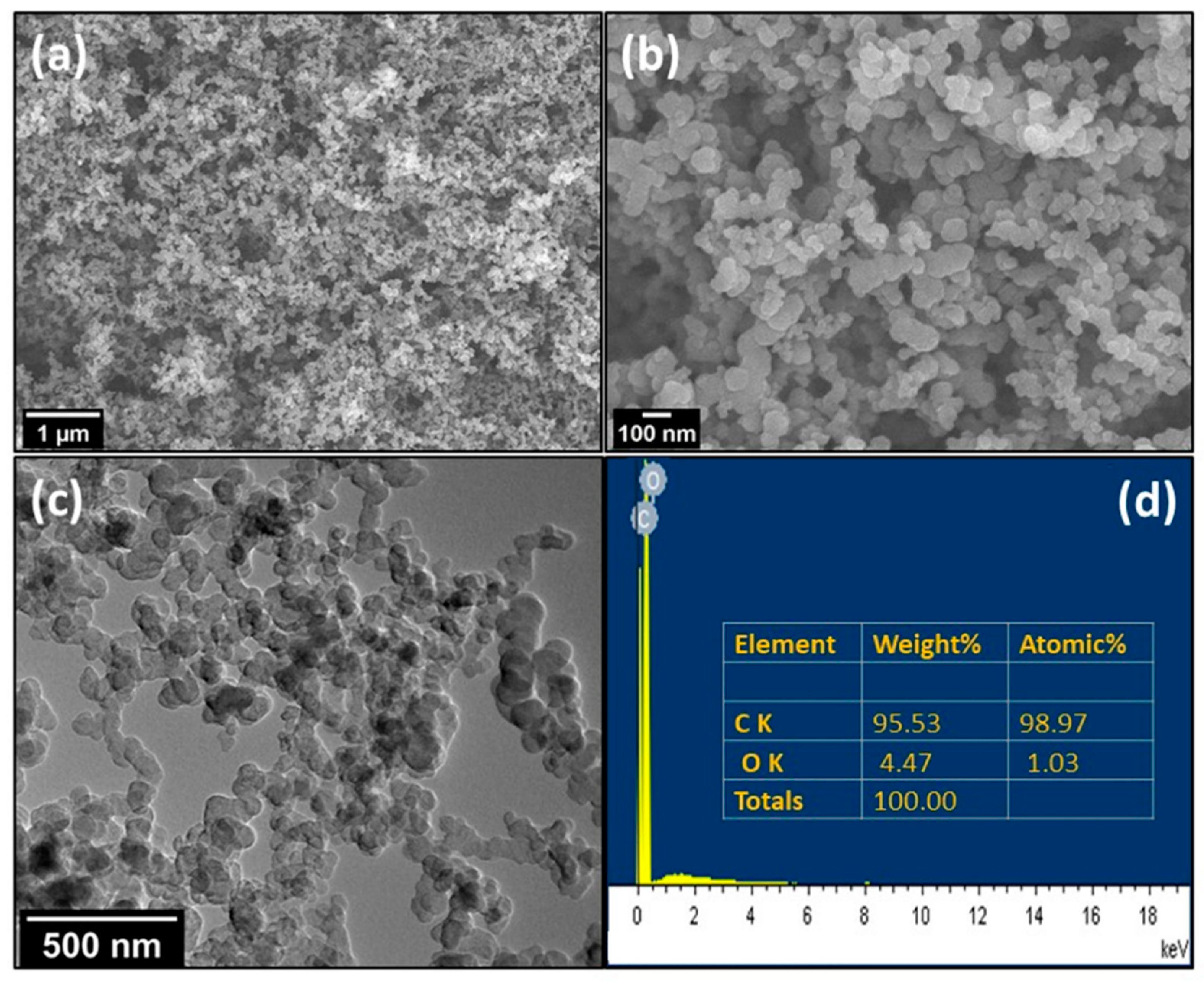
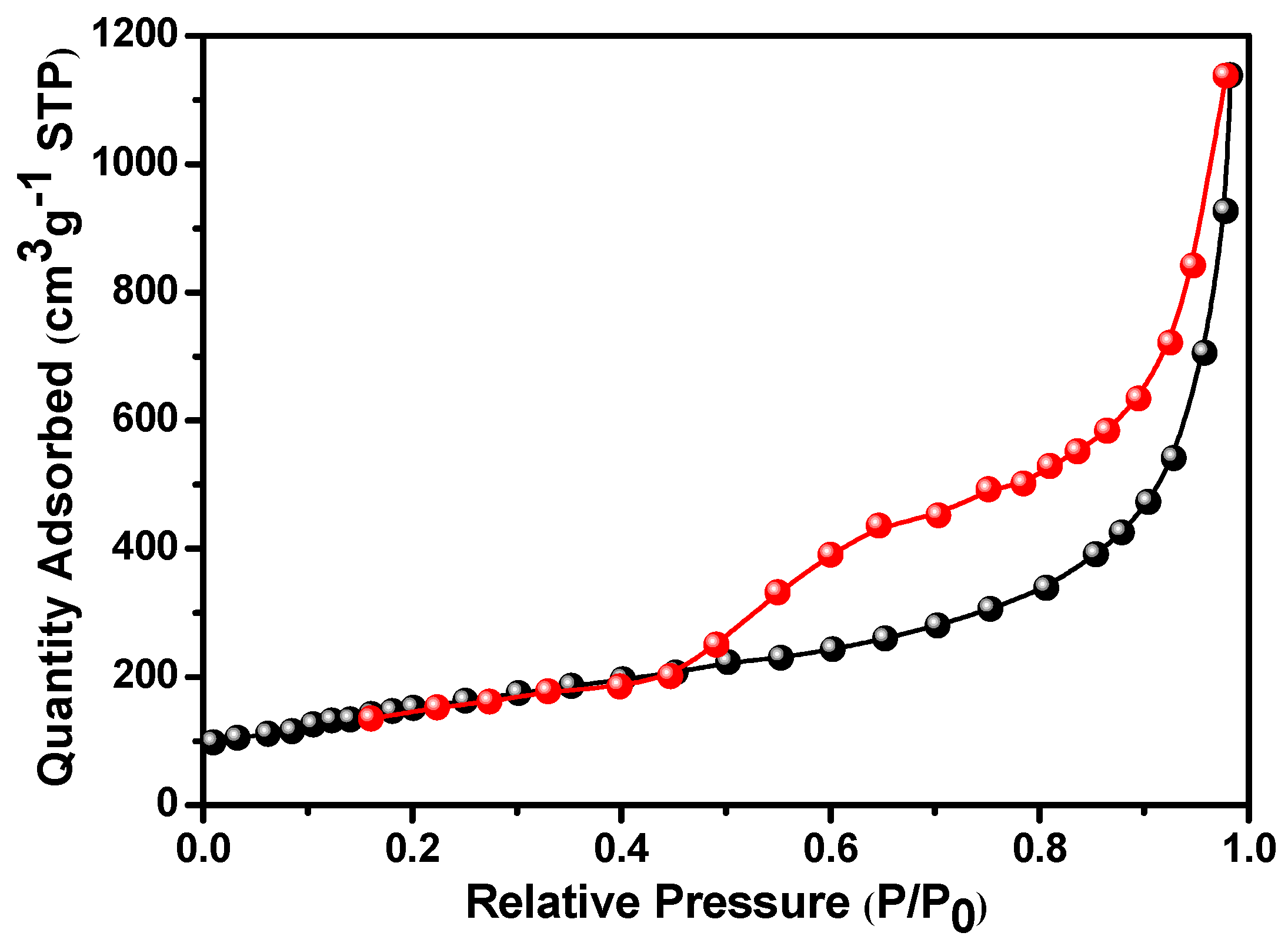
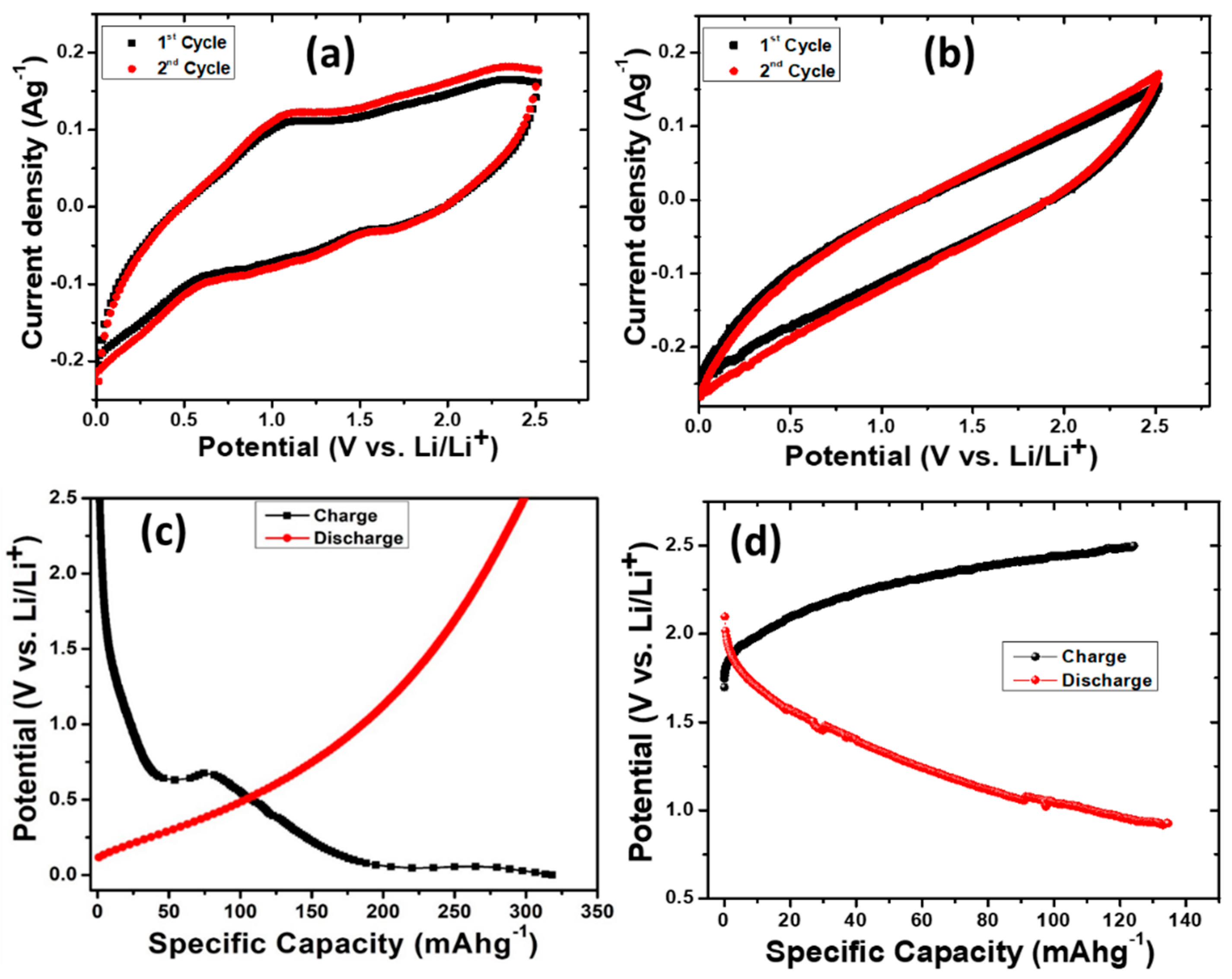
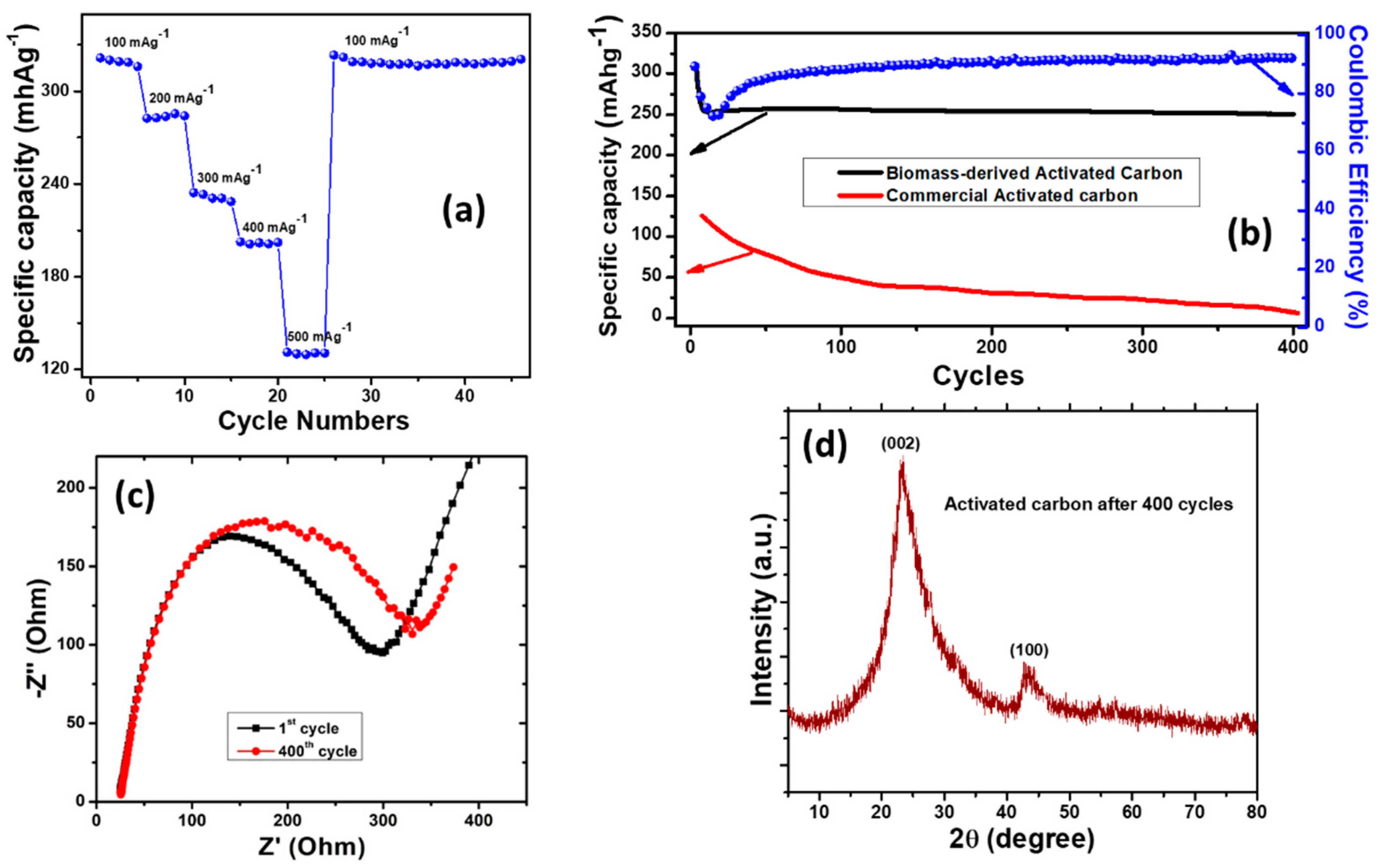
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybylin, E.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Ding, R.; Huang, Y.; Li Liao, Q.; Wei, T.; Liu, Y.; Huang, Y.; He, H. Carbon Anode Materials for Rechargeable Alkali Metal Ion Batteries and in-situ Characterization Techniques. Front. Chem. 2020, 8, 607504. [Google Scholar] [CrossRef]
- Ahmed, F.; Kumar, S.; Shaalan, N.M.; Saber, O.; Rehman, S.; Aljaafari, A.; Abuhimd, H.; Alshahrani, M. Synergistic Effect of Hexagonal Boron Nitride-Coated Separators and Multi-Walled Carbon Nanotube Anodes for Thermally Stable Lithium-Ion Batteries. Crystals 2022, 12, 125. [Google Scholar] [CrossRef]
- Farquhar, A.K.; Smith, S.R.; Dyck, C.V.; McCreery, R.L. Large Capacity Enhancement of Carbon Electrodes by Solution Processing for High Density Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 10211. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, R.; Zhou, Z.; Yan, S.; Dong, X. Novel Sodium Niobate-Based Lead-Free Ceramics as New Environment-Friendly Energy Storage Materials with High Energy Density, High Power Density, and Excellent Stability. ACS Sustain. Chem. Eng. 2018, 6, 12755. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, T.; Wang, L.; Ma, L.; Hu, Y.; Chen, R.; Wang, Y.; Wang, C.; Yan, W.; Tie, Z.; et al. High energy density hybrid lithium-ion capacitor enabled by Co3ZnC@ N-doped carbon nanopolyhedra anode and microporous carbon cathode. Energy Storage Mater. 2018, 14, 246. [Google Scholar] [CrossRef]
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290. [Google Scholar] [CrossRef]
- Hou, X.; Shi, H.; Chang, T.; Hou, K.; Feng, L.; Suo, G.; Ye, X.; Zhang, L.; Yang, Y.; Wang, W. Hollow opening nanoflowers MoS2-CuS-EG cathodes for high-performance hybrid Mg/Li-ion batteries. Chem. Eng. J. 2021, 409, 128271. [Google Scholar] [CrossRef]
- Ju, Z.; Jin, C.; Cai, X.; Sheng, O.; Wang, J.; Luo, J.; Yuan, H.; Lu, G.; Tao, X.; Liang, Z. Cationic Interfacial Layer toward a LiF-Enriched Interphase for Stable Li Metal Batteries. ACS Energy Lett. 2022, 8, 486–493. [Google Scholar] [CrossRef]
- Zhao, J.; Hong, M.; Ju, Z.; Yan, X.; Gai, Y.; Liang, Z. Durable Lithium Metal Anodes Enabled by Interfacial Layers Based on Mechanically Interlocked Networks Capable of Energy Dissipation. Angew. Chem. Int. Ed. 2022, 61, e202214386. [Google Scholar] [CrossRef]
- Ju, Z.; Xie, Q.; Sheng, O.; Wu, X.; Tan, Y.; Hong, M.; Tao, X.; Liang, Z. Biomass-Derived Anion-Anchoring Nano-CaCO3 Coating for Regulating Ion Transport on Li Metal Surface. Nano Lett. 2022, 22, 5473–5480. [Google Scholar] [CrossRef]
- Downie, L.E.; Krause, L.J.; Burns, J.C.; Jensen, L.D.; Chevrier, V.L. In Situ Detection of Lithium Plating on Graphite Electrodes by Electrochemical Calorimetry. J. Electrochem. Soc. 2013, 160, A588–A594. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Liaw, B.Y.; Metzler, V.; Zhang, J. A review of lithium deposition in lithium-ion and lithium metal secondary batteries. J. Power Sources 2014, 254, 168–182. [Google Scholar] [CrossRef]
- Endo, M.; Kim, C.; Nishimura, K.; Fujino, T.; Miyashita, K. Recent development of carbon materials for Li ion batteries. Carbon 2000, 38, 183–197. [Google Scholar] [CrossRef]
- Yoo, H.D.; Ryu, J.H.; Park, S.; Park, Y.; Ka, B.H.; Oh, S.M. Expanded graphite negative electrode for lithium-ion batteries. J. Electrochem. Sci. Technol. 2011, 2, 45–50. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Qiu, J.; Li, M.; Ming, H.; Zhang, S.; Zhang, T. Controllable synthesis of silicon/carbon hollow microspheres using renewable sources for high energy lithium-ion battery. J. Solid State Chem. 2021, 296, 121968. [Google Scholar] [CrossRef]
- Yu, J.; Luo, J.-D.; Zhang, H.; Zhang, Z.; Wei, J.; Yang, Z. Renewable agaric-based hierarchically porous cocoon-like MnO/Carbon composites enable high-energy and high-rate Li-ion batteries. Electrochim. Acta 2019, 322, 134757. [Google Scholar] [CrossRef]
- Kalyani, P.; Anitha, A. Biomass carbon & its prospects in electrochemical energy systems. Int. J. Hydrogen Energy 2013, 38, 4034–4045. [Google Scholar]
- Yao, Y.; Wu, F. Naturally derived nanostructured materials from biomass for rechargeable lithium/sodium batteries. Nano Energy 2015, 17, 91–103. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Tian, N.; Qin, A.; Liao, L.; Du, R.; Wei, C. Biomass carbon derived from sisal fiber as anode material for lithium-ion batteries. Mater. Lett. 2015, 142, 193–196. [Google Scholar] [CrossRef]
- Kim, H.; Guccini, V.; Lu, H.; Salazar-Alvarez, G.; Lindbergh, G.; Cornell, A. Lithium ion battery separators based on carboxylated cellulose nanofibers from wood. ACS Appl. Energy Mater. 2018, 2, 1241. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Zhang, M.; Li, M.; Li, T.; Yuan, G.; Liu, Y.; Zhang, X.; Cheng, X. Li-ion charge storage performance of wood-derived carbon fibers@ MnO as a battery anode. Chin. Chem. Lett. 2021, 33, 1091. [Google Scholar] [CrossRef]
- Arun, S.; Kiran, K.U.V.; Kumar, S.M.; Karnan, M.; Sathish, M.; Mayavan, S. Biomass-Derived Carbon for High-Performance Batteries: From Structure to Properties. J. Energy Storage 2021, 34, 102225. [Google Scholar] [CrossRef]
- Saha, A.; Sharabani, T.; Evenstein, E.; Nessim, G.D.; Noked, M.; Sharma, R. Probing electrochemical behaviour of lignocellulosic, orange peel derived hard carbon as anode for sodium ion battery. J. Electrochem. Soc. 2020, 167, 090505. [Google Scholar] [CrossRef]
- Um, J.H.; Ahn, C.-Y.; Kim, J.; Jeong, M.; Sung, Y.-E.; Cho, Y.-H.; Kim, S.-S.; Yoon, W.-S. From grass to battery anode: Agricultural biomass hemp-derived carbon for lithium storage. RSC Adv. 2018, 8, 32231. [Google Scholar] [CrossRef]
- Anarghya, D.; Anantha, M.S.; Venkatesh, K.; Santosh, M.S.; Priya, M.G.; Muralidhara, H.B. Bermuda grass derived nitrogen-doped carbon as electrocatalyst in graphite felt electrode to increase the efficiency of all-iron redox flow batteries. J. Electroanal. Chem. 2020, 878, 114577. [Google Scholar] [CrossRef]
- Xue, M.; Tan, Y.; Ren, Z.; Li, B.; Zhang, C. Mangosteen peel-derived porous carbon: Synthesis and its application in the sulfur cathode for lithium sulfur battery. J. Mater. Sci. 2018, 53, 11062. [Google Scholar] [CrossRef]
- Demir, E.; Aydin, M.; Arie, A.A.; Demir-Cakan, R. Apricot shell derived hard carbons and their tin oxide composites as anode materials for sodium-ion batteries. J. Alloys Compd. 2019, 788, 1093. [Google Scholar] [CrossRef]
- Bharti, V.; Gangadharan, A.; Rao, T.N.; Sharma, C.S. Carbon soot over layered sulfur impregnated coconut husk derived carbon: An efficient polysulfide suppressor for lithium sulfur battery. Mater. Today Commun. 2020, 22, 100717. [Google Scholar] [CrossRef]
- Narayanan, D.P.; Balasubramanyan, S.; Cherikkallinmel, S.K.; Vadery, V.; Sankaran, S.; Narayanan, B.N. A Green Approach for the Synthesis of Coconut Husk Ash—Twisted Graphene Nanocomposites: Novel Catalysts for Solvent-Free Biginelli Reaction. ChemistrySelect 2019, 4, 4785. [Google Scholar] [CrossRef]
- Yu, K.; Li, J.; Qi, H.; Liang, C. High-capacity activated carbon anode material for lithium-ion batteries prepared from rice husk by a facile method. Diam. Relat. Mater. 2018, 86, 139–145. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Z.; Pan, F.; Zhong, X.; Liu, X.; Gu, L.; Yu, Y. Phosphorus-doped porous carbon derived from rice husk as anode for lithium ion batteries. RSC Adv. 2015, 5, 55136–55142. [Google Scholar] [CrossRef]
- Li, W.; Nazhipkyzy, M.; Bandosz, T.J. Inorganic matter in rice husk derived carbon and its effect on the capacitive performance. J. Energy Chem. 2020, 57, 639–649. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Feng, N.; Qiao, L.; Li, X.; He, D.J. A new carbonaceous material derived from biomass source peels as an improved anode for lithium ion batteries. Anal. Appl. Pyrol. 2013, 100, 181–185. [Google Scholar] [CrossRef]
- Fey, G.; Lee, D.; Lin, Y.; Kumar, T.P. High-capacity disordered carbons derived from peanut shells as lithium-intercalating anode materials. Synth. Met. 2003, 139, 71–80. [Google Scholar] [CrossRef]
- Unur, E.; Brutti, S.; Panero, S.; Scrosati, B. Nanoporous carbons from hydrothermally treated biomass as anode materials for lithium ion batteries. Microporous Mesoporous Mater. 2013, 174, 25–33. [Google Scholar] [CrossRef]
- Jayaraman, S.; Jain, A.; Ulaganathan, M.; Edison, E.; Srinivasan, M.P.; Balasubramanian, R. Li-ion vs. Na-ion capacitors: A performance evaluation with coconut shell derived mesoporous carbon and natural plant based hard carbon. Chem. Eng. J. 2017, 316, 506–513. [Google Scholar] [CrossRef]
- Sennu, P.; Arun, N.; Madhavi, S.; Aravindan, V.; Lee, Y.-S. All carbon based high energy lithium-ion capacitors from biomass: The role of crystallinity. J. Power Sources 2019, 414, 96–102. [Google Scholar] [CrossRef]
- Tao, L.; Zheng, Y.; Zhang, Y.; Ma, H.; Di, M.; Zheng, Z. Liquefied walnut shell-derived carbon nanofibrous mats as highly efficient anode materials for lithium ion batteries. RSC Adv. 2017, 7, 27113–27120. [Google Scholar] [CrossRef]
- Fang, T.; Yu, X.; Zhang, X.; Li, Y.; Liang, X.; Liao, L.; Li, B. A Comparative Investigation on Lithium Storage Performance of Carbon Microsphere Originated from Agriculture Bio-waste Materials: Sunflower Stalk and Walnut Shell. Waste Biomass Valor. 2020, 11, 6981–6992. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Basak, T.; Srinivasan, R. Microwave heating characteristics of graphite based powder mixtures. Int. Commun. Heat Mass Transfer 2013, 48, 22–27. [Google Scholar] [CrossRef]
- Faheem, A.; Nishat, A.; Anwar, M.S.; Rehan, D.; Bon, H.K. Morphological evolution of ZnO nanostructures and their aspect ratio-induced enhancement in photocatalytic properties. RSC Adv. 2014, 4, 29249–29263. [Google Scholar]
- Musso, S.; Giorcelli, M.; Pavese, M.; Bianco, S.; Rovere, M.; Tagliaferro, A. Improving macroscopic physical and mechanical properties of thick layers of aligned multiwall carbon nanotubes by annealing treatment. Diam. Relat. Mater. 2008, 17, 542–547. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, T.P.; Ramesh, R.; Thomas, S.; Jeong, S.K.; Nahm, J.S. Pyrolitic Carbon from Biomass Precursors as Anode Materials for Lithium Batteries. Mater. Sci. Eng. A 2006, 430, 132–137. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.C.; Holden, J.M.; Jishi, R.A. Vibrational modes of carbon nanotubes; spectroscopy and theory. Carbon 1995, 33, 959. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in grapheme. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Awano, H.; Brodd, R.; Cho, J.; Dimov, N.; Gotoh, K.; Horiba, T.; Kikuya, K.; Kono, M.; Kozawa, A.; Maeda, M.; et al. Lithium-Ion Batteries; Yoshio, M., Brodd, R., Kozawa, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Zhiyu, W.; Deyan, L.; Freddy, Y.C.B.; Xiong, W.L. Fast Formation of SnO2 Nanoboxes with Enhanced Lithium Storage Capability. J. Am. Chem. Soc. 2011, 133, 4738. [Google Scholar]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for Lithium Insertion in Carbonaceous Materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Pol, V.G.; Thackeray, M.M. Spherical Carbon Particles and Carbon Nanotubes Prepared by Autogenic Reactions: Evaluation as Anodes in Lithium Electrochemical Cells. Energy Environ. Sci. 2011, 4, 1904–1912. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novak, P.J.A.M. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Sato, K.; Noguchi, M.; Demachi, A.; Oki, N.; Endo, M. A mechanism of lithium storage in disordered carbons. Science 1994, 264, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Reimers, J.N.; Dahn, J.R. Effect of turbostratic disorder in graphitic carbon hosts on the intercalation of lithium. Phys. Rev. B Condens. Matter Mater. Phys. 1995, 51, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T. Lithium insertion in high capacity carbonaceous materials. J. Electrochem. Soc. 1995, 142, 2581. [Google Scholar] [CrossRef]
- Zheng, T.; Xing, W.; Dahn, J.R. Carbons prepared from coals for anodes of lithium-ion cells. Carbon 1996, 34, 1501–1507. [Google Scholar] [CrossRef]
- Kim, K.; Adams, R.A.; Kim, P.J.; Arora, A.; Martinez, E.; Youngblood, J.P.; Pol, V.G. Li-Ion Storage in an Amorphous, Solid, Spheroidal Carbon Anode Produced by Dry-Autoclaving of Coffee Oil. Carbon 2018, 133, 62–68. [Google Scholar] [CrossRef]
- Hong, K.L.; Qie, L.; Zeng, R.; Yi, Z.Q.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.J.; Zhang, W.X.; et al. Biomass Derived Hard Carbon Used as a High Performance Anode Material for Sodium Ion Batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X.S. Biomass Derived Carbon Nanoparticle as Anodes for High Performance Sodium and Lithium Ion Batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Lee, J.W.; Song, T.; Paik, U. 3D-interconnected nanoporous RGO-CNT structure for supercapacitors application. Electrochim. Acta 2014, 125, 536–542. [Google Scholar] [CrossRef]
- Jin, H.; Yu, H.; Li, H.; Davey, K.; Song, T.; Paik, U.; Qiao, S.Z. MXene Analogue: A 2D Nitridene Solid Solution for High-Rate Hydrogen Production. Angew. Chem. Int. Ed. 2022, 61, e202203850. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.Z. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 2018, 12, 12761–12769. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, F.; Almutairi, G.; Hasan, P.M.Z.; Rehman, S.; Kumar, S.; Shaalan, N.M.; Aljaafari, A.; Alshoaibi, A.; AlOtaibi, B.; Khan, K. Fabrication of a Biomass-Derived Activated Carbon-Based Anode for High-Performance Li-Ion Batteries. Micromachines 2023, 14, 192. https://doi.org/10.3390/mi14010192
Ahmed F, Almutairi G, Hasan PMZ, Rehman S, Kumar S, Shaalan NM, Aljaafari A, Alshoaibi A, AlOtaibi B, Khan K. Fabrication of a Biomass-Derived Activated Carbon-Based Anode for High-Performance Li-Ion Batteries. Micromachines. 2023; 14(1):192. https://doi.org/10.3390/mi14010192
Chicago/Turabian StyleAhmed, Faheem, Ghazzai Almutairi, Prince M. Z. Hasan, Sarish Rehman, Shalendra Kumar, Nagih M. Shaalan, Abdullah Aljaafari, Adil Alshoaibi, Bandar AlOtaibi, and Kaffayatullah Khan. 2023. "Fabrication of a Biomass-Derived Activated Carbon-Based Anode for High-Performance Li-Ion Batteries" Micromachines 14, no. 1: 192. https://doi.org/10.3390/mi14010192
APA StyleAhmed, F., Almutairi, G., Hasan, P. M. Z., Rehman, S., Kumar, S., Shaalan, N. M., Aljaafari, A., Alshoaibi, A., AlOtaibi, B., & Khan, K. (2023). Fabrication of a Biomass-Derived Activated Carbon-Based Anode for High-Performance Li-Ion Batteries. Micromachines, 14(1), 192. https://doi.org/10.3390/mi14010192











