Cold Laser Micro-Machining of PDMS as an Encapsulation Layer for Soft Implantable Neural Interface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Neural Electrode
2.2. Excimer Laser
2.3. Orthogonal Experimental Design
2.4. Electrochemical Impedance Measurement and Analysis
3. Results and Discussion
3.1. Orthogonal Experimental Analysis of EIS
3.1.1. Range Analysis
3.1.2. Mean Analysis
3.2. Orthogonal Experimental Analysis Based on Equivalent Circuit Model
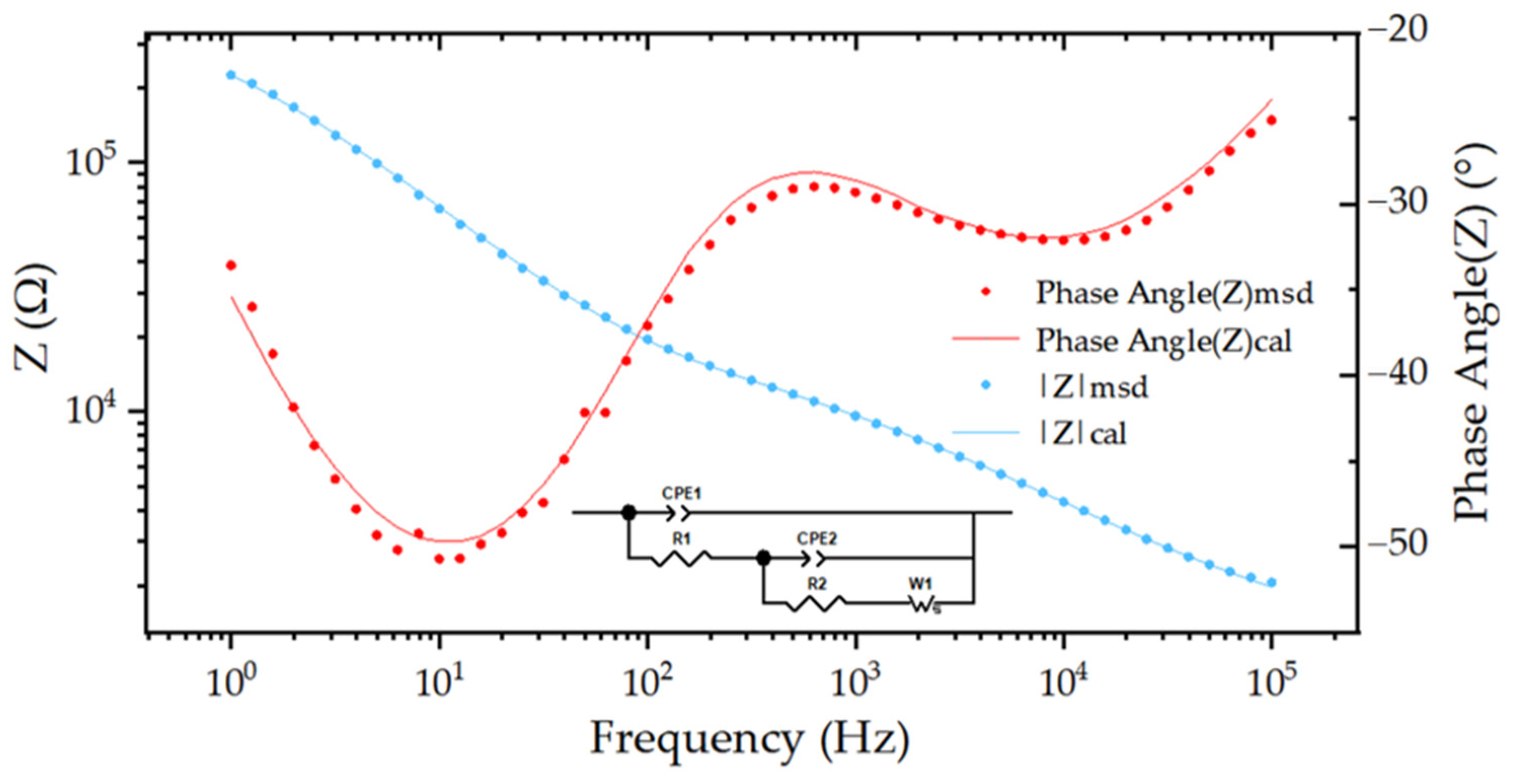
3.2.1. Construction of Equivalent Circuit Model
3.2.2. Range Analysis
3.2.3. Mean Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Zhao, Y.; Hao, W.; Zhang, X.D.; Ming, D. Micro- and nanotechnology for neural electrode-tissue interfaces. Biosens. Bioelectron. 2020, 170, 112645. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Lycke, R.; Ganji, M.; Xie, C.; Luan, L. Ultraflexible Neural Electrodes for Long-Lasting Intracortical Recording. iScience 2020, 23, 101387. [Google Scholar] [CrossRef] [PubMed]
- Fairfield, J.A. Nanostructured Materials for Neural Electrical Interfaces. Adv. Funct. Mater. 2018, 28, 1701145. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Guger, C.; Muller, G.; Krausz, G.; Neuper, C. Brain oscillations control hand orthosis in a tetraplegic. Neurosci. Lett. 2000, 292, 211–214. [Google Scholar] [CrossRef]
- Lacour, S.P.; Benmerah, S.; Tarte, E.; FitzGerald, J.; Serra, J.; McMahon, S.; Fawcett, J.; Graudejus, O.; Yu, Z.; Morrison, B. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med. Biol. Eng. Comput. 2010, 48, 945–954. [Google Scholar] [CrossRef]
- Lötters, J.C.; Olthuis, W.; Veltink, P.H.; Bergveld, P. The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications. J. Micromech. Microeng. 1997, 7, 145. [Google Scholar] [CrossRef]
- Abbasi, F.; Mirzadeh, H.; Katbab, A.A. Modification of polysiloxane polymers for biomedical applications: A review. Soc. Chem. Ind. 2001, 50, 1279–1287. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Guo, L.; Guvanasen, G.S.; Liu, X.; Tuthill, C.; Nichols, T.R.; DeWeerth, S.P. A PDMS-based integrated stretchable microelectrode array (isMEA) for neural and muscular surface interfacing. IEEE Trans. Biomed. Circuits Syst. 2012, 7, 1–10. [Google Scholar]
- Kim, J.-M.; Oh, D.-R.; Sanchez, J.; Kim, S.-H.; Seo, J.-M. Fabrication of polydimethylsiloxane (PDMS)-based multielectrode array for neural interface. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1716–1719. [Google Scholar]
- Delivopoulos, E.; Chew, D.J.; Minev, I.R.; Fawcett, J.W.; Lacour, S.P. Concurrent recordings of bladder afferents from multiple nerves using a microfabricated PDMS microchannel electrode array. Lab Chip 2012, 12, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Delivopoulos, E.; Minev, I.R.; Lacour, S.P. Evaluation of negative photo-patternable PDMS for the encapsulation of neural electrodes. In Proceedings of the 2011 5th International IEEE/EMBS Conference on Neural Engineering, Cancun, Mexico, 27 April 2011–1 May 2011; pp. 490–494. [Google Scholar]
- Ochoa, M.; Wei, P.; Wolley, A.J.; Otto, K.J.; Ziaie, B. A hybrid PDMS-Parylene subdural multi-electrode array. Biomed. Microdevices 2013, 15, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Babaroud, N.B.; Dekker, R.; Serdijn, W.; Giagka, V. PDMS-Parylene adhesion improvement via ceramic interlayers to strengthen the encapsulation of active neural implants. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3399–3402. [Google Scholar]
- Ordonez, J.S.; Boehler, C.; Schuettler, M.; Stieglitz, T. Silicone rubber and thin-film polyimide for hybrid neural interfaces—A MEMS-based adhesion promotion technique. In Proceedings of the 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER), San Diego, CA, USA, 6–8 November 2013; pp. 872–875. [Google Scholar]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L. Electronic dura mater for long-term multimodal neural interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef]
- Yeon, P.; Rajan, S.K.; Falcone, J.; Gonzalez, J.L.; May, G.S.; Bellamkonda, R.V.; Brand, O.; Bakir, M.S.; Ghovanloo, M. Microfabrication, Coil Characterization, and Hermetic Packaging of Millimeter-Sized Free-Floating Neural Probes. IEEE Sens. J. 2021, 21, 13837–13848. [Google Scholar] [CrossRef]
- Li, X.R.; Song, Y.L.; Xiao, G.H.; Xie, J.Y.; Dai, Y.C.; Xing, Y.; He, E.H.; Wang, Y.; Xu, S.W.; Zhang, L.L.; et al. Flexible Electrocorticography Electrode Array for Epileptiform Electrical Activity Recording under Glutamate and GABA Modulation on the Primary Somatosensory Cortex of Rats. Micromachines 2020, 11, 732. [Google Scholar] [CrossRef]
- Trevathan, J.K.; Baumgart, I.W.; Nicolai, E.N.; Gosink, B.A.; Asp, A.J.; Settell, M.L.; Polaconda, S.R.; Malerick, K.D.; Brodnick, S.K.; Zeng, W.F.; et al. An Injectable Neural Stimulation Electrode Made from an In-Body Curing Polymer/Metal Composite. Adv. Healthc. Mater. 2019, 8, 1900892. [Google Scholar] [CrossRef]
- Lee, W.R.; Im, C.; Park, H.Y.; Seo, J.M.; Kim, J.M. Fabrication of Convex PDMS-Parylene Microstructures for Conformal Contact of Planar Micro-Electrode Array. Polymers 2019, 11, 1436. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Wang, K.; Li, S.W.; Zhang, P.; Shen, Y.; Fu, Y.; Zhang, Y.; Zhou, J.; Wang, C.Y. Polydimethylsiloxane (PDMS)-Based Flexible Optical Electrodes with Conductive Composite Hydrogels Integrated Probe for Optogenetics. J. Biomed. Nanotechnol. 2018, 14, 1099–1106. [Google Scholar] [CrossRef]
- Adly, N.; Weidlich, S.; Seyock, S.; Brings, F.; Yakushenko, A.; Offenhausser, A.; Wolfrum, B. Printed microelectrode arrays on soft materials: From PDMS to hydrogels. NPJ Flex. Electron. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Biswas, S.; Sikdar, D.; Das, D.; Mahadevappa, M.; Das, S. PDMS based multielectrode arrays for superior in-vitro retinal stimulation and recording. Biomed. Microdevices 2017, 19, 1–14. [Google Scholar] [CrossRef]
- Garra, J.; Long, T.; Currie, J.; Schneider, T.; White, R.; Paranjape, M. Dry etching of polydimethylsiloxane for microfluidic systems. J. Vac. Sci. Technol. A 2002, 20, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Dubowski, J.; Julier, M.; Sproule, G.; Mason, B. Laser-assisted dry etching ablation for microstructuring of III-V semiconductors. MRS Online Proc. Libr. (OPL) 1995, 397, 509. [Google Scholar] [CrossRef]
- Wang, J.; Niino, H.; Yabe, A. Micromachining of quartz crystal with excimer lasers by laser-induced backside wet etching. Appl. Phys. A 1999, 69, S271–S273. [Google Scholar] [CrossRef]
- Liang, C.; Su, W.; Sun, X.; Hu, Y.; Duan, J.A. Femtosecond Laser Patterning Wettability-Assisted PDMS for Fabrication of Flexible Silver Nanowires Electrodes. Adv. Mater. Interfaces 2021, 8, 2100608. [Google Scholar] [CrossRef]
- Saadat, M.; Taylor, M.; Hughes, A.; Hajiyavand, A.M. Rapid prototyping method for 3D PDMS microfluidic devices using a red femtosecond laser. Adv. Mech. Eng. 2020, 12, 1687814020982713. [Google Scholar] [CrossRef]
- Yong, J.L.; Chen, F.; Huo, J.L.; Fang, Y.; Yang, Q.; Zhang, J.Z.; Hou, X. Femtosecond laser induced underwater superaerophilic and superaerophobic PDMS sheets with through microholes for selective passage of air bubbles and further collection of underwater gas. Nanoscale 2018, 10, 3688–3696. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Hayashi, S.; Katayama, A.; Nedyalkov, N.; Terakawa, M. Femtosecond Laser-Based Modification of PDMS to Electrically Conductive Silicon Carbide. Nanomaterials 2018, 8, 558. [Google Scholar] [CrossRef]
- Yong, J.L.; Chen, F.; Yang, Q.; Du, G.Q.; Bian, H.; Zhang, D.S.; Si, J.H.; Yun, F.; Hou, X. Rapid Fabrication of Large-Area Concave Micro lens Arrays on PDMS by a Femtosecond Laser. ACS Appl. Mater. Interfaces 2013, 5, 9382–9385. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Z.; Hou, Z.; Song, J. Processing and Profile Control of Microhole Array for PDMS Mask with Femtosecond Laser. Micromachines 2022, 13, 340. [Google Scholar] [CrossRef]
- Wolfe, D.B.; Ashcom, J.B.; Hwang, J.C.; Schaffer, C.B.; Mazur, E.; Whitesides, G.M. Customization of poly(dimethylsiloxane) stamps by micromachining using a femtosecond-pulsed laser. Adv. Mater. 2003, 15, 62. [Google Scholar] [CrossRef]
- Kim, T.N.; Campbell, K.; Groisman, A.; Kleinfeld, D.; Schaffer, C.B. Femtosecond laser-drilled capillary integrated into a microfluidic device. Appl. Phys. Lett. 2005, 86, 201106. [Google Scholar] [CrossRef] [Green Version]
- Borasi, L.; Casamenti, E.; Charvet, R.; Denereaz, C.; Pollonghini, S.; Deillon, L.; Yang, T.; Ebrahim, F.; Mortensen, A.; Bellouard, Y. 3D metal freeform micromanufacturing. J. Manuf. Process. 2021, 68, 867–876. [Google Scholar] [CrossRef]
- Qi, L.; Ruck, C.; Spychalski, G.; King, B.; Wu, B.; Zhao, Y. Writing Wrinkles on Poly(dimethylsiloxane) (PDMS) by Surface Oxidation with a CO2 Laser Engraver. ACS Appl. Mater. Interfaces. 2018, 10, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.T. Fabricating plasma bonded microfluidic chips by CO2 laser machining of PDMS by the application of viscoelastic particle focusing and droplet generation. J. Manuf. Process. 2022, 73, 260–268. [Google Scholar] [CrossRef]
- Qiang, Y.-X.; Zhu, C.-H.; Wu, Y.-P.; Cui, S.; Liu, Y. Bio-inspired semi-transparent silver nanowire conductor based on a vein network with excellent electromechanical and photothermal properties. RSC Adv. 2018, 8, 23066–23076. [Google Scholar] [CrossRef]
- Wu, C.; Xu, J.; Zhang, T.; Xin, G.; Li, M.; Rong, Y.; Zhang, G.; Huang, Y. Precision cutting of PDMS film with UV-nanosecond laser based on heat generation-diffusion regulation. Opt. Laser Technol. 2022, 145, 107462. [Google Scholar] [CrossRef]
- Hohnholz, A.; Obata, K.; Nakajima, Y.; Koch, J.; Terakawa, M.; Suttmann, O.; Overmeyer, L. Hybrid UV laser direct writing of UV-curable PDMS thin film using aerosol jet printing. Appl. Phys. A 2019, 125, 1–6. [Google Scholar] [CrossRef]
- Yan, Z.; Huang, X.; Yang, C. Rapid prototyping of single-layer microfluidic PDMS devices with abrupt depth variations under non-clean-room conditions by using laser ablation and UV-curable polymer. Microfluid. Nanofluid. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Lippert, T.K.; Bennett, L.; Kunz, T.; Hahn, C.; Wokaun, A.J.; Furutani, H.; Fukumura, H.; Masuhara, H.M.; Nakamura, T.; Yabe, A. Photopolymers designed for high-resolution laser ablation at a specific irradiation wavelength. In Proceedings of the Excimer Lasers, Optics, and Applications, San Jose, CA, USA, 31 March 1997; pp. 135–143. [Google Scholar]
- Athanassiou, A.; Lassithiotaki, M.; Anglos, D.; Georgiou, S.; Fotakis, C. A comparative study of the photochemical modifications effected in the UV laser ablation of doped polymer substrates. Appl. Surf. Sci. 2000, 154, 89–94. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Haag, R.; Bowden, N.; Whitesides, G.M. Generation of micrometer-sized patterns for microanalytical applications using a laser direct-write method and microcontact printing. Anal. Chem. 1998, 70, 4645–4652. [Google Scholar] [CrossRef]
- Kusaka, Y.; Hirata, A.; Ushijima, H. Direct adhesion contrast patterning on PDMS substrate by ArF excimer laser scanning for on-demand printing of functional layers. Int. J. Adv. Manuf. Technol. 2018, 99, 859–865. [Google Scholar] [CrossRef]
- Zhu, J.J.; Chew, D.A.S.; Lv, S.N.; Wu, W.W. Optimization method for building envelope design to minimize carbon emissions of building operational energy consumption using orthogonal experimental design (OED). Habitat. Int. 2013, 37, 148–154. [Google Scholar] [CrossRef]
- Ballantyne, K.; Van Oorschot, R.; Mitchell, R. Reduce optimisation time and effort: Taguchi experimental design methods. Forensic Sci. Int. Genet. Suppl. Ser. 2008, 1, 7–8. [Google Scholar] [CrossRef]
- Harris, A.R.; Paolini, A.G. Correlation of impedance and effective electrode area of iridium oxide neural electrodes. Aust. J. Chem. 2017, 70, 1016–1024. [Google Scholar] [CrossRef]
- Harris, A.R.; Hutchinson, R.; Molino, P.J.; Kapsa, R.M.I.; Clark, G.M.; Paolini, A.G.; Wallace, G.G. Correlation of Impedance and Effective Electrode Area of Dextran Sulfate Doped PEDOT Modified Electrodes. J. Electrochem. Soc. 2016, 163, H534–H540. [Google Scholar] [CrossRef]
- Harris, A.R.; Molino, P.J.; Kapsa, R.M.; Clark, G.M.; Paolini, A.G.; Wallace, G.G. Correlation of the impedance and effective electrode area of doped PEDOT modified electrodes for brain-machine interfaces. Analyst 2015, 140, 3164–3174. [Google Scholar] [CrossRef]
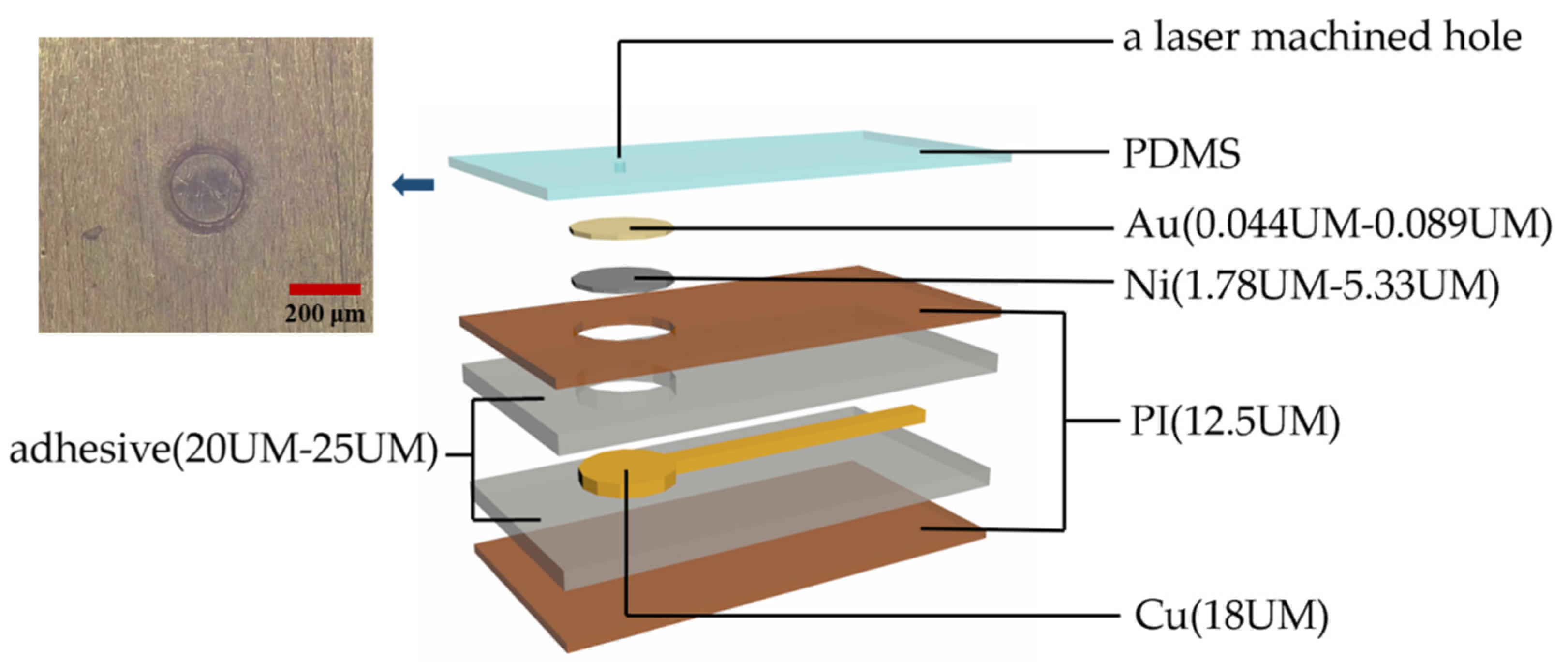
| Low Pulse Energy (<3 mJ) | High Pulse Energy (>5 mJ) | |
|---|---|---|
| small number (<400 times) | PDMS is not penetrated | electrode is damaged |
| large number (>600 times) | PDMS cutting edges are not neat | electrode is damaged |
| Level | (A) Pulse Energy (mJ) | (B) Number (Times) | (C) Repetition Rate (Hz) |
|---|---|---|---|
| 1 | 3 | 400 | 100 |
| 2 | 4 | 500 | 200 |
| 3 | 5 | 600 | 300 |
| Experimental Label | (A) Pulse Energy (mJ) | (B) Number (Times) | (C) Repetition Rate (Hz) |
|---|---|---|---|
| 1 | 3 | 400 | 100 |
| 2 | 3 | 500 | 200 |
| 3 | 3 | 600 | 300 |
| 4 | 4 | 400 | 200 |
| 5 | 4 | 500 | 300 |
| 6 | 4 | 600 | 100 |
| 7 | 5 | 400 | 300 |
| 8 | 5 | 500 | 100 |
| 9 | 5 | 600 | 200 |
| 200 * 450 μm | 1 Hz (Ω) | 100 Hz (Ω) | 1 KHz (Ω) | 10 KHz (Ω) | 100 KHz (Ω) |
|---|---|---|---|---|---|
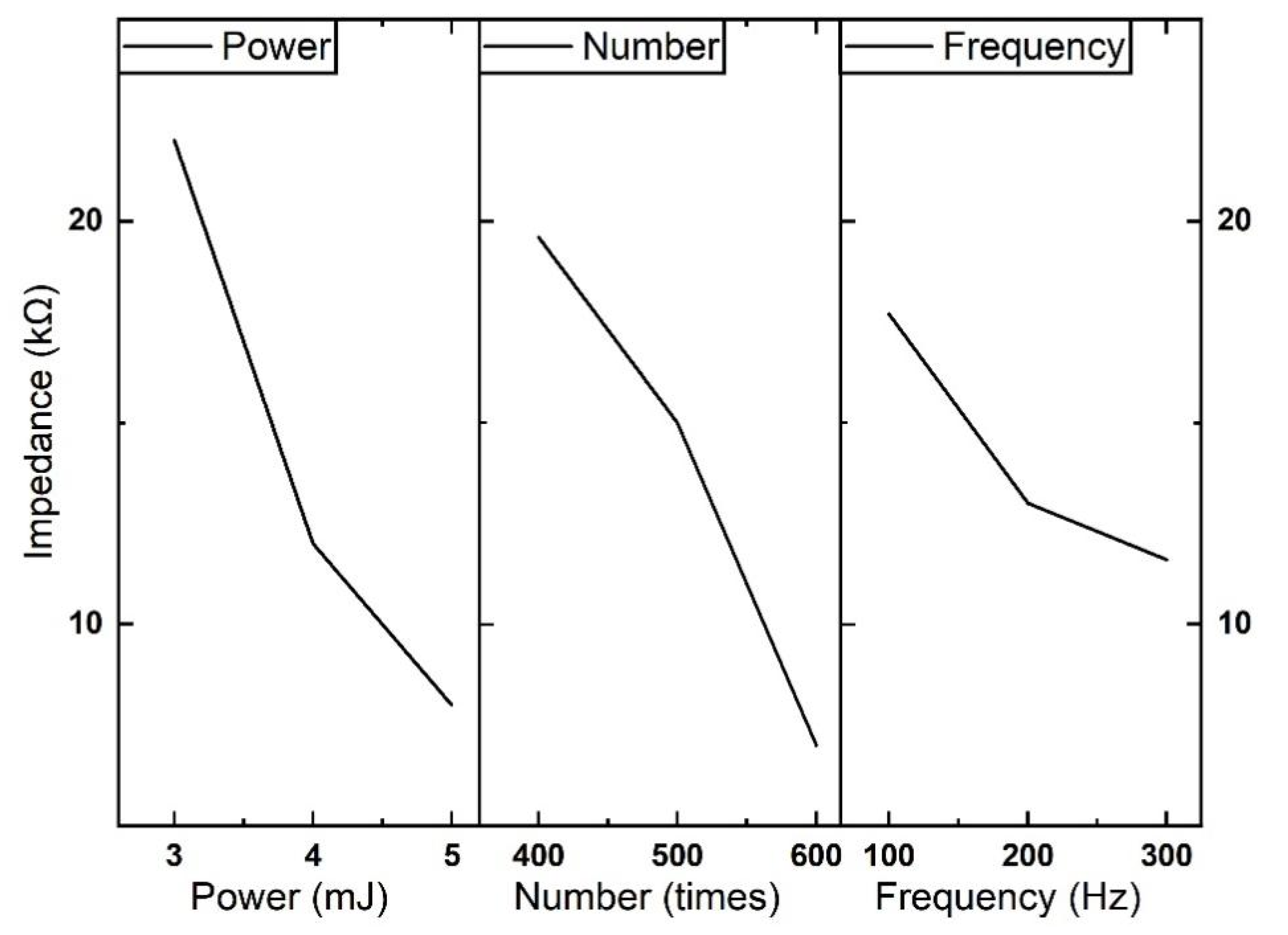
| (A) Pulse energy | 51,247.3 | 25,884.9 | 14,031.7 | 7414.3 | 2230.9 |
| (B) Number | 50,614.4 | 20,682.5 | 12,419.0 | 6670.6 | 2506.1 |
| (C) Repetition rate | 34,187.2 | 10,744.1 | 6065.7 | 3012.6 | 609.6 |
| Dominant factor | A > B > C | A > B > C | A > B > C | A > B > C | B > A > C |
| 200 μm | 1 Hz (Ω) | 100 Hz (Ω) | 1 KHz (Ω) | 10 KHz (Ω) | 100 KHz (Ω) |
|---|---|---|---|---|---|
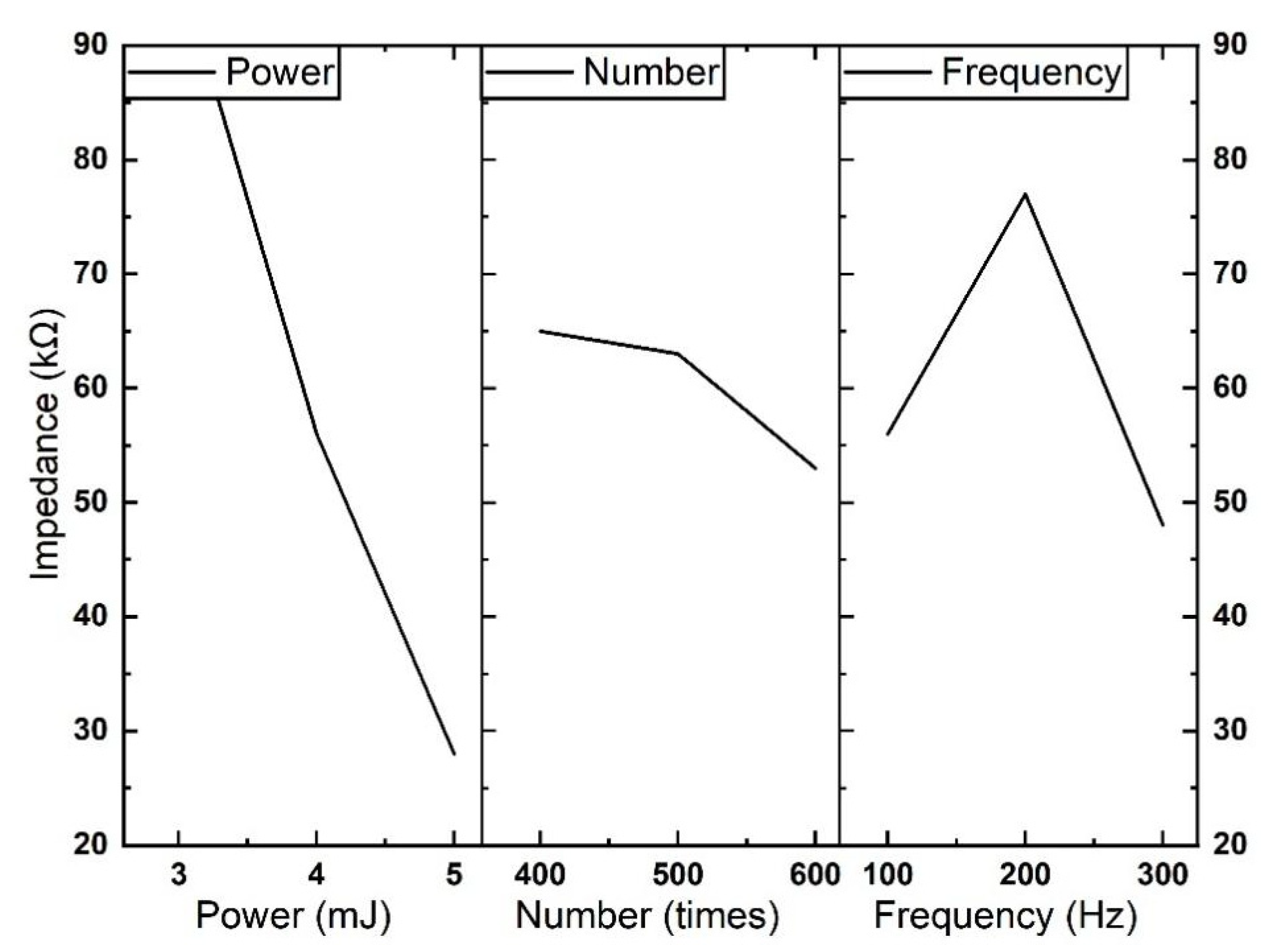
| (A) Pulse energy | 338,858.1 | 165,371.1 | 69,091.4 | 29,252.6 | 9197.4 |
| (B) Number | 71,222.3 | 33,870.8 | 12,170.4 | 5193.7 | 2462.5 |
| (C) Repetition rate | 140,860.4 | 63,946.0 | 29,342.0 | 11,636.4 | 4932.8 |
| Dominant factor | A > C > B | A > C > B | A > C > B | A > C > B | A > C > B |
| 200 * 450 μm | 1 Hz (Ω) | 100 Hz (Ω) | 1 KHz (Ω) | 10 KHz (Ω) | 100 KHz (Ω) |
|---|---|---|---|---|---|
| (A1) 3 mJ | 89,721.5 | 41,472.2 | 22,061.0 | 11,808.7 | 4986.2 |
| (A2) 4 mJ | 58,563.3 | 24,742.0 | 12,327.4 | 5561.9 | 2755.3 |
| (A3) 5 mJ | 38,474.2 | 15,587.3 | 8029.3 | 4394.4 | 2809.0 |
| (B1) 400 times | 87,945.6 | 36,177.0 | 19,685.7 | 10,300.0 | 4647.5 |
| (B2) 500 times | 61,482.2 | 30,129.9 | 15,465.4 | 7835.6 | 3761.4 |
| (B3) 600 times | 37,331.2 | 15,494.5 | 7266.7 | 3629.4 | 2141.5 |
| (C1) 100 Hz | 83,848.9 | 33,829.2 | 17,694.1 | 8859.4 | 3818.6 |
| (C2) 200 Hz | 49,661.7 | 24,887.2 | 13,095.2 | 7058.7 | 3522.8 |
| (C3) 300 Hz | 53,248.3 | 23,085.1 | 11,628.4 | 5846.9 | 3209.0 |
| Best combination | A3B3C2 | A3B3C3 | A3B3C3 | A3B3C3 | A2B3C3 |
| 200 μm | 1 Hz (Ω) | 100 Hz (Ω) | 1 KHz (Ω) | 10 KHz (Ω) | 100 KHz (Ω) |
|---|---|---|---|---|---|
| (A1) 3 mJ | 479,222.6 | 231,745.8 | 97,450.9 | 39,149.3 | 12,563.4 |
| (A2) 4 mJ | 232,688.2 | 144,667.0 | 56,782.2 | 16,139.2 | 4769.2 |
| (A3) 5 mJ | 140,364.5 | 66,374.7 | 28,359.5 | 9896.7 | 3366.0 |
| (B1) 400 times | 279,028.0 | 168,608.7 | 65,751.1 | 20,443.0 | 5756.0 |
| (B2) 500 times | 322,234.8 | 139,440.8 | 63,260.8 | 24,967.9 | 8218.4 |
| (B3) 600 times | 251,012.5 | 134,737.9 | 53,580.7 | 19,774.3 | 6724.2 |
| (C1) 100 Hz | 255,215.3 | 143,257.6 | 56,096.1 | 16,784.8 | 4376.9 |
| (C2) 200 Hz | 368,960.2 | 181,737.9 | 77,919.2 | 28,421.2 | 9309.7 |
| (C3) 300 Hz | 228,099.8 | 117,792.0 | 48,577.3 | 19,979.1 | 7011.9 |
| Best combination | A3B3C3 | A3B3C3 | A3B3C3 | A3B3C1 | A3B1C1 |
| 1 Hz (Ω) | 100 Hz (Ω) | 10 kHz (Ω) | 100 kHz (Ω) | |
|---|---|---|---|---|
| Total average | 173,172.4 | 87,431.5 | 14,491.7 | 5208.2 |
| 200 * 450 μm | R1(×104) (Ω) | R2 (×104) (Ω) | CPE1 (×10−10) (F) | CPE2 (×10−8) (F) | W (×105) (F) |
|---|---|---|---|---|---|
| (A) Pulse energy | 0.72 | 2.53 | 1.13 | 1.98 | 0.60 |
| (B) Number | 0.63 | 1.90 | 1.14 | 2.97 | 0.94 |
| (C) Repetition rate | 0.18 | 0.80 | 0.27 | 1.43 | 0.78 |
| Dominant factor | A > B > C | A > B > C | B > A > C | B > A > C | B> A > C |
| 200 μm | R1 (×104) (Ω) | R2(×104) (Ω) | CPE1 (×10−10) (F) | CPE2 (×10−9) (F) | W (×105) (F) |
|---|---|---|---|---|---|
| (A) Pulse energy | 4.18 | 22.43 | 2.10 | 2.97 | 3.27 |
| (B) Number | 0.20 | 3.07 | 0.94 | 3.50 | 1.50 |
| (C) Repetition rate | 1.02 | 5.13 | 3.31 | 1.43 | 2.07 |
| Dominant factor | A > B > C | A > C > B | C > A > B | B > A > C | A > C > B |
| 200 * 450 μm | R1 (×104) (Ω) | R2 (×104) (Ω) | CPE1 (×10−10) (F) | CPE2 (×10−8) (F) | W (×105) (F) |
|---|---|---|---|---|---|
| (A1) 3 mJ | 1.10 | 3.43 | 3.33 | 3.43 | 1.39 |
| (A2) 4 mJ | 0.53 | 2.40 | 3.83 | 1.45 | 1.13 |
| (A3) 5 mJ | 0.38 | 0.90 | 2.70 | 3.23 | 0.79 |
| (B1) 400 times | 0.93 | 2.37 | 2.63 | 1.53 | 1.63 |
| (B2) 500 times | 0.78 | 3.13 | 3.47 | 2.09 | 0.99 |
| (B3) 600 times | 0.30 | 1.23 | 3.77 | 4.50 | 0.69 |
| (C1) 100 Hz | 0.78 | 2.33 | 3.17 | 1.93 | 1.57 |
| (C2) 200 Hz | 0.63 | 1.80 | 3.27 | 3.37 | 0.79 |
| (C3) 300 Hz | 0.60 | 2.60 | 3.43 | 2.82 | 0.96 |
| Best combination | A3B3C3 | A3B3C2 | A3B1C1 | A2B1C1 | A3B3C2 |
| 200 μm | R1 (×104) (Ω) | R2(×104) (Ω) | CPE1 (×10−10) (F) | CPE2 (×10−9) (F) | W (×105) (F) |
|---|---|---|---|---|---|
| (A1) 3 mJ | 5.47 | 28.37 | 2.46 | 6.47 | 5.77 |
| (A2) 4 mJ | 2.30 | 14.13 | 4.55 | 7.77 | 3.17 |
| (A3) 5 mJ | 1.28 | 5.93 | 4.30 | 9.43 | 2.50 |
| (B1) 400 times | 2.93 | 17.77 | 4.30 | 6.40 | 3.47 |
| (B2) 500 times | 3.13 | 14.70 | 3.36 | 7.37 | 4.73 |
| (B3) 600 times | 2.98 | 15.97 | 3.66 | 9.90 | 3.23 |
| (C1) 100 Hz | 2.83 | 15.03 | 5.66 | 7.33 | 3.57 |
| (C2) 200 Hz | 3.62 | 19.27 | 2.35 | 7.57 | 4.97 |
| (C3) 300 Hz | 2.60 | 14.13 | 3.30 | 8.77 | 2.90 |
| Best combination | A3B1C3 | A3B2C3 | A1B2C2 | A1B1C1 | A3B3C3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, Y.; Bin, J.; Niu, L.; Zhang, J.; Liu, L.; Wang, A.; Tao, J.; Liang, J.; Zhang, L.; et al. Cold Laser Micro-Machining of PDMS as an Encapsulation Layer for Soft Implantable Neural Interface. Micromachines 2022, 13, 1484. https://doi.org/10.3390/mi13091484
Wang M, Zhang Y, Bin J, Niu L, Zhang J, Liu L, Wang A, Tao J, Liang J, Zhang L, et al. Cold Laser Micro-Machining of PDMS as an Encapsulation Layer for Soft Implantable Neural Interface. Micromachines. 2022; 13(9):1484. https://doi.org/10.3390/mi13091484
Chicago/Turabian StyleWang, Minjie, Yuan Zhang, Jianxiong Bin, Lan Niu, Jing Zhang, Lusheng Liu, Aiping Wang, Jin Tao, Jingqiu Liang, Lihua Zhang, and et al. 2022. "Cold Laser Micro-Machining of PDMS as an Encapsulation Layer for Soft Implantable Neural Interface" Micromachines 13, no. 9: 1484. https://doi.org/10.3390/mi13091484
APA StyleWang, M., Zhang, Y., Bin, J., Niu, L., Zhang, J., Liu, L., Wang, A., Tao, J., Liang, J., Zhang, L., & Kang, X. (2022). Cold Laser Micro-Machining of PDMS as an Encapsulation Layer for Soft Implantable Neural Interface. Micromachines, 13(9), 1484. https://doi.org/10.3390/mi13091484






