Abstract
A non-invasive laser fiber-optic method based on infrared sensors for heart rate (Hr) recording was applied to assess the physiological condition of Pinna nobilis. During 2017, the specimens of P. nobilis were sampled at three sites within the Boka Kotorska Bay, Montenegro and used for ex situ experiments with short-term reduction/restoration of ambient salinity to evaluate their physiological adaptive capacity based on heart rate recovery time (Trec). Mean Trec for specimens from Sv. Nedjelja (reference site), Dobrota and Sv. Stasije were 72 ± 3, 91 ± 7 and 117 ± 15 min, while the coefficients of variation (CV) were 0.12, 0.13 and 0.17, respectively. Resting heart rate (Hrrest) and Trec showed statistically significant differences between the groups of mussels from Dobrota and Sv. Stasije in comparison to the reference site. Statistically significant correlations were observed between Trec and shell length/width, which was not the case in comparison between Hrrest and shell length/width. The lower adaptive capacity within the P. nobilis specimens from Dobrota and Sv. Stasije in comparison to the reference site could occur due to stress induced by deterioration of environmental conditions, which could have led to impairment of the physiological state of the mussels evaluated by Hr. All the specimens of P. nobilis survived the experimental treatments; afterwards, they were successfully transplanted at the Dobrota site. The experimental unit with sensor technology applied in this study can provide Hr recording in real time and could have an application in monitoring the physiological/health state of P. nobilis individuals maintained in aquaria.
1. Introduction
Current trends of global climate change, such as extreme draughts or strong rainfall, contributed to more intensive fluctuations in coastal salinity, influencing marine organisms [1,2]. Estuarine bivalves are particularly sensitive to hyposalinity conditions due to activity reduction, and high-energy demands to maintain ion homeostasis and avoid irreversible cell damage [3,4]. However, mussels have interesting physiological mechanisms of adaptation to variable salinity [5]. The heart rate (Hr) of marine mussels as a physiological biomarker measured by non-invasive infrared-based sensors [6] proved to be a reliable indicator of environmental salinity changes [7,8,9,10]. Moreover, a similar methodology for Hr registration, the fiber-optic method [11], has an application in the assessment of aquatic ecosystem health by investigation of a mussel’s physiological condition. This was achieved by ex situ standardized test based on the calculation of heart rate recovery time (Trec) after rapid change in ambient water salinity as a stress stimulus [12]. A shorter Trec in mussels from the clean site in comparison with the polluted one as an indication of higher adaptive capacity and good health condition was the main hypothesis, evaluated in many studies [13,14,15,16,17,18].
The pen shell Pinna nobilis (Linnaeus, 1758) is an endemic Mediterranean bivalve; its large shell can reach 120 cm of antero–posterior length [19], burrowed in marine sediment at depths ranging from 0.5 to 60 m [20]. P. nobilis became endangered due to fishing, habitat degradation and marine pollution [21]. Moreover, since late 2016, disease amongst P. nobilis caused by the protozoan parasite Haplosporidium pinnae sp. nov. [22] and/or mycobacterial disease [23] has been occurring, starting from Spain and spreading all over the Mediterranean, with a mortality rate of up to 100% [24,25]. Consequently, P. nobilis is marked as Critically Endangered in the IUCN Red List of Threatened Species [26]. The remaining populations of P. nobilis on the western Mediterranean coast of Spain and France have survived under the fluctuating environment of lagoons and estuarine [27,28,29,30]. The Boka Kotorska Bay (Adriatic Sea) is exposed to many sources of freshwater inputs which can strongly modify the pattern of the seawater temperature, salinity and currents [31]. Besides the physiological studies on pen shell gaping activity [32,33,34], respiration rates [35] and osmoregulation [36], there is a lack of studies on the influence of environmental parameters on P. nobilis Hr.
Considering all of the aforementioned, the main aim of this study was to assess P. nobilis’ health condition in the Boka Kotorska Bay by a non-invasive fiber-optic method for Hr recording of mussels, under short-term ex situ salinity reduction test. In addition, the aim was to compare the Hrrest and Trec of shells with different sizes. Moreover, one of the purposes was the transplantation of P. nobilis specimens from a more to a less dense population toward the goal of species’ protection. The study was carried out during 2017 as part of the PinnaSpot project: the study, protection and possible breeding of the pen shell (Pinna nobilis) in the Boka Kotorska Bay, two years before the occurrence of the mass mortality event (MME) of P. nobilis in the Adriatic Sea [37].
2. Materials and Methods
2.1. Specimen Collection and Relocation
Since P. nobilis is endangered and protected under ANNEX II of the Barcelona Convention and ANNEX IV of the EC Habitats Directive 92/43/EEC, before the implementation of any activities envisaged by the PinnaSpot project, including the present study, a confirmation letter from the relevant state authorities in Montenegro was obtained (enclosed). During the summer of 2017, 34 P. nobilis specimens were sampled from three sites: Sv. Nedjelja (42°27′30.80″ N 18°40′26.70″ E; depth = 2 m; n = 14); Dobrota (42°26′13.50″ N 18°45′47.32″ E; depth = 5 m; n = 11) and Sv. Stasije (42°28′4.14″ N 18°45′44.28″ E; depth = 5 m; n = 9) within the Boka Kotorska Bay, Adriatic Sea, Montenegro (Figure 1). Sv. Nedjelja was used as the reference site due to the exceptionally high density of P. nobilis in this location [38], which indicates suitable environmental conditions for their development. Moreover, P. nobilis is a reliable bioindicator species for benthic coastal ecosystems [39]. Sv. Nedjelja is situated in the middle part of the bay, featured by a sandy bottom, a higher level of seawater exchange and lower salinity fluctuations during the year. P. nobilis population at this site was situated within the seagrass meadows of Cymodocea nodosa (Ucria) Aschers. The Dobrota and Sv. Stasije sites feature muddy sediments, a lower level of seawater exchange due to a higher inner positioning within the bay and higher seasonal salinity oscillations caused by freshwater inflow from the land and underwater springs. The seagrass communities were not developed at the Dobrota site while the P. nobilis population in Sv. Stasije was settled within the Possidonia oceanica (L.) Delile meadows. During the extraction of P. nobilis specimens from their natural habitat, it was important to avoid shell damage and maintain the byssus threads intact. This was achieved by a metal hand trowel which helped to maintain a certain amount of sediment around the buried part of the shell. The residual sediment was gently removed by hand to avoid seawater turbidity, since the specimens of P. nobilis were placed in tanks at 18 °C for transportation. Then, in 10–60 min, the fan mussels were transferred to the laboratory of the Institute of Marine Biology, Kotor, and placed in aquaria with clean seawater, constant aeration, temperature 20 ± 2 °C and salinity 29–36‰ for acclimation. The temperature and salinity were measured with a WTW Multi 350i probe (WTW GmbH; Weilheim, Germany), while the antero–posterior shell length and width of specimens were measured with an aluminum caliper. After laboratory manipulations (further in text) P. nobilis specimens were transplanted into the sediment at a 5 m depth and approximately 1 m distance between each other at the Dobrota site. It was important to bury them in sediment at least up to half of the shell in an appropriate orientation to minimize resistance to hydrodynamic forces. The whole extraction/transplantation procedure was conducted according to the protocol by [40,41].

Figure 1.
Sampling sites in the Boka Kotorska Bay, Montenegro.
2.2. Heart Rate Analyses
2.2.1. Heart Rate Recording
The Hr recording of the mussels was carried out by a non-invasive laser fiber-optic method developed in 1999 at the Research Center of Ecological Safety, Russian Academy of Sciences, St. Petersburg, Russia [11]. The applied method is based on photoplethysmography (PPG). PPG is a simple and inexpensive technology that includes a light source and a photodetector, often used for Hr monitoring [42]; it is focused on changes in light intensity reflected from or transmitted through the tissue, providing information on the heartbeats [43]. The whole procedure for the cardiac activity registration of benthic invertebrates was thoroughly explained by [15]. Briefly, the experimental unit includes eight PPG devices (Photoplethismograph, RIC “Eco-Contour”; Russia) allowing for the simultaneous recording of cardiac activity of eight mussels. P. nobilis specimens were polished by gentle sandpaper in a small region above the heart area to attach sensor holders by waterproof epoxy glue (Figure 2). The connection with the IR light source and receiver placed in the PPG device was made by fiber-optic cables. The sensor detected IR light which was reflected from the heart area and the data on periodical changes of the heart volume were transferred to a personal computer. After amplification and analog to digital conversion, the signal was processed by VarPulse 9.0 software (St. Peterburg, Russia), used for analyses of cardiac intervals [44].

Figure 2.
Pinna nobilis shell: 1—fiber-optic cable, 2—sensor attached above the heart area.
2.2.2. Hyposalinity Test
After the acclimation of the mussels, we carried out two experiments per site. For each one, 4–7 pen shell specimens were placed in seawater tanks for hyposalinity test, while one specimen per experiment in a separate aquarium was used as a control. Only one P. nobilis specimen was used as a control due to a lower number of specimens sampled at the two sites and the equipment limitation to eight animals per experiment. The standardized experimental procedure for marine species, which includes seawater salinity reduction, was conducted according to [12,15]. The hyposalinity test started by a gentle addition of distilled water to the seawater aquarium to reduce the salinity by 50%, as the ranges of the physiological tolerance limits for different species were defined previously [15]. Then, lower salinity values were measured, the salt solution was prepared based on calculation and added to the aquarium after approximately 1 h, to restore the background salinity determined prior to the test. Before the addition of distilled water, we recorded the Hr values in clean seawater for at least 3 h to establish normal Hr-resting response (Hrrest) in defined environmental conditions. Establishing a normal resting response is required in physiological assays due to considerable interindividual variability in physiological measurements [45].
2.2.3. Calculations of Trec and CV
The calculations of Trec and CV were based on the result graphs of Hr pattern in MS Excel for each specimen separately (Figure S1) and presented as mean ± standard error within the groups of mussels from the sampling sites. Trec was defined as the time–distance between two points (end of salinity restoration and re-achieving the stable Hr values recorded prior to the test), while CV was determined as the relation between standard deviation (SD) and mean value of Hr measured 1 h after the end of salinity restoration in the group of specimens for each experiment [16].
2.3. Statistical Analyses
Statistical analysis of the results obtained in six experiments was performed by Statistica 7.0 Software (StatSoft, Inc., Tulsa, OK, USA) [46]. The Kolmogorov–Smirnov test for normality of distribution was used prior to statistical analysis. Considering that the data were not in line with the requirements for the application of parametric tests, differences between each group and corresponding reference points were tested using the Mann–Whitney U test. Correlation analyses were carried out using the Spearman correlation test with a significance level p < 0.05.
3. Results
The temperature and salinity of seawater in the aquaria, before, during and after the hyposalinity test are presented in Table 1, while the results for mean Hr of P. nobilis from all of the sampling sites are summarized in Figure 3. In all six experiments, the Hr of control specimens was stable by the end of recording (Figure 3). For both of the experiments within the groups of mussels from Sv. Nedjelja—reference site (Figure 3a,b) and from Dobrota (Figure 3a,b), mean Hr decreased by hyposalinity while the restoration of background salinity induced Hr elevation. On the other hand, in both of the experiments with mussels from Sv. Stasije (Figure 3e), hyposalinity caused an increase in the mean Hr, while in the second (Figure 3f), a mean Hr increase was observed after salinity restoration as well. In all of the experiments, immediately after hyposalinity onset, faster or slower, the mean Hr pattern showed a characteristic ridge shape (Figure 3), which is more visible on the Hr example of a single specimen (Figure S1). However, Hr of mussels showed interindividual variability within the groups, expressed as different responses during the hyposalinity test (SD, Figure 3). Regardless of their size, after initial Hr increase followed by decline during the lower salinity, in a few cases the Hr decrease did not occur. The observed Hr differences were statistically significant for each P. nobilis specimen in comparison between the periods before and during hyposalinity exposure. In the mussels sampled from Sv. Nedjelja, the mean Trec (both experiments) was 72 ± 3 min, while in the mussels from Dobrota and Sv. Stasije, it was 91 ± 7 and 117 ± 15 min, respectively. Mean values of CV for both experiments within the groups of specimens from Sv. Nedjelja, Dobrota and Sv. Stasije were 0.12, 0.13 and 0.17, respectively.

Table 1.
Seawater temperature and salinity in aquaria with Pinna nobilis specimens sampled from the sites Sv. Nedelja, Dobrota and Sv. Stasije, measured before hyposalinity test (background), during the 50% reduction by distilled water (salinity reduction) and after the restoration by salt addition (salinity restoration). Abbreviations: Exp.—experiment; Temp.—temperature.
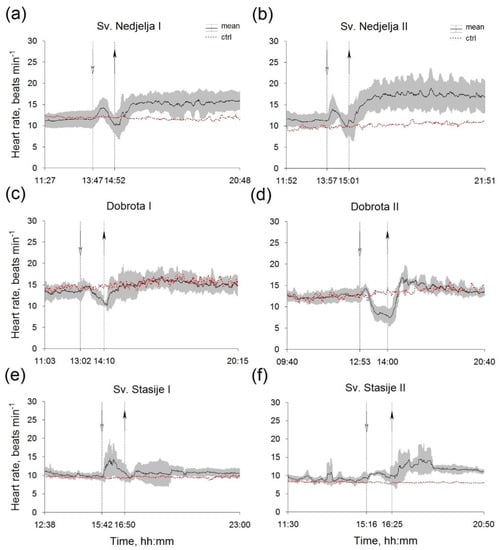
Figure 3.
Mean HR within groups of Pinna nobilis specimens sampled from Sv. Nedjelja (a,b), Dobrota (c,d) and Sv. Stasije (e,f), before, during (between dashed lines) and after experimental treatment. Abbreviations: ctrl—control (red dotted lines); white head arrows—onset of salinity change by distilled water; black head arrows—onset of restoration of initial salinity values by salt addition; I—the first experiment; II—the second experiment.
There was no statistically significant difference (p > 0.05) of tested parameters in comparison between the two experiments carried out within the groups of mussels from the same sampling sites. The shell length of mussels from Dobrota and Sv. Stasije was significantly higher in comparison to Sv. Nedjelja, and the specimens from Sv. Stasije were significantly longer in comparison to Dobrota (Figure 4a). Statistically significant differences in shell width between the Sv. Nedjelja and Dobrota sites were not observed, while the specimens from Sv. Stasije were significantly wider in comparison to other sites (Figure 4b).
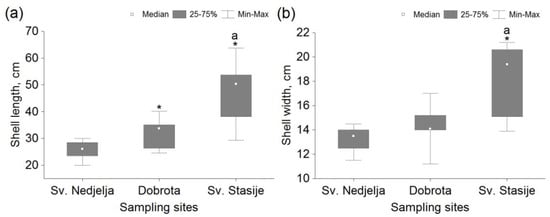
Figure 4.
Antero–posterior shell length (a) and width (b) of Pinna nobilis sampled from Sv. Nedjelja (n = 12), Dobrota (n = 9) and Sv. Stasije (n = 7) within the Boka Kotorska Bay. *—statistically significant difference (p < 0.05) in comparison with the reference site (Sv. Nedjelja); a—statistically significant difference (p < 0.05) in comparison with the other sampling site; n—number of specimens.
Mean Hrrest was significantly higher in mussels from Dobrota in comparison to other sites, while mussels from Sv. Stasije showed a significantly lower value of mean Hrrest in comparison to Sv. Nedjelja (Figure 5a). There was no significant difference in Trec between the specimens from the Dobrota and Sv. Stasije sites, however specimens from both sites showed significantly longer Trec in comparison to Sv. Nedjelja (Figure 5b).

Figure 5.
(a)—resting heart rate (Hrrest); (b)—heart rate recovery time (Trec) within a group of Pinna nobilis specimens sampled from Sv. Nedjelja, Dobrota and Sv. Stasije. *—statistically significant difference (p < 0.05) in comparison with the reference site (Sv. Nedjelja); a—statistically significant difference (p < 0.05) in comparison with the other sampling site.
Furthermore, a significant correlation (p < 0.05) was observed between the data for Trec and shell length (p = 0.000528; r = 0.59; Figure 6a), and Trec and shell width (p = 0.0000875; r = 0.65; Figure 6b). On the other hand, in comparison between Hrrest and shell length, a significant correlation was not observed (p = 0.029318; r = −0.36; Figure S2a), nor between Hrrest and shell width (p = 0.033571; r = −0.35; Figure S2b).
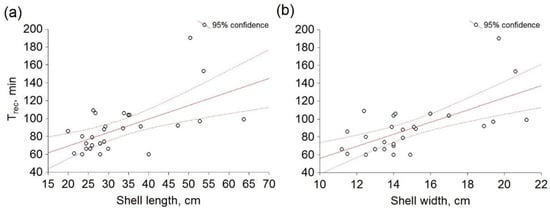
Figure 6.
Relation between heart rate recovery time (Trec) and antero–posterior (a) shell length, (b) shell width of Pinna nobilis. Circles represent mean values obtained in six independent experiments.
4. Discussion
If we compare the Hr pattern of P. nobilis in the control with control experiments of M. galloprovincialis (L.) [47,48], a lower Hrrest and a very stable Hr of P. nobilis can be observed for a longer period without the Hr oscillations recorded in Mytilus. The reason could be the size difference between these two mollusk species, since the large shells of the freshwater mussel Cristaria plicata (Leach, 1815) showed lower mean values of Hrrest [49]. Moreover, Hr of marine mussels Perna viridis (Linnaeus, 1758) [50] and Chlamys farreri (Jones et Preston, 1904) [51] showed a negative correlation with shell size. The reason for different shell sizes of P. nobilis specimens sampled from the three sites at the Boka Kotorska Bay was population structure. The Sv. Nedjelja and Sv. Stasije sites were mainly inhabited by younger/smaller and older/larger specimens, respectively, while at the Dobrota site, the population was comprised of small- to middle-sized specimens. In this study it was shown that the significantly longer and wider shells of P. nobilis specimens from Sv. Stasije have significantly lower Hrrest in comparison to other sites. However, a significant correlation between the shell size and Hrrest for all of the P. nobilis specimens was not observed, which supports our initial assumption to compare Hr within the groups of mussels with different shell sizes. Furthermore, the gradual Hr increase in P. nobilis at the onset of the hyposalinity test (ridge-shaped Hr pattern) in comparison to the faster response of Mytilus [13,15] could be explained by the larger volume of seawater with higher salinity which remains for longer inside the large shell of P. nobilis upon the valve closure. Isolation response to environmental salinity reduction by closing shell valves accompanied with Hr decrease occurs due to the restriction of the gas exchange and aerobic metabolism [52]. In a few cases, the Hr of P. nobilis showed only the increase phase, despite the fact that a further decline in Hr value was expected for marine species based on the hyposalinity tests in the aforementioned studies. The observed Hr differences between the P. nobilis specimens contributed to interindividual variability as the response to hyposalinity conditions. Thus, it would be recommendable to prolong the duration of the hyposalinity test to 1.5–2 h to have higher uniformity in the Hr response for this species and probably others with a larger shell size as well.
Significantly shorter Trec of the P. nobilis specimens from the reference site in comparison with Dobrota and Sv. Stasije showed a higher adaptive capacity and good health condition which is in compliance with our premise of a good ecological status at this site featured by a very high density of P. nobilis population [38]. In addition, the good health state of rock crabs from clean sites was confirmed by Hr and other physiological parameters [53]. In other hyposalinity or hypersalinity studies performed on different marine and freshwater mollusks [13,15,16,18], the Trec values for the reference sites were in a similar range as the Trec of P. nobilis measured in this study. According to recommendations [12], the specimens from Dobrota and Sv. Stasije with significantly longer Trec in comparison with the reference site had a lower compensatory response to stress probably due to poor health condition. Considering the close relationship between P. nobilis and the sea bottom, the higher content of certain trace elements detected in the sediments at the Dobrota site [54], and the increased lead concentration in seawater, sediments and the seagrass P. oceanica found at Sv. Stasije [55] could contribute to the accumulation of these contaminants in the fan mussels’ tissues and affect their physiology. Particularly, trace element contamination in the tissues of the Mediterranean mussels from the Boka Kotorska Bay induced longer Trec after the hyposalinity test [16]. In this study, we have not performed any toxicological analyses due to the existing sediment data of the investigated sites and to avoid tissue sampling of endangered species, such as P. nobilis. However, the lower adaptive capacity, defined as the poor health state [12,15], of P. nobilis specimens indicated by Hr response could be caused by deterioration of environmental conditions at the studied sites.
On the other hand, the specimens from all of the three sites showed very low CV values specific for healthy individuals inhabiting clean environments which indicated that the differences in the health status of these individuals are not dramatic. In general, the significant correlation between Trec and the shell size of P. nobilis is in compliance with data on CV. However, despite the significant difference in the shell size between the specimens from Dobrota and Sv. Stasije, a significant difference in Trec was not observed. Accordingly, these data showed that the significantly longer Trec of the specimens from the studied sites in comparison to the reference site was not a consequence of shell size.
As far as we know, these are the first data on the Hr of P. nobilis. Based on our experience, P. nobilis is a very suitable model organism for studying cardiac activity by non-invasive PPG method. Despite its vulnerability in nature, P. nobilis showed a sufficient level of resilience during handling. Moreover, the P. nobilis shell is firm, flat and thin above the heart area which contributes to more precise sensor positioning and better Hr signal transduction. Furthermore, based on the results of this study, the most appropriate size of P. nobilis specimens for Hr monitoring purposes belongs to the size class, 30–40 cm. The smaller P. nobilis individuals needed more time for shell surface preparation due to densely spaced spines near the heart region, while the larger individuals were less suitable for handling since these shells needed more space and larger amounts of seawater for maintenance in the laboratory tanks.
After laboratory analyses, the specimens were transplanted in a more distant environment from the open sea with a lower level of seawater exchange at the inner part of the Boka Kotorska Bay on the Dobrota site due to the possibility of P. nobilis MME spreading from the western Mediterranean [24] at that time. Another reason in favor of this decision was the unhindered growth of the P. nobilis population from Dobrota under a lower salinity regime [56], knowing that salinity is a limitation factor for the spread of P. nobilis MME [27]. It was also beneficial to select an environment already inhabited by P. nobilis to increase transplantation success [40]. All the specimens survived for at least two years, until Spring 2019 and P. nobilis MME appeared in the Montenegrin coast (our unpublished data), which indicates a well-performed transplantation.
P. nobilis Hr, featured by specific response to stress, could be suggested as a potential biomarker of distress, such as marine pollution or poor ex situ maintenance conditions of individuals maintained indoors. The experimental unit used in this study is particularly suitable for this purpose, because it is capable of real-time observation of cardiac activity in 10 s intervals and continuous long-term registration [44]. By using this Hr recording methodology, it is also possible to reveal early signs of physiological impairment in rescued individuals to quickly undertake the measures needed for survival in captivity. The method could be used to separate diseased and healthy individuals, which would not show external symptoms of disease in the initial phases of the infection, reducing the possibilities of cross-infections and increasing the chances of survival of uninfected individuals [57]. This is especially so since similar sensor technology applied on blue mussels indicated Hr changes as the response to Himasthla elongata (Mehlis, 1831) parasite infection [58]. More studies should be completed in this regard, to differentiate Hr between healthy and infected individuals by H. pinnae. The method is also easily adaptable to monitor other commercial and non-commercial species of sessile invertebrates to check their health status.
Thus, investigation focused on the maintenance of rescued individuals in captivity should be prioritized, studying their adaptive capacity under physico-chemical parameters variations by means of non-invasive techniques. It could be important for future repopulation trials in order to make the right decisions on the selection of environments suitable for the growth of P. nobilis but constrained or restricted for pathogen development. Moreover, an earlier long-term study [59] found that hyposalinity suppressed the distribution of a haplosporidian parasite in oyster beds. The same was observed in the case of P. nobilis within the littoral lagoons and deltas of Spain and France in the aforementioned studies. Accordingly, the ex situ monitoring of P. nobilis‘ physiological state under short and intensive hyposalinity could be important to obtain new insights of species’ salinity tolerance toward development of potential prophylactic treatment against H. pinnae, needed for the survival of already infected specimens, since [57] reported some positive responses to temperature and salinity treatments of the P. nobilis in captivity infected by H. pinnae.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/mi13091549/s1, Figure S1: Example of the heart rate pattern of pen shell P. nobilis in clean sea water (Hrrest), during ≈ 1 h of 50% salinity reduction by distilled water (dH2O) and after salt addition. Abbreviations: Trec—heart rate recovery time; Figure S2: Relation between resting heart rate (Hrrest) and antero–posterior (a) shell length, (b) shell width of P. nobilis. Circles represent mean values obtained in six independent experiments.
Author Contributions
Conceptualization, Z.G. and J.R.G.-M.; methodology, R.M.; software, R.M.; validation, J.R.G.-M., N.V. and Z.G.; formal analysis, Z.G.; investigation, R.M.; resources, D.J.; data curation, Z.G.; writing—original draft preparation, R.M.; writing—review and editing, J.R.G.-M.; visualization, Z.G.; supervision, N.V.; project administration, D.J.; funding acquisition, D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Prince Albert II of Monaco Foundation as part of the project, “The Study, Protection and Possible Breeding of Pen Shell (Pinna nobilis) in the Boka Kotorska Bay”, project code: BF/HEM 15-1662, Financing agreement N° 1796.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to SRCES RAS, St. Petersburg, Russia and the EPA Montenegro for providing experimental equipment. The authors are grateful to Luka Gačić, who provided improvements to our English.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Attrill, M.J. A testable linear model for diversity trends in estuaries. J. Anim. Ecol. 2002, 71, 262–269. [Google Scholar] [CrossRef]
- Philippart, C.J.; Anadón, R.; Danovaro, R.; Dippner, J.W.; Drinkwater, K.F.; Hawkins, S.J.; Oguzg, T.; O’Sullivanh, G.; Reid, P.C. Impacts of cli-mate change on European marine ecosystems: Observations, expectations and indicators. J. Exp. Mar. Biol. Ecol. 2011, 400, 52–69. [Google Scholar] [CrossRef]
- Peteiro, L.G.; Woodin, S.; Wethey, D.; Costas-Costas, D.; Martínez-Casal, A.; Olabarria, C.; Vázquez, E. Responses to salinity stress in bivalves: Evidence of ontogenetic changes in energetic physiology on Cerastoderma edule. Sci. Rep. 2018, 8, 8329. [Google Scholar] [CrossRef] [PubMed]
- Pourmozaffar, S.; Jahromi, S.T.; Rameshi, H.; Sadeghi, A.; Bagheri, T.; Behzadi, S.; Gozari, M.; Zahedi, M.R.; Lazarjani, S.A. The role of salinity in physiological responses of bivalves. Rev. Aquac. 2019, 12, 1548–1566. [Google Scholar] [CrossRef]
- Berger, V.J.; Kharazova, A.D. Mechanisms of salinity adaptations in marine molluscs. In Interactions and Adaptation Strategies of Marine Organisms; Springer: Dordrecht, The Netherlands, 1997; pp. 115–126. [Google Scholar]
- Depledge, M.; Andersen, B. A computer-aided physiological monitoring system for continuous, long-term recording of cardiac activity in selected invertebrates. Comp. Biochem. Physiol. Part A Physiol. 1990, 96, 473–477. [Google Scholar] [CrossRef]
- Bakhmet, I.; Berger, V.; Khalaman, V. The effect of salinity change on the heart rate of Mytilus edulis specimens from different ecological zones. J. Exp. Mar. Biol. Ecol. 2005, 318, 121–126. [Google Scholar] [CrossRef]
- Bakhmet, I.N.; Komendantov, A.J.; Smurov, A.O. Effect of salinity change on cardiac activity in Hiatella arctica and Modiolus modiolus, in the White Sea. Polar Biol. 2012, 35, 143–148. [Google Scholar] [CrossRef]
- Bakhmet, I.; Fokina, N.; Ruokolainen, T. Changes of heart rate and lipid composition in Mytilus edulis and Modiolus modiolus caused by crude oil pollution and low salinity effects. J. Xenobiotics 2021, 11, 46–60. [Google Scholar] [CrossRef]
- Sarà, G.; De Pirro, M. Heart beat rate adaptations to varying salinity of two intertidal Mediterranean bivalves: The invasive Brachidontes pharaonis and the native Mytilaster minimus. Ital. J. Zool. 2011, 78, 193–197. [Google Scholar] [CrossRef]
- Fedotov, V.P.; Kholodkevich, S.V.; Strochilo, A.G. Study of contractile activity of the crayfish heart with the aid of a new non-invasive technique. J. Evol. Biochem. Physiol. 2000, 36, 288–293. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Kuznetsova, T.V.; Lehtonen, K.K.; Kurakin, A.S. Experiences on ecological status assessment of the Gulf of Bothnia different sites based on cardiac activity biomarkers of caged mussels (Mytilus edulis). In Proceedings of the ICES Annual Science Conference, Gdańsk, Poland, 19–23 September 2011; Volume 19, p. 12. [Google Scholar]
- Martinović, R.; Kurakin, A.S.; Kholodkevich, S.V.; Gačić, Z.; Kljajić, Z. Preliminary results of sea water quality assessment based on physiological biomarkers in part of the Boka Kotorska Bay. Water Res. Manag. 2013, 3, 31–34. [Google Scholar]
- Turja, R.; Höher, N.; Snoeijs, P.; Baršienė, J.; Butrimavičienė, L.; Kuznetsova, T.; Kholodkevich, S.; Devier, M.-H.; Budzinski, H.; Lehtonen, K.K. A multibiomarker approach to the assessment of pollution impacts in two Baltic Sea coastal areas in Sweden using caged mussels (Mytilus trossulus). Sci. Total Environ. 2014, 473, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Kholodkevich, S.; Kuznetsova, T.V.; Sharov, A.N.; Kurakin, A.S.; Lips, U.; Kolesova, N.; Lehtonen, K.K. Applicability of a bioelectronic cardiac monitoring system for the detection of biological effects of pollution in bioindicator species in the Gulf of Finland. J. Mar. Syst. 2017, 171, 151–158. [Google Scholar] [CrossRef]
- Kholodkevich, S.; Sharov, A.; Kuznetsova, T.; Kurakin, A.; Joksimović, D.; Nikolić, M. Physiological testing of Mytilus galloprovincialis for the environmental assessing of coastal marine areas: A case study in Boka Kotorska Bay (the Adriatic Sea). Chem. Ecol. 2019, 35, 631–643. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Sharov, A.N.; Chuiko, G.M.; Kuznetsova, T.V.; Gapeeva, M.V.; Lozhkina, R.A. Quality Assessment of Freshwater Ecosystems by the Functional State of Bivalved Mollusks. Water Resour. 2019, 46, 249–257. [Google Scholar] [CrossRef]
- Nikolic, M.; Kuznetsova, T.; Kholodkevich, S.; Gvozdenovic, S.; Mandic, M.; Joksimovic, D.; Teodorovic, I. Cardiac activity in the Mediterranean mussel (Mytilus galloprovincialis Lamarck, 1819) as a biomarker for assessing sea water quality in Boka Kotorska Bay, South Adriatic Sea. Mediterr. Mar. Sci. 2019, 20, 680–687. [Google Scholar] [CrossRef]
- Zavodnik, D.; Hrs-Brenko, M.; Legac, M. Synopsis on the fan shell Pinna nobilis L. in the eastern Adriatic Sea. In Les Espèces Marines à Protéger en Méditerranée; Boudouresque, C.F., Avon, M., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1991; pp. 169–178. [Google Scholar]
- Butler, A.; Vicente, N.; De Gaulejac, B. Ecology of the pterioid bivalves Pinna bicolor Gmelin and Pinna nobilis L. Mar. Life 1993, 3, 37–45. [Google Scholar]
- Basso, L.; Vázquez-Luis, M.; García-March, J.R.; Deudero, S.; Alvarez, E.; Vicente, N.; Duarte, C.M.; Hendriks, I.E. The pen shell, Pinna nobilis: A review of population status and recommended research priorities in the Mediterranean Sea. Adv. Mar. Biol. 2015, 71, 109–160. [Google Scholar]
- Catanese, G.; Grau, A.; Valencia, J.M.; Garcia-March, J.R.; Vázquez-Luis, M.; Alvarez, E.; Deudero, S.; Darriba, S.; Carballal, M.J.; Villalba, A. Haplosporidium pinnae sp. nov., a haplosporidan parasite associated with mass mortalities of the fan mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Pathol. 2018, 157, 9–24. [Google Scholar] [CrossRef]
- Carella, F.; Aceto, S.; Pollaro, F.; Miccio, A.; Iaria, C.; Carrasco, N.; Prado, P.; De Vico, G. A mycobacterial disease is associated with the silent mass mortality of the pen shell Pinna nobilis along the Tyrrhenian coastline of Italy. Sci. Rep. 2019, 9, 2725. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Álvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jiménez, S.; Kersting, D.; Moreno, D.; Pérez, M.; et al. S.O.S. Pinna nobilis: A Mass Mortality Event in Western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 220. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsirintanis, K.; Tsaparis, D.; Doukas, D.; Sini, M.; Athanassopoulou, F.; Κolygas, M.N.; Tontis, D.; Koutsoubas, D.; Bakopoulos, V. The cryptogenic parasite Haplosporidium pinnae invades the Aegean Sea and causes the collapse of Pinna nobilis populations. Aquat. Invasions 2019, 14, 150–164. [Google Scholar] [CrossRef]
- Kersting, D.; Benabdi, M.; Čižmek, H.; Grau, A.; Jimenez, C.; Katsanevakis, S.; Öztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna nobilis. The IUCN Red List of Threatened Species 2019; IUCN Red List: London, UK, 2019. [Google Scholar] [CrossRef]
- Cabanellas-Reboredo, M.; Vázquez-Luis, M.; Mourre, B.; Álvarez, E.; Deudero, S.; Amores, Á.; Addis, P.; Ballesteros, E.; Barrajón, A.; Coppa, S.; et al. Tracking a mass mortality outbreak of pen shell Pinna nobilis populations: A collaborative effort of scientists and citizens. Sci. Rep. 2019, 9, 13355. [Google Scholar] [CrossRef] [PubMed]
- Simide, R.; Couvray, S.; Vicente, N. Présence de Pinna nobilis (L. 1758) dans l’étang littoral de Diana (Corse). Marinelife-revue.fr 2019, 1–4. [Google Scholar]
- Foulquié, M.; de la Grandrive, R.D.; Dalias, N.; Vicente, N. Inventaire et état de santé des populations de Pinna nobilis (L. 1758) dans l’étang de Thau (Hérault, France). Marinelife-revue.fr 2020, 1–25. [Google Scholar]
- Peyran, C.; Morage, T.; Nebot-Colomer, E.; Iwankow, G.; Planes, S. Unexpected residual habitats raise hope for the survival of the fan mussel Pinna nobilis along the Occitan coast (Northwest Mediterranean Sea). Endanger. Species Res. 2022, 48, 123–137. [Google Scholar] [CrossRef]
- Bellafiore, D.; Guarnieri, A.; Grilli, F.; Penna, P.; Bortoluzzi, G.; Giglio, F.; Pinardi, N. Study of the hydrodynamical processes in the Boka Kotorska Bay with a finite element model. Dyn. Atmos. Ocean. 2011, 52, 298–321. [Google Scholar] [CrossRef]
- García-March, J.R.; Sanchís Solsona, M.Á.; García-Carrascosa, A.M. Shell gaping behaviour of Pinna nobilis L.; 1758: Circadian and circalunar rhythms revealed by in situ monitoring. Mar. Biol. 2008, 153, 689–698. [Google Scholar] [CrossRef]
- Garcia-March, J.R.; Jiménez, S.; Sanchis, M.A.; Monleon, S.; Lees, J.; Surge, D.; Tena-Medialdea, J. In situ biomonitoring shows seasonal patterns and environmentally mediated gaping activity in the bivalve, Pinna nobilis. Mar. Biol. 2016, 163, 29. [Google Scholar] [CrossRef]
- Hernandis, S.; Garcia-March, J.; Sanchis, M.Á.; Monleón, S.; Vicente, N.; Tena, J. Temperature regulates the switch be-tween light-synchronized and unsynchronized activity patterns in the subtidal bivalve Pinna nobilis. Mediterr. Mar. Sci. 2018, 19, 366–375. [Google Scholar]
- Trigos, S.; García-March, J.R.; Vicente, N.; Tena, J.; Torres, J. Respiration rates of the fan mussel Pinna nobilis at different temperatures. J. Molluscan Stud. 2014, 81, 217–222. [Google Scholar] [CrossRef]
- Cappello, T.; Maisano, M.; Giannetto, A.; Natalotto, A.; Parrino, V.; Mauceri, A.; Spanò, N. Pen shell Pinna nobilis L. (Mollusca: Bivalvia) from different peculiar environments: Adaptive mechanisms of osmoregulation and neurotransmission. Eur. Zool. J. 2019, 86, 333–342. [Google Scholar] [CrossRef]
- Čižmek, H.; Čolić, B.; Gračan, R.; Grau, A.; Catanese, G. An emergency situation for pen shells in the Mediterranean: The Adriatic Sea, one of the last Pinna nobilis shelters, is now affected by a mass mortality event. J. Invertebr. Pathol. 2020, 173, 107388. [Google Scholar] [CrossRef]
- Mačić, V.; Hernandis Caballero, S.; Vicente, N.; García March, J.R.; Tena Medialdea, J.; Martinović, R.; Joksimović, D.; Drakulović, D.; Petović, S. Exceptionally high density of Pinna nobilis L. 1758 in the Boka Kotorska Bay (Montenegro). In Proceedings of the 13rd European Conference on Scientific Diving, Funchal, Madeira, Portugal, 22–23 March 2017. [Google Scholar]
- Marrocco, V.; Zangaro, F.; Sicuro, A.; Pinna, M. A scaling down mapping of Pinna nobilis (Linnaeus, 1758) through the combination of scientific literature, NATURA 2000, grey literature and citizen science data. Nat. Conserv. 2019, 33, 43–53. [Google Scholar] [CrossRef]
- Garcia-March, J.R.; Vicente, N. Protocol to Study and Monitor Pinna nobilis Populations within Marine Protected Areas; MEPA: La Valette, France, 2007. [Google Scholar]
- Trigos-Santos, S.; Vicente, N. Transplantation protocol for the fan mussel Pinna nobilis in different types of substrate. Mar. Life 2016, 18, 55–61. [Google Scholar]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195. [Google Scholar]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Kholodkevich, S.V.; Ivanov, A.V.; Kurakin, A.S.; Kornienko, E.L.; Fedotov, V.P. Real time biomonitoring of surface water toxicity level at water supply stations. Environ. Bioindic. 2008, 3, 23–34. [Google Scholar] [CrossRef]
- Handy, R.D.; Depledge, M.H. Physiological responses: Their measurement and use as environmental biomarkers in ecotoxicology. Ecotoxicology 1999, 8, 329–349. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 7. 2004. Available online: www.statsoft.com (accessed on 20 August 2022).
- Martinović, R.; Kolarević, S.; Kračun-Kolarević, M.; Kostić, J.; Marković, S.; Gačić, Z.; Kljajić, Z.; Vuković-Gačić, B. Genotoxic potential and heart rate disorders in the Mediterranean mussel Mytilus galloprovincialis exposed to Superdispersant-25 and dispersed diesel oil. Mar. Environ. Res. 2015, 108, 83–90. [Google Scholar] [CrossRef]
- Martinović, R.; Kolarević, S.; Kračun-Kolarević, M.; Kostić, J.; Jokanović, S.; Gačić, Z.; Joksimović, D.; Đurović, M.; Kljajić, Z.; Vuković-Gačić, B. Comparative assessment of cardiac activity and DNA damage in haemocytes of the Mediterranean mussel Mytilus galloprovincialis in exposure to tributyltin chloride. Environ. Toxicol. Pharmacol. 2016, 47, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Zarykhta, V.V.; Kuznetsova, T.V.; Sharov, A.N.; Kholodkevich, S.V.; Zhaohan, Z.; Yujie, F. Cardiac Activity in the Bivalve Mollusc Cristaria plicata from the River Songhua (China). J. Evol. Biochem. Physiol. 2019, 55, 423–425. [Google Scholar] [CrossRef]
- Nicholson, S. Ecophysiological aspects of cardiac activity in the subtropical mussel Perna viridis (L.) (Bivalvia: Mytilidae). J. Exp. Mar. Biol. Ecol. 2002, 267, 207–222. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, L.; Li, Y.; Zhu, X.; Li, Y.; Guo, H.; Bao, Z.; Wang, S. Development of Novel Cardiac Indices and Assessment of Factors Affecting Cardiac Activity in a Bivalve Mollusc Chlamys farreri. Front. Physiol. 2019, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Braby, C.E.; Somero, G.N. Following the heart: Temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J. Exp. Biol. 2006, 209, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Depledge, M.H.; Lundebye, A.K. Physiological monitoring of contaminant effects in individual rock crabs, Hemigrapsus edwardsi: The ecotoxicological significance of variability in response. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 113, 277–282. [Google Scholar] [CrossRef]
- Joksimović, D.; Perošević, A.; Castelli, A.; Pestorić, B.; Šuković, D.; Đurović, D. Assessment of heavy metal pollution in surface sediments of the Montenegrin coast: A 10-year review. J. Soils Sediments 2020, 20, 2598–2607. [Google Scholar] [CrossRef]
- Joksimović, D.; Stanković, S. Accumulation of trace metals in marine organisms of the southeastern Adriatic coast, Montenegro. J. Serb. Chem. Soc. 2012, 77, 105–117. [Google Scholar] [CrossRef]
- Martinović, R.; Petović, S.; Joksimović, D.; Bunet, R.; Couvray, S.; Kirchhofer, D.; Simide, R.; Garcia-March, J.R.; Tena-Medialdea, J.; Castelli, A.; et al. Recruitment and Growth of the Fan Mussel Pinna nobilis in the Montenegrin Adriatic Coast and Comparison with the Western Mediterranean. In The Montenegrin Adriatic Coast; Springer: Cham, Switzerland, 2021; pp. 193–213. [Google Scholar] [CrossRef]
- García-March, J.R.; Tena, J.; Henandis, S.; Vázquez-Luis, M.; López, D.; Téllez, C.; Prado, P.; Navas, J.I.; Bernal, J.; Catanese, G.; et al. Can we save a marine species affected by a highly infective, highly lethal, waterborne disease from extinction? Biol. Conserv. 2020, 243, 108498. [Google Scholar] [CrossRef]
- Bakhmet, I.; Nikolaev, K.; Levakin, I.; Ekimov, D. Influence of Himasthla elongata (Trematoda: Echinostomatidae) metacercariae on heart rate in blue mussels (Mytilus edulis). J. Invertebr. Pathol. 2019, 166, 107220. [Google Scholar] [CrossRef]
- Haskin, H.H.; Ford, S.E. Haplosporidium nelsoni (MSX) on delaware bay seed oyster beds: A host-parasite relationship along a salinity gradient. J. Invertebr. Pathol. 1982, 40, 388–405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).