Small-Molecule Fluorescent Probes for Detecting Several Abnormally Expressed Substances in Tumors
Abstract
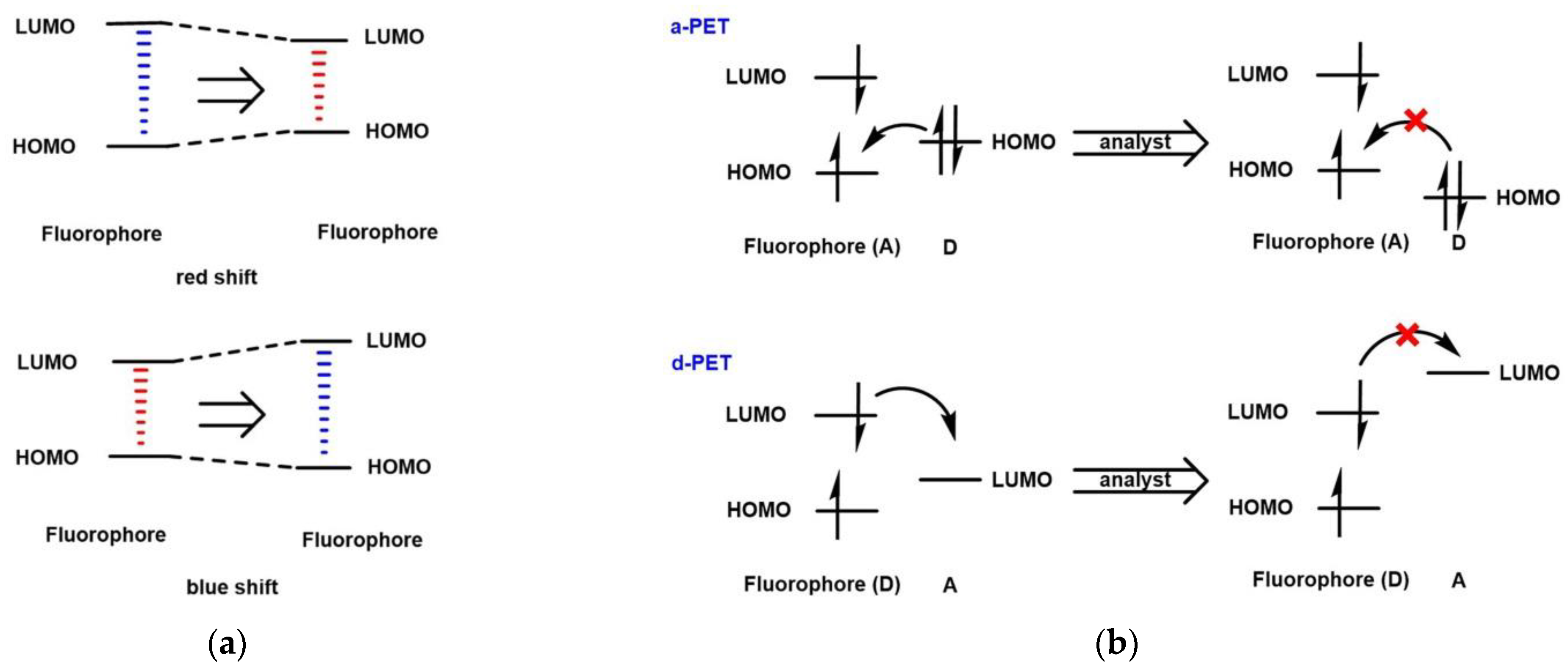
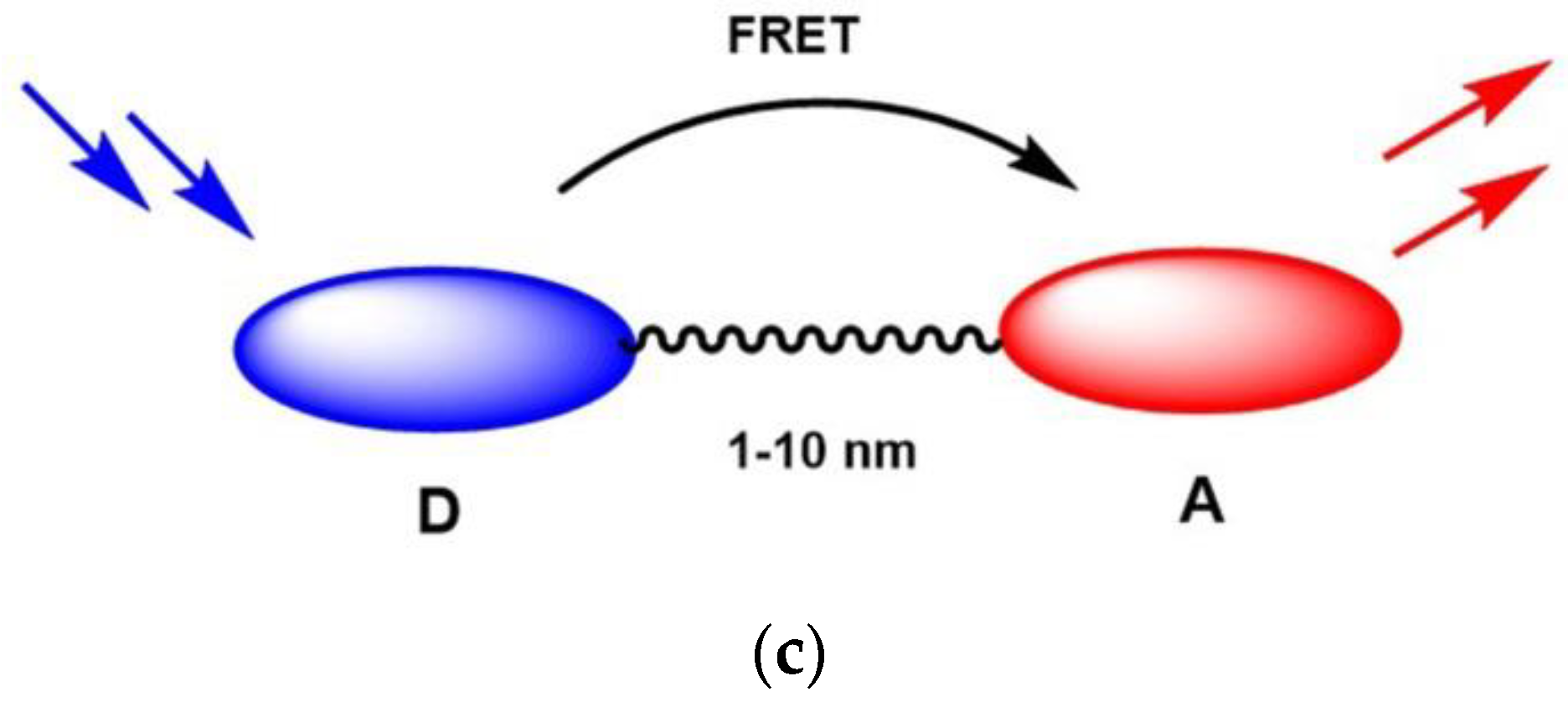
:1. Introduction
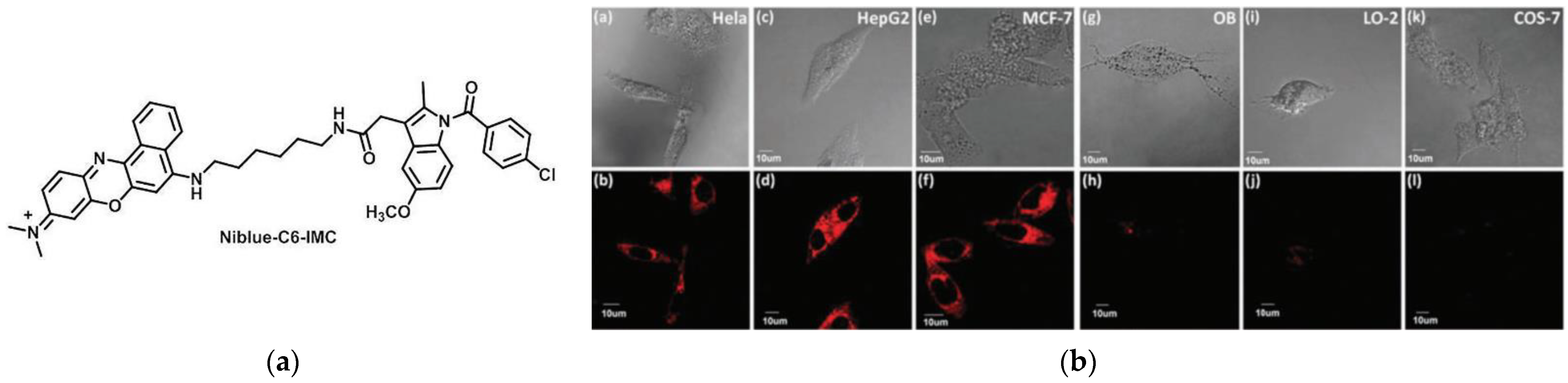
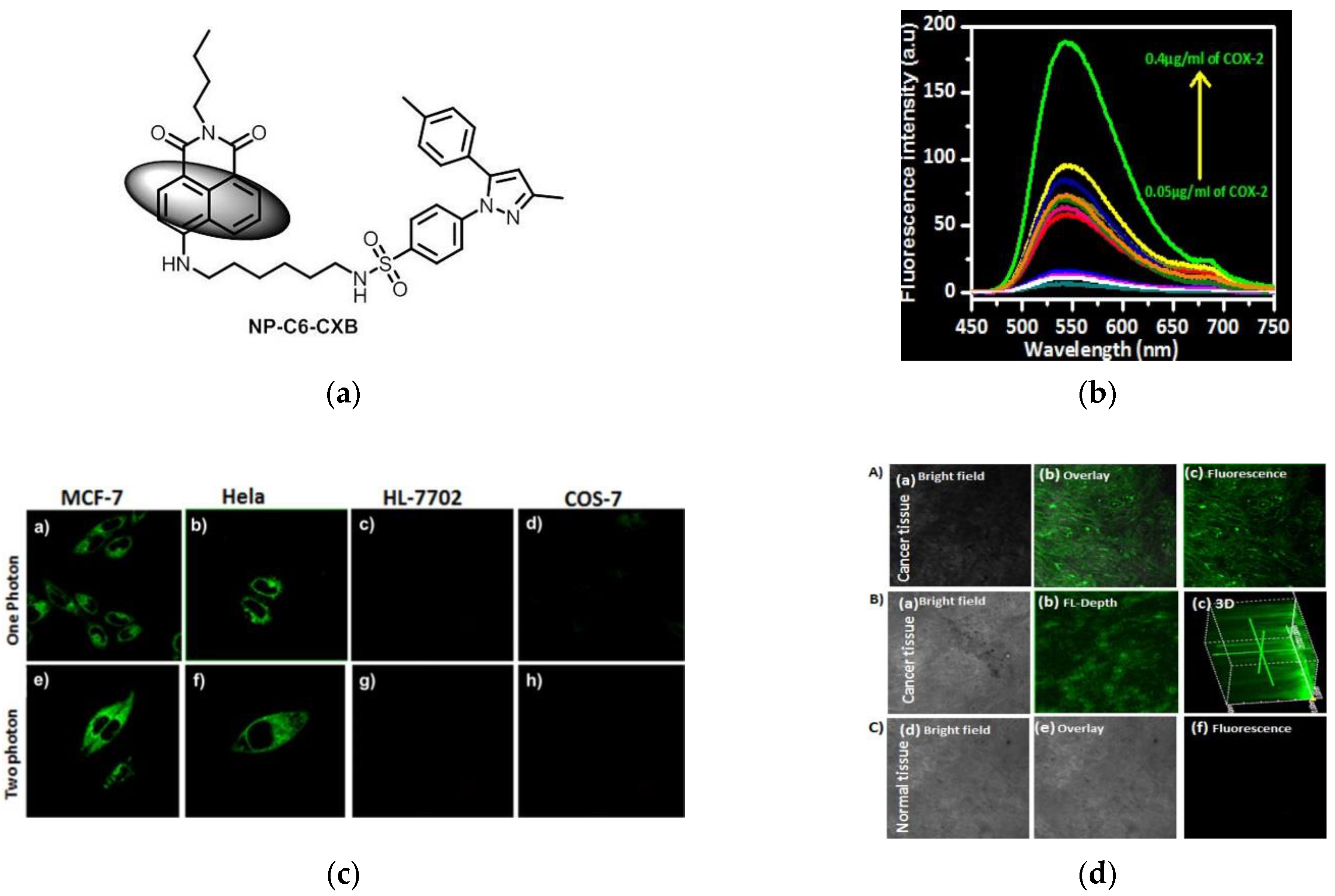
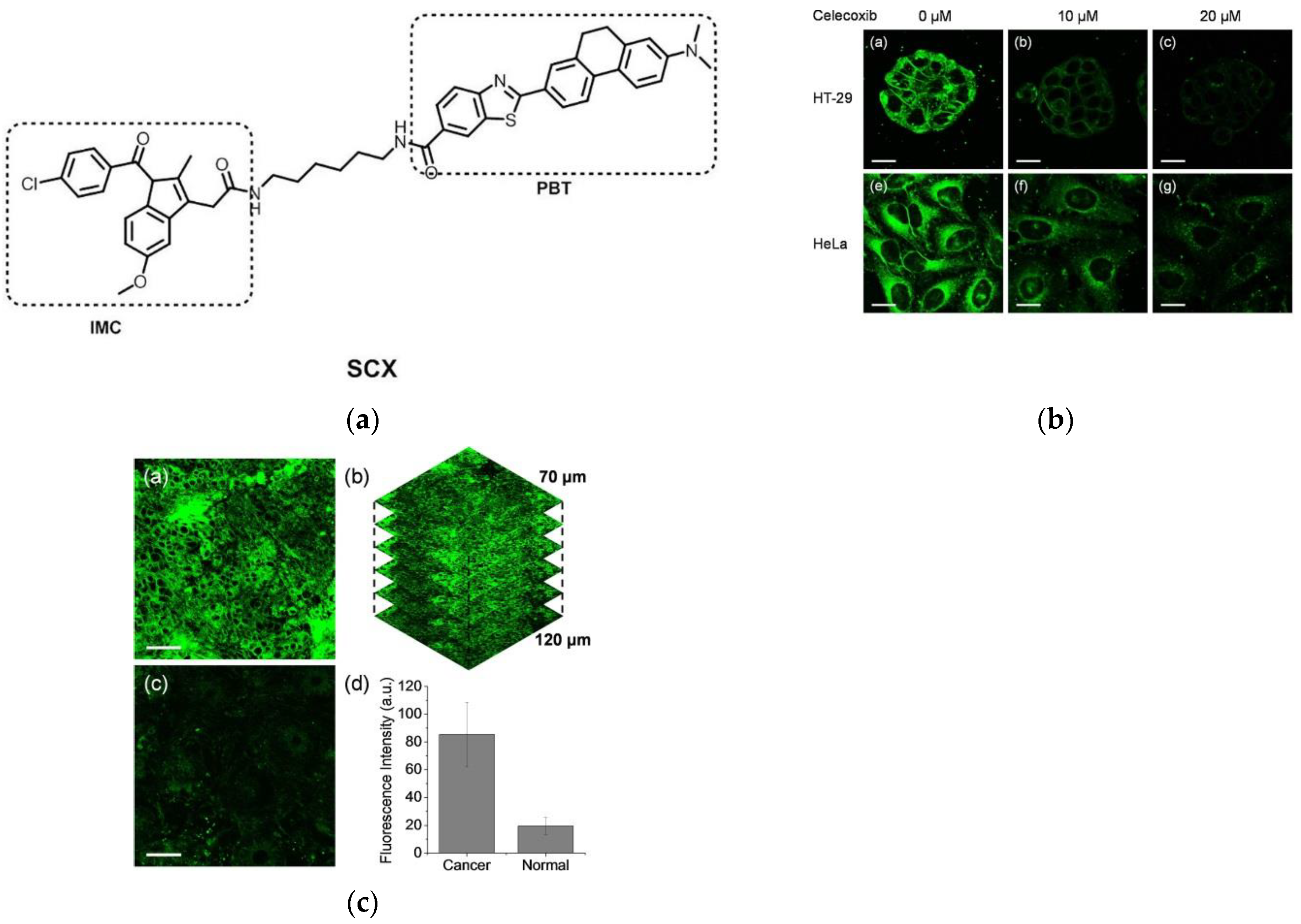
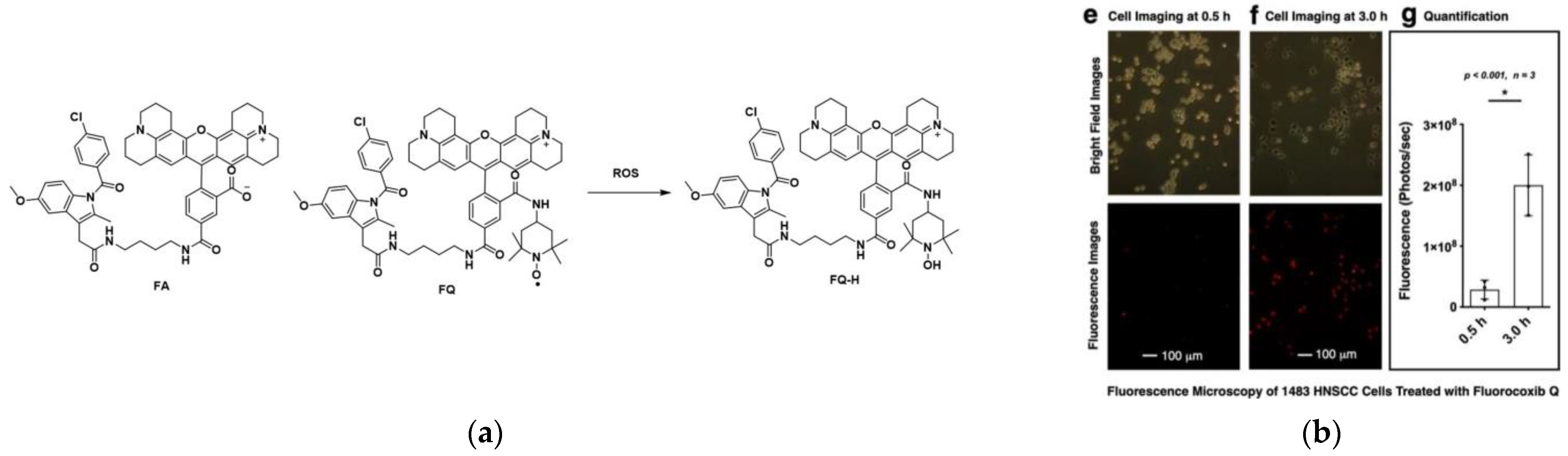
2. Fluorescent Probe for Detecting Cyclooxygenase-2 (COX-2) Enzymes
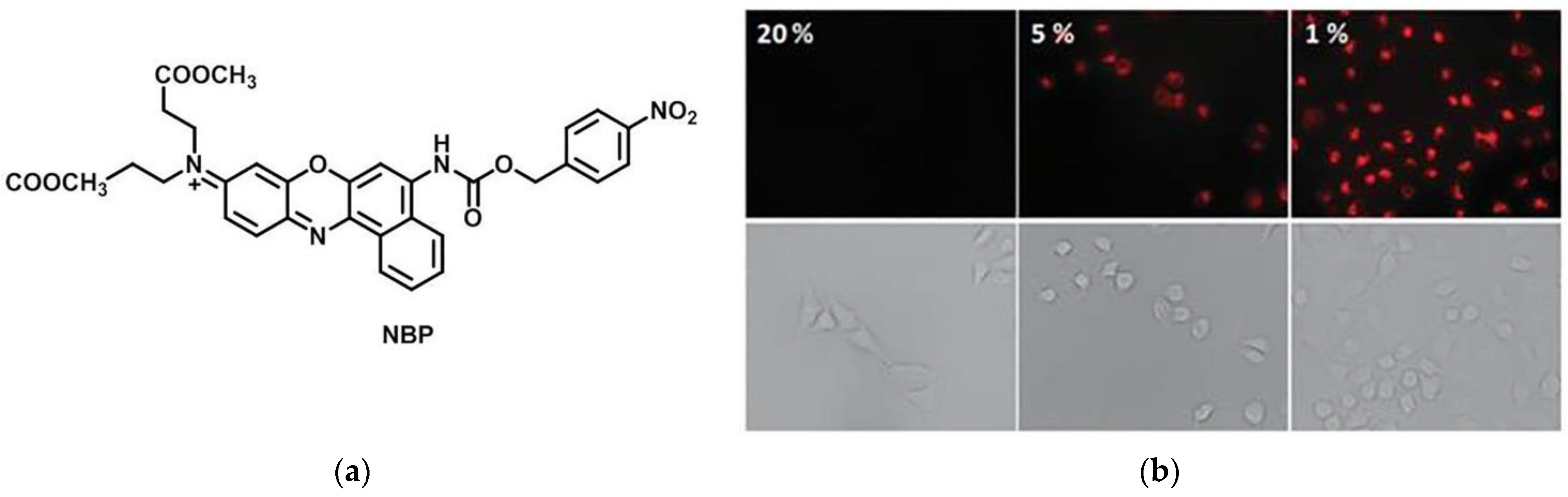
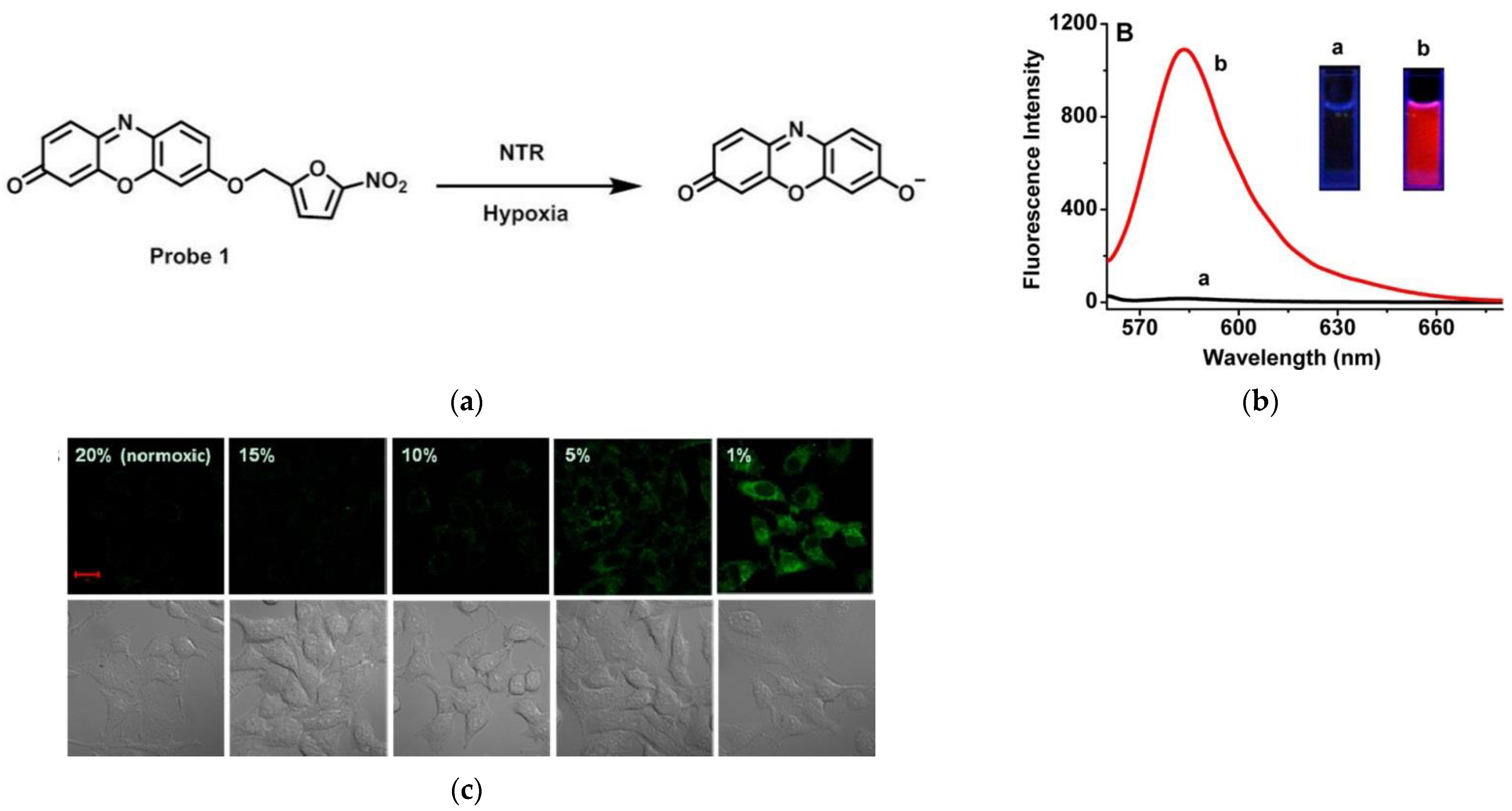
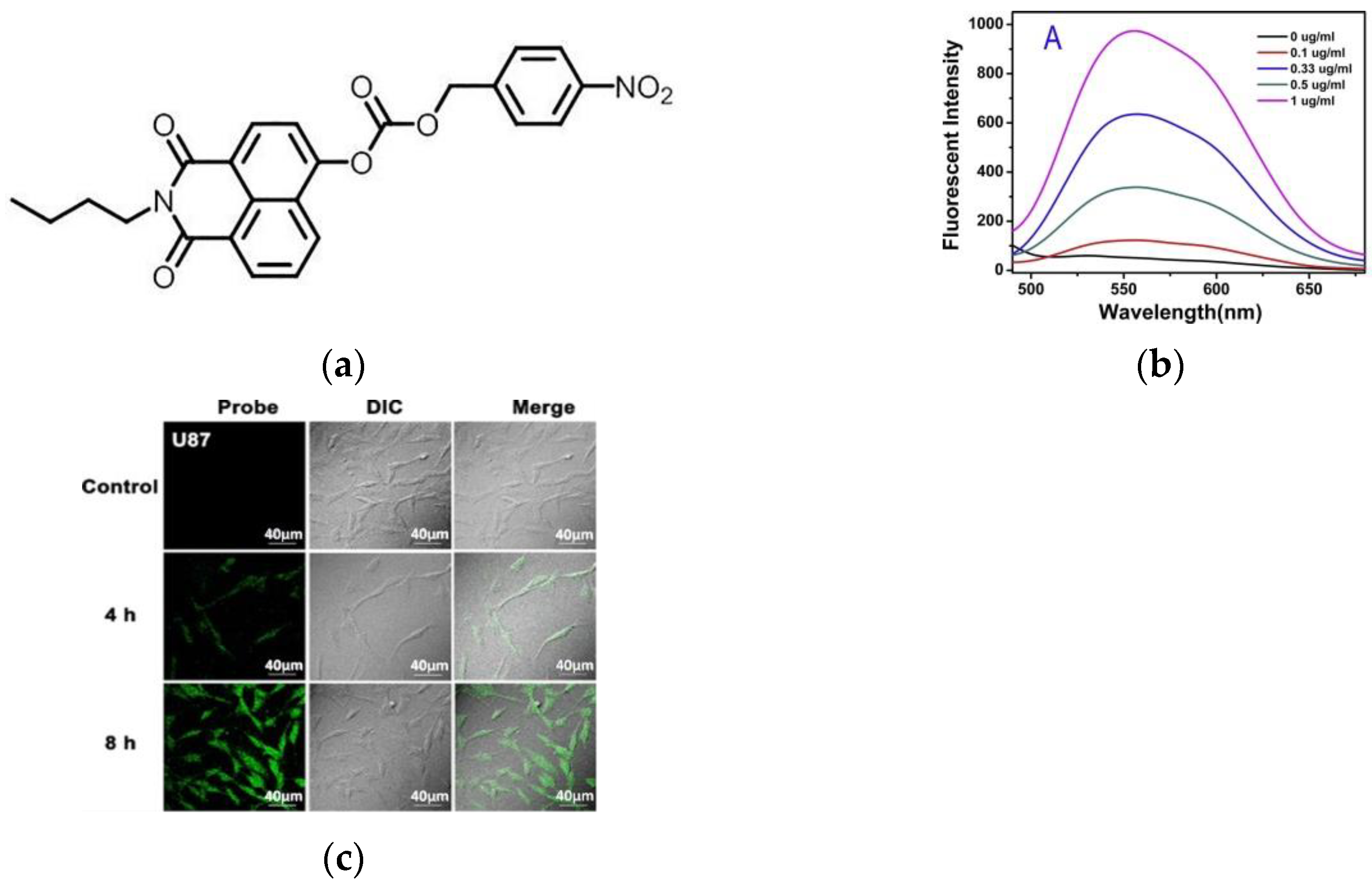
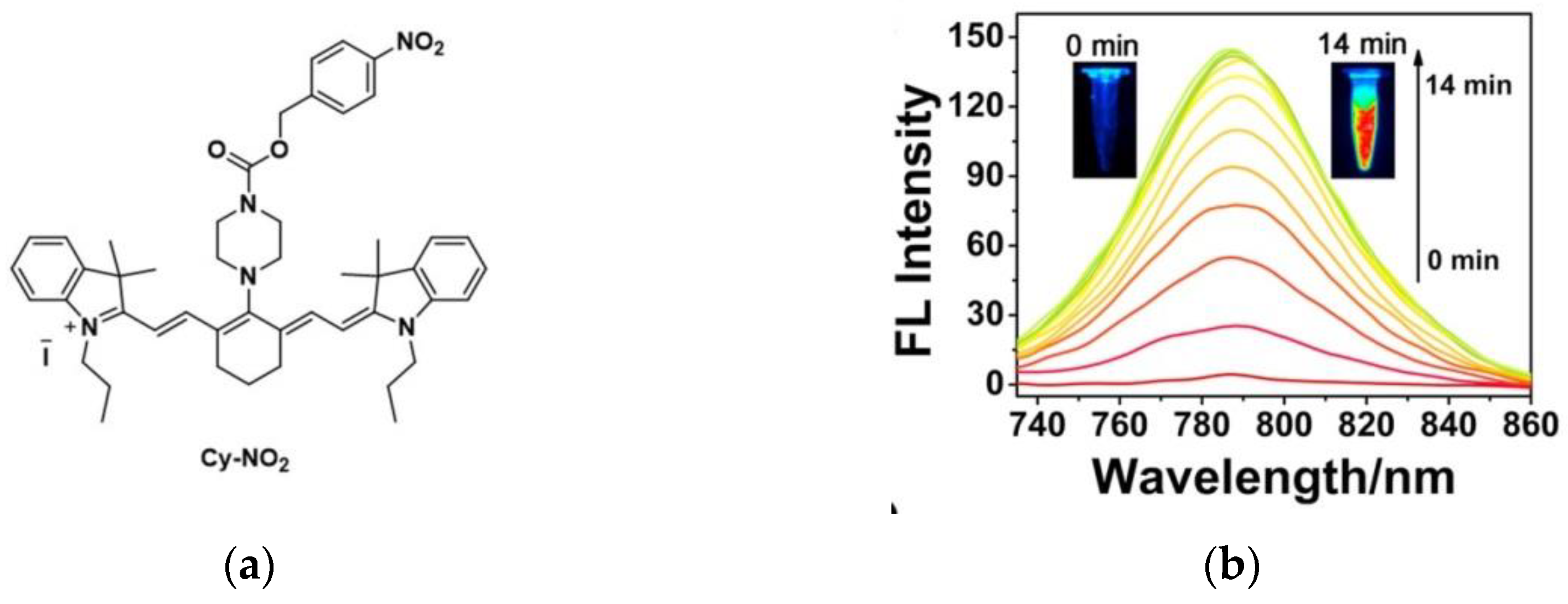
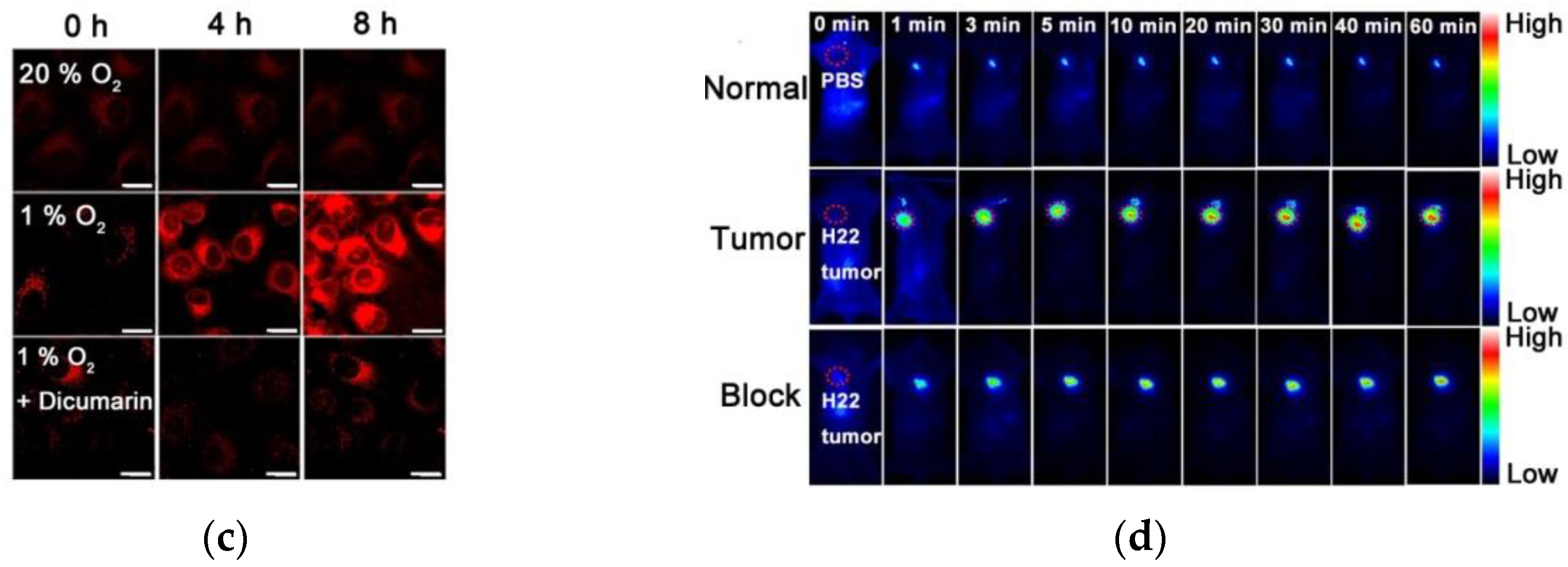
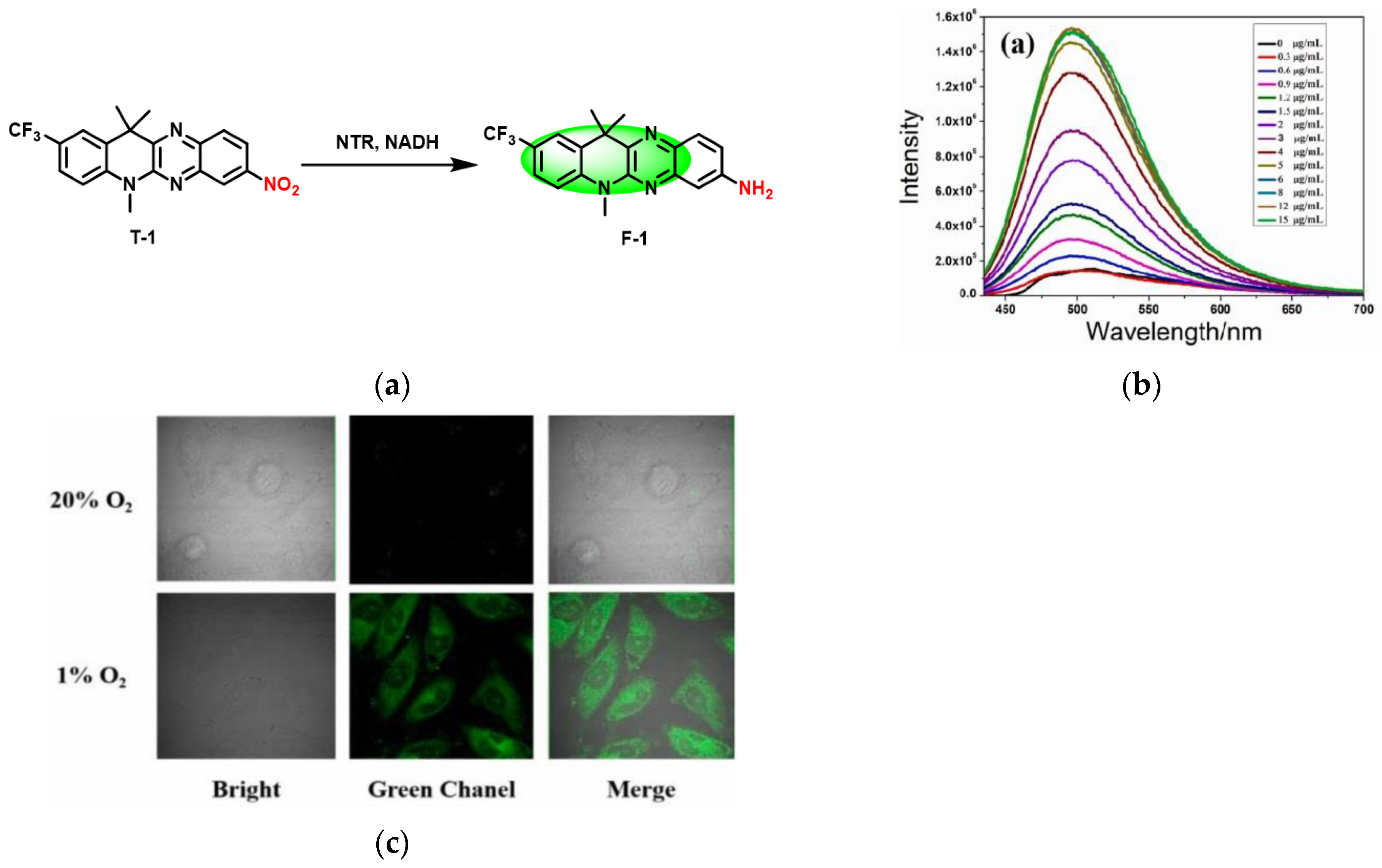
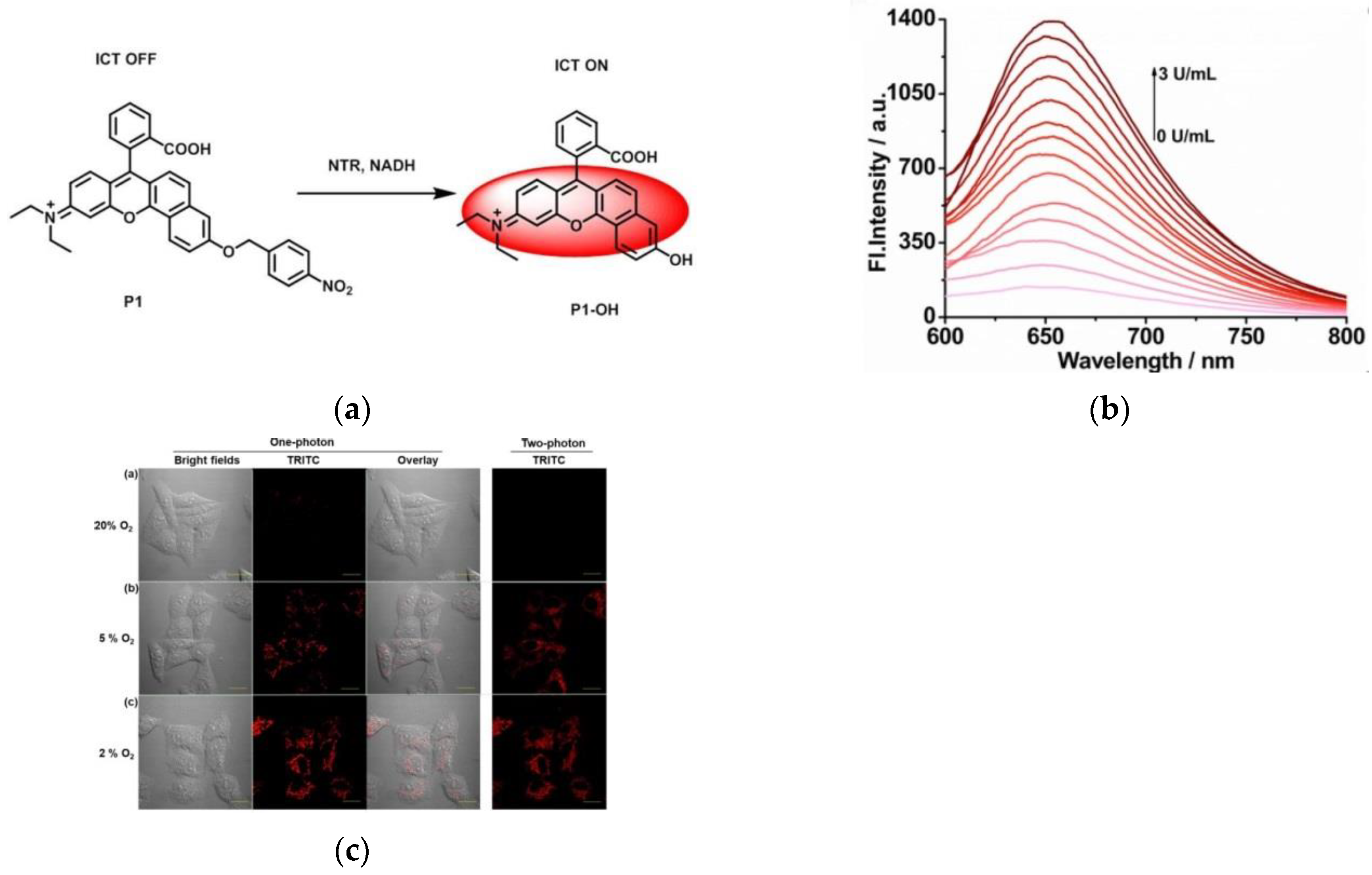
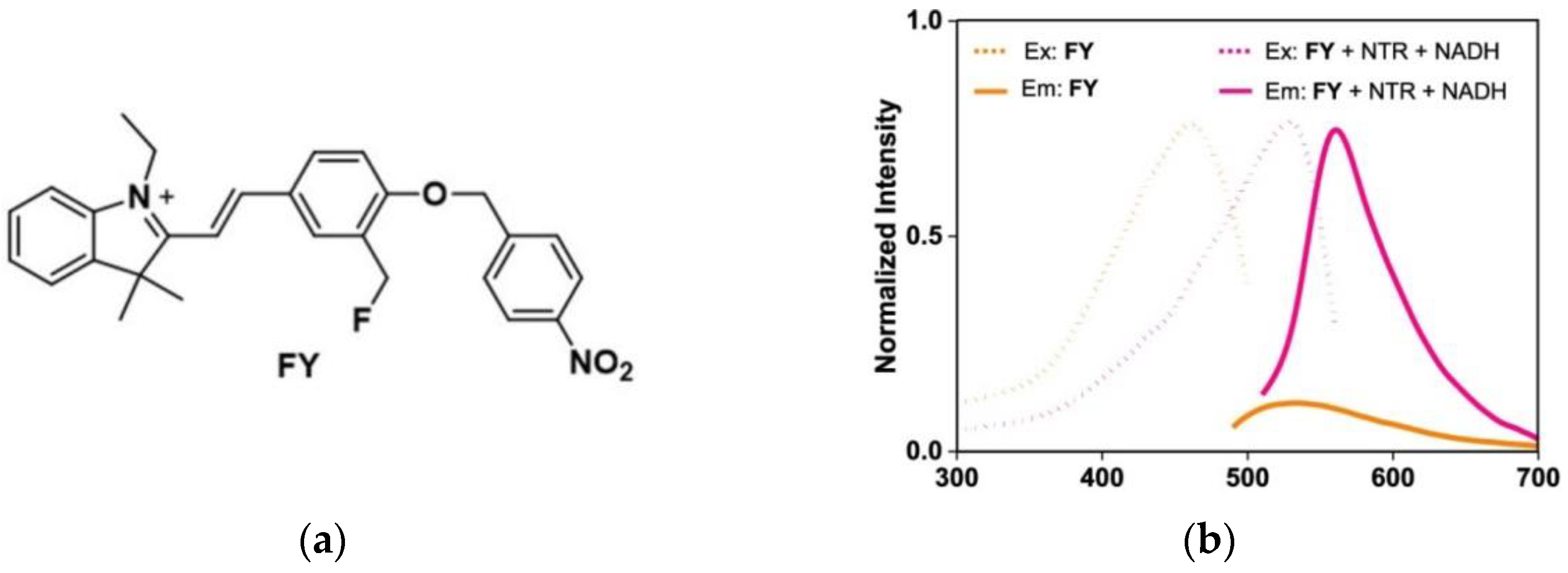
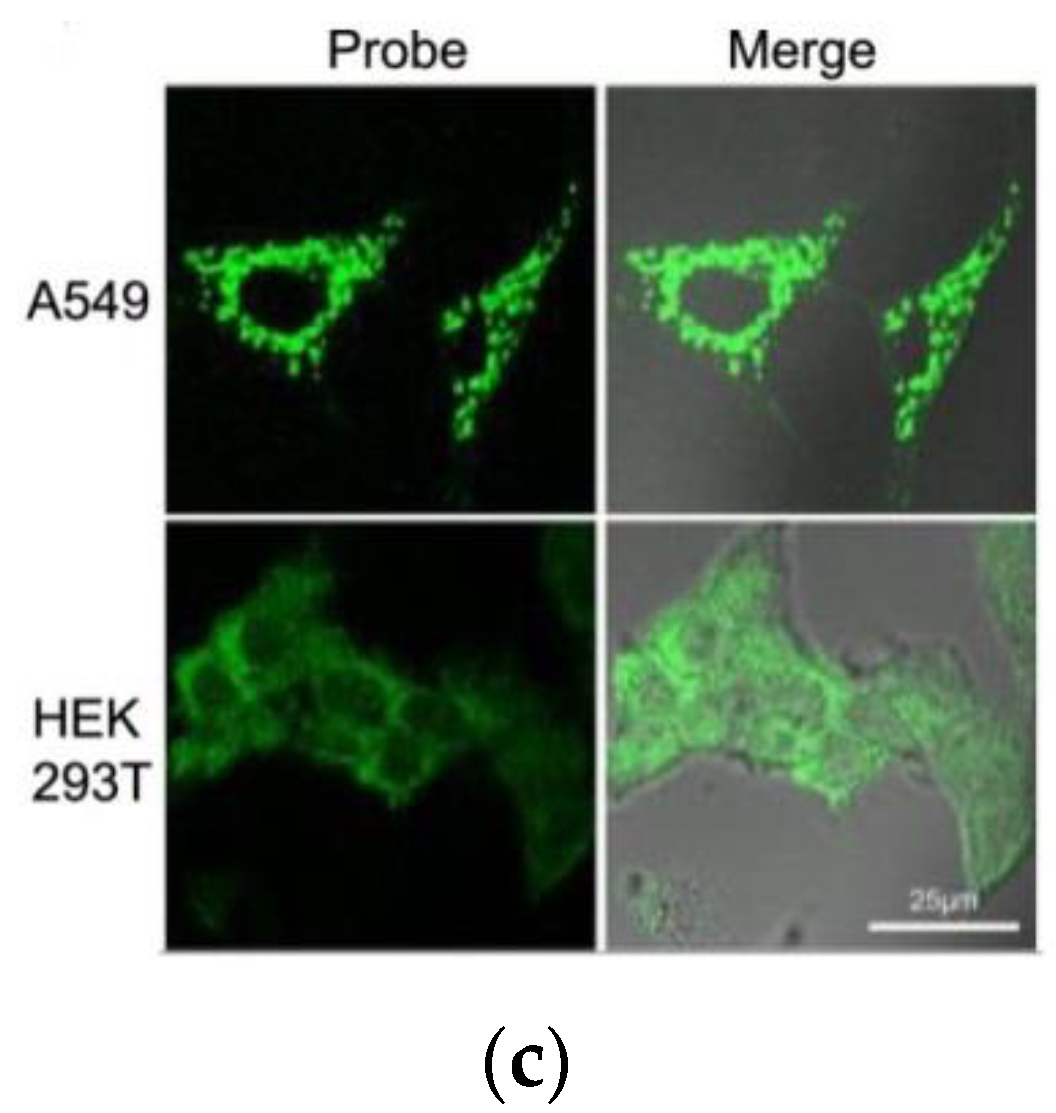
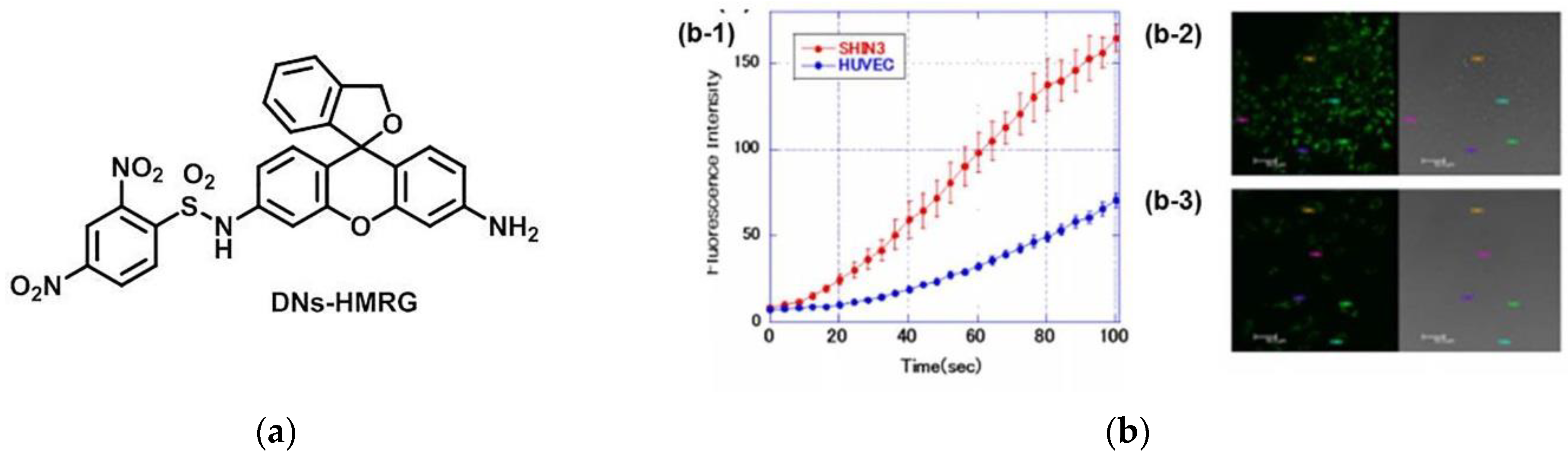
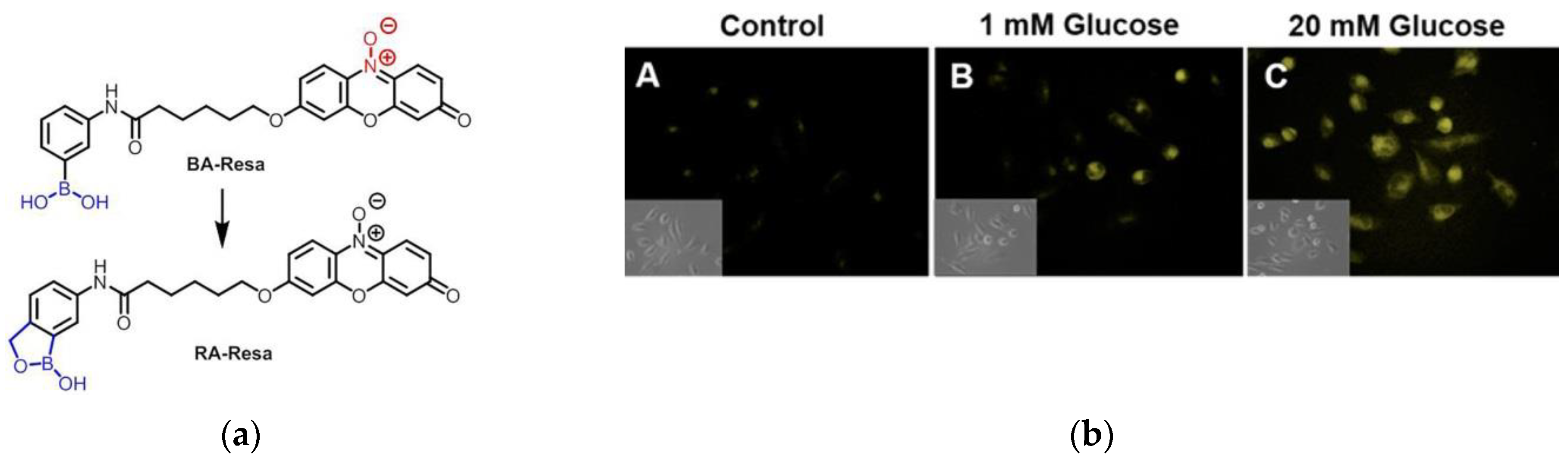
3. Fluorescent Probe for Detecting Nitroreductase (NTR) Enzymes in Tumor Cells/Tissues
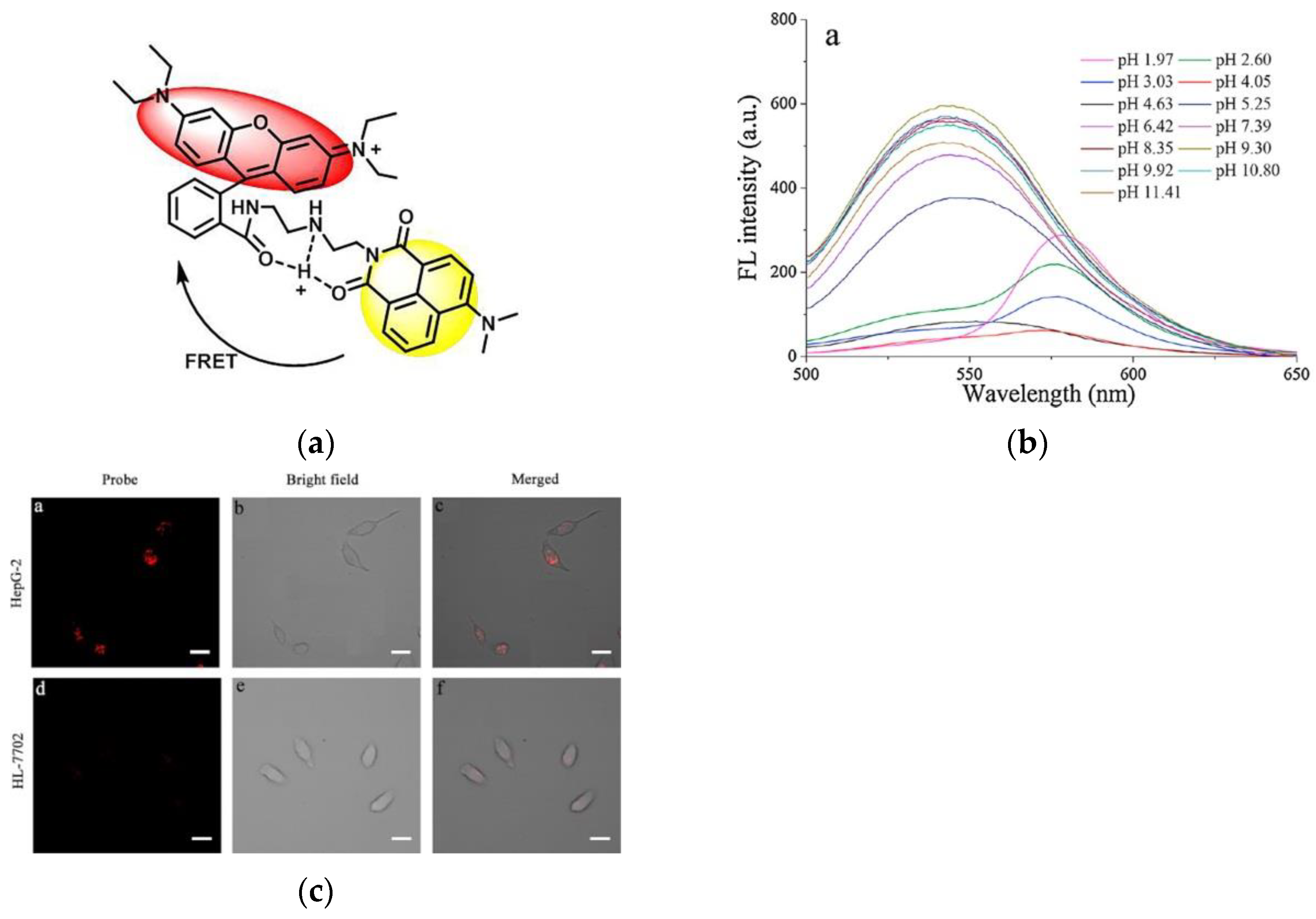
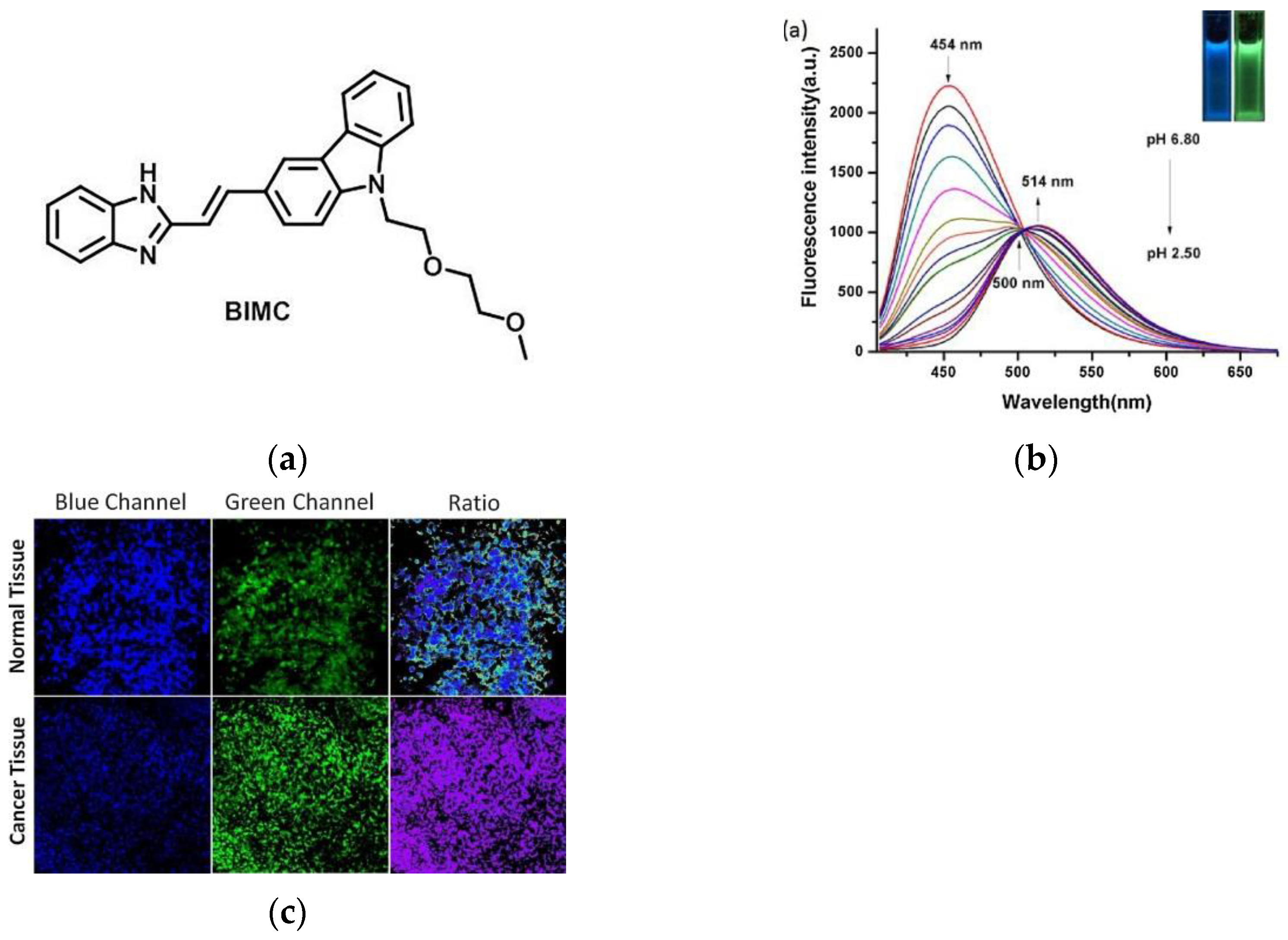
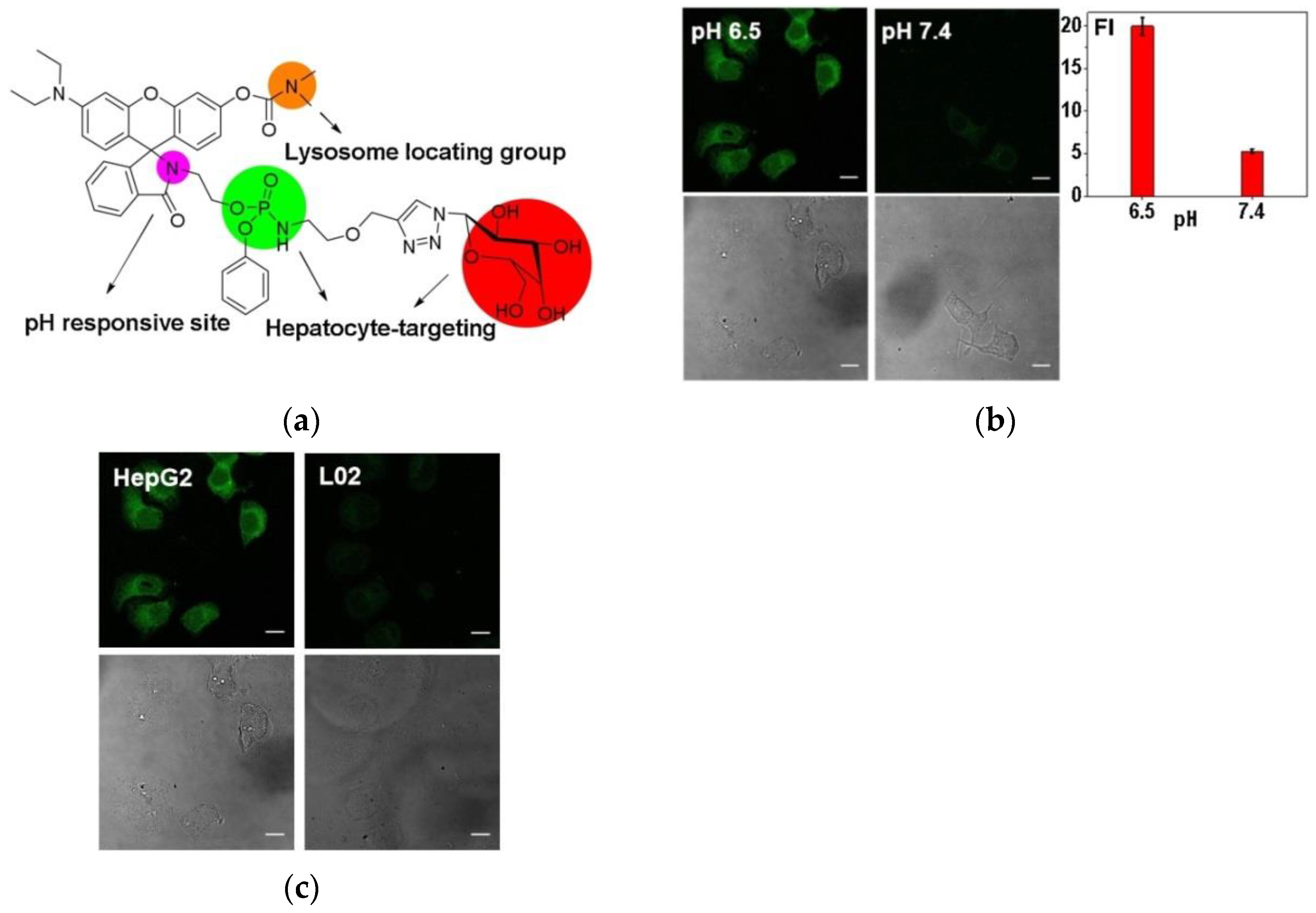
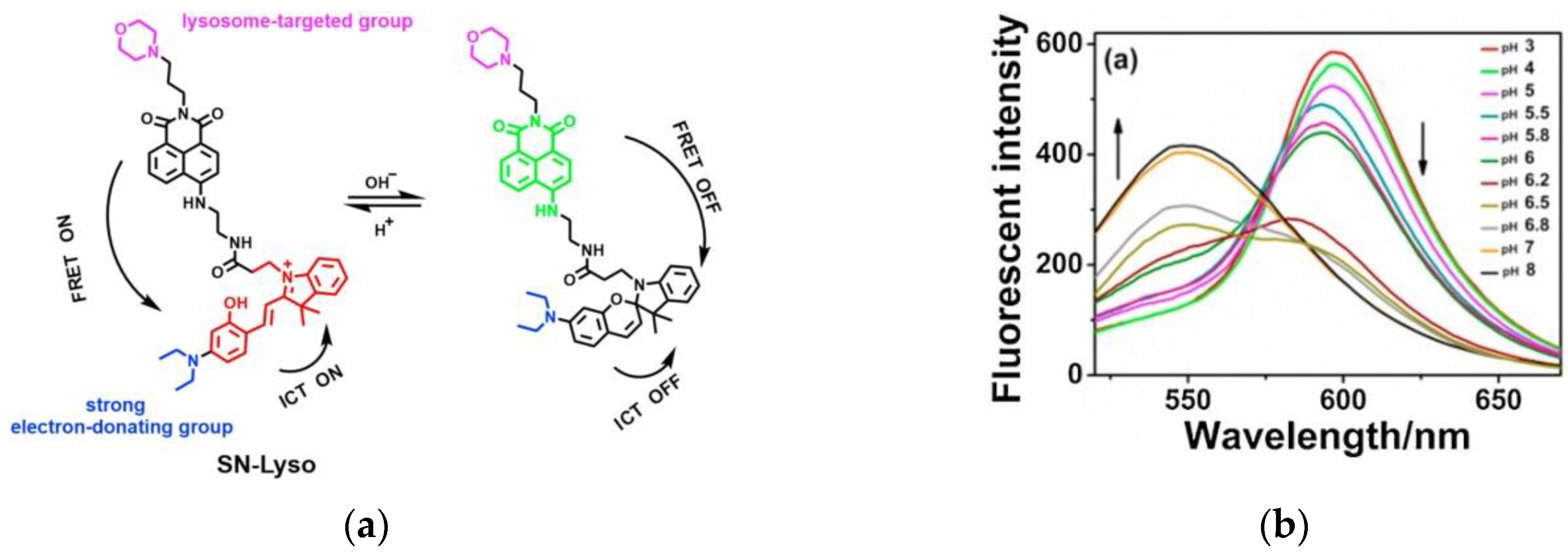
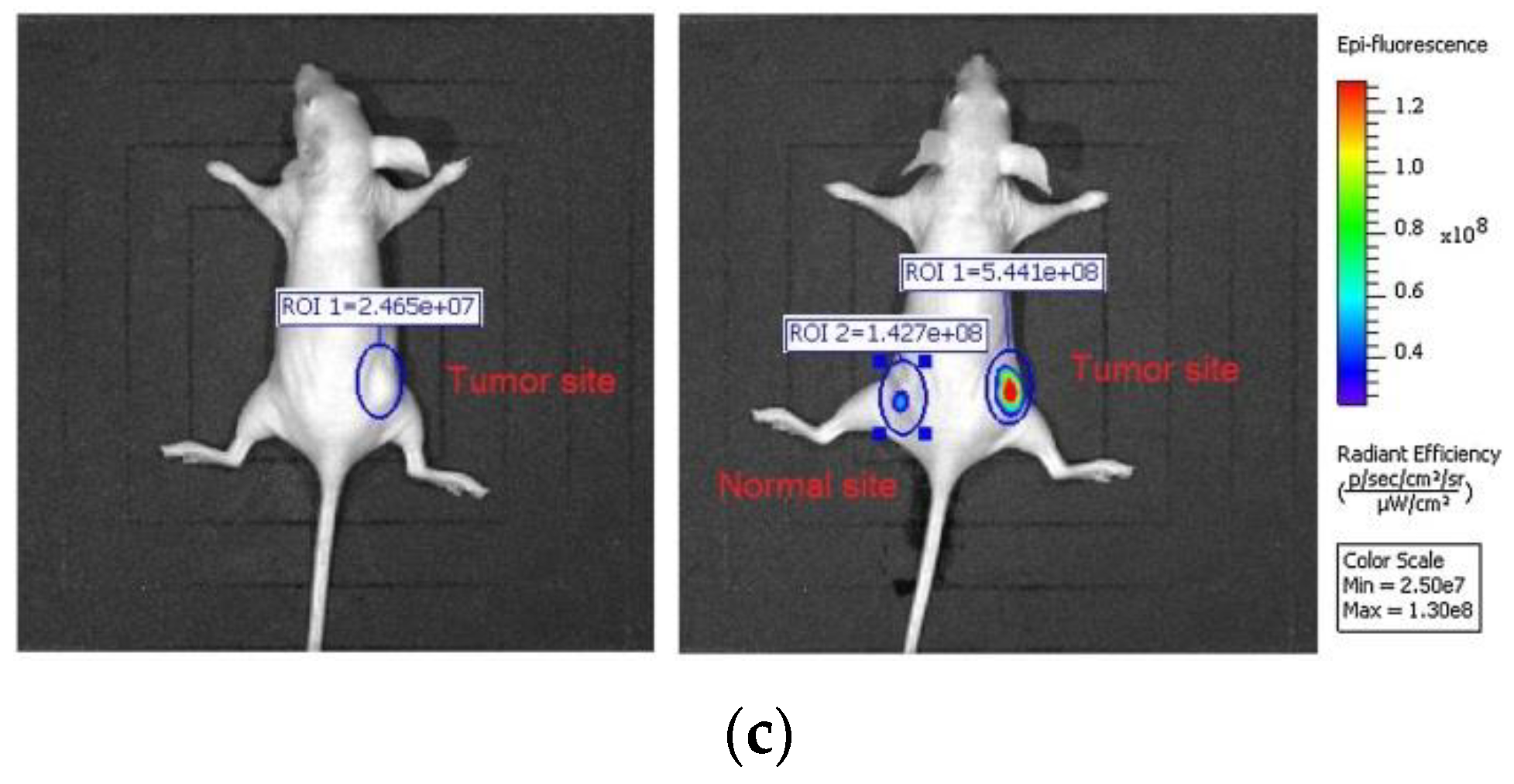
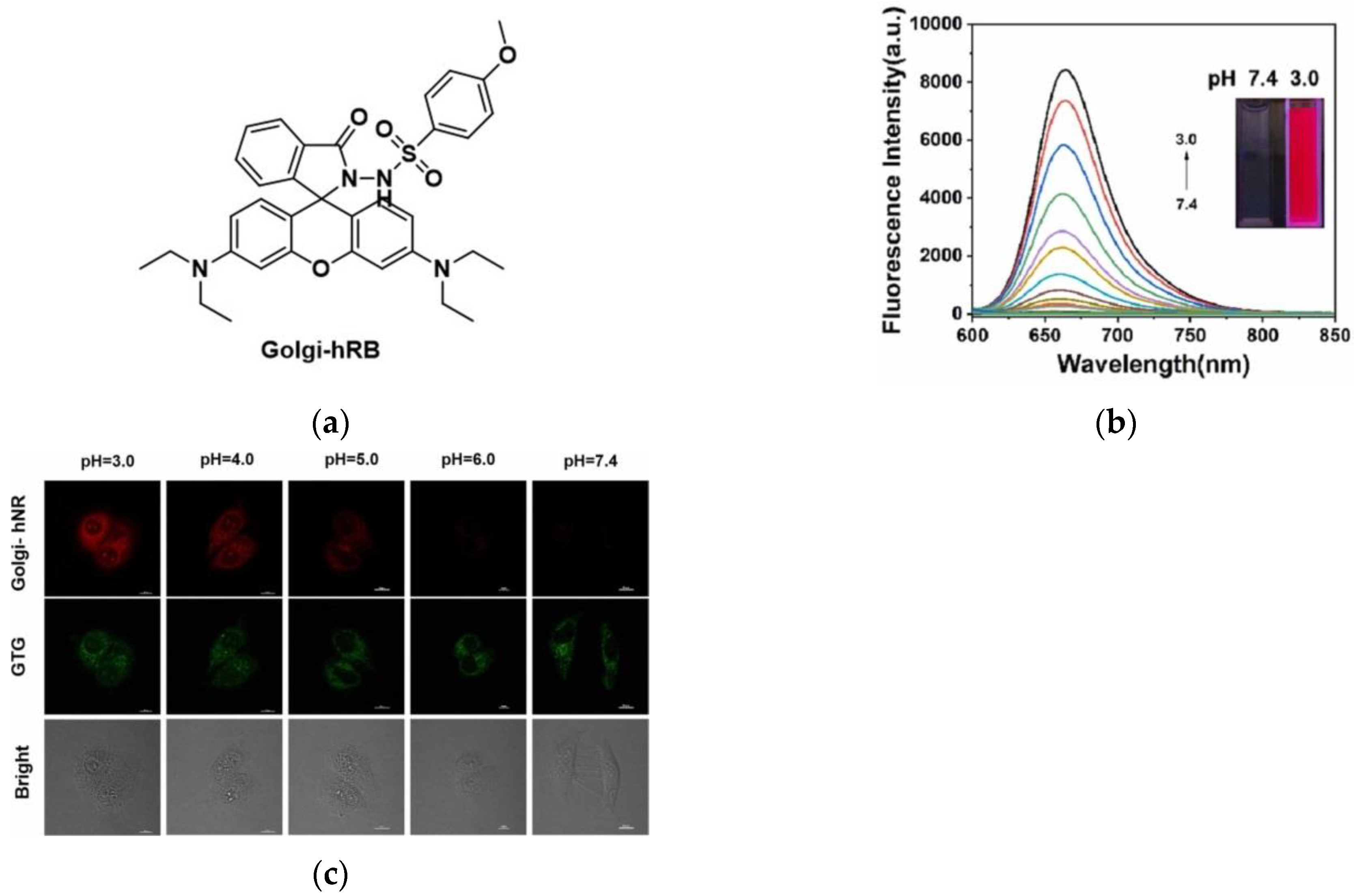
4. Fluorescent Probe for Detecting pH
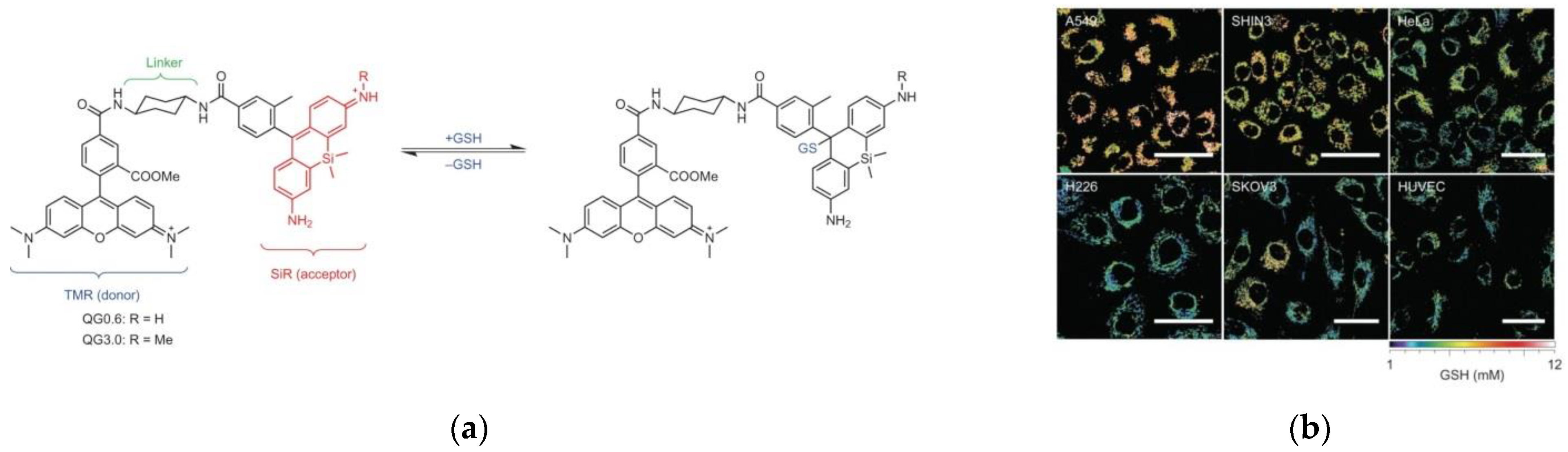
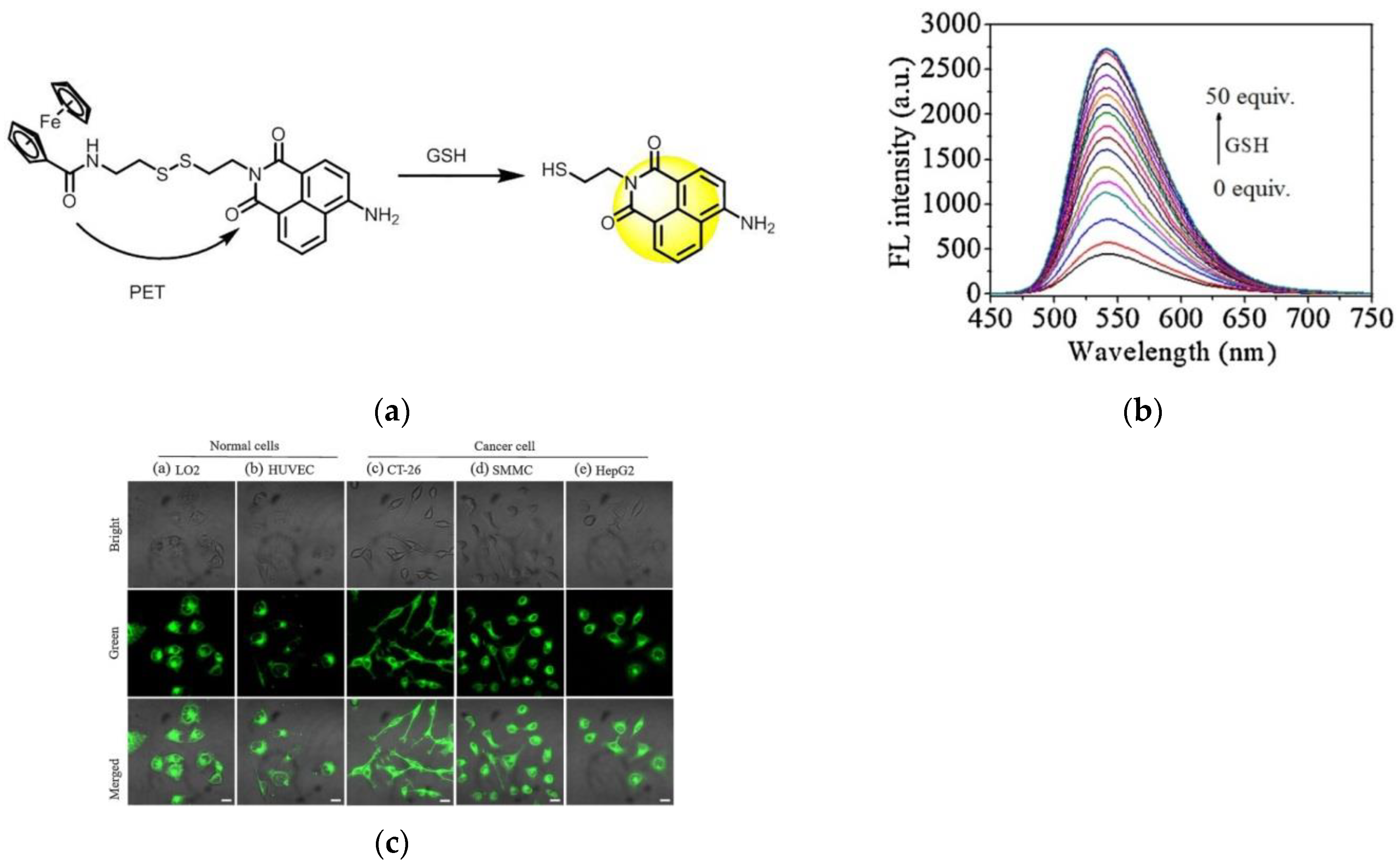
5. Fluorescent Probe for Detecting Glutathione (GSH)
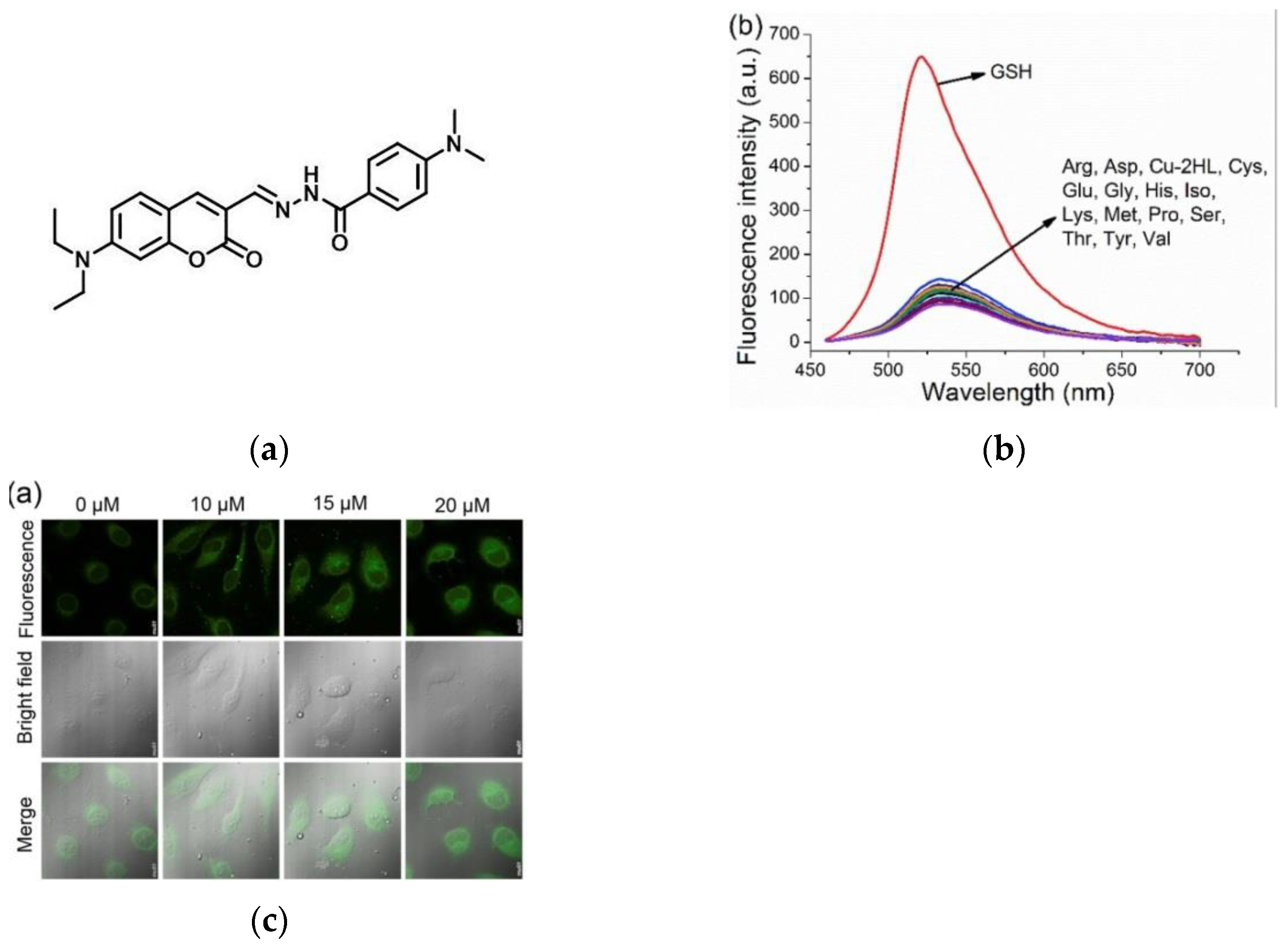
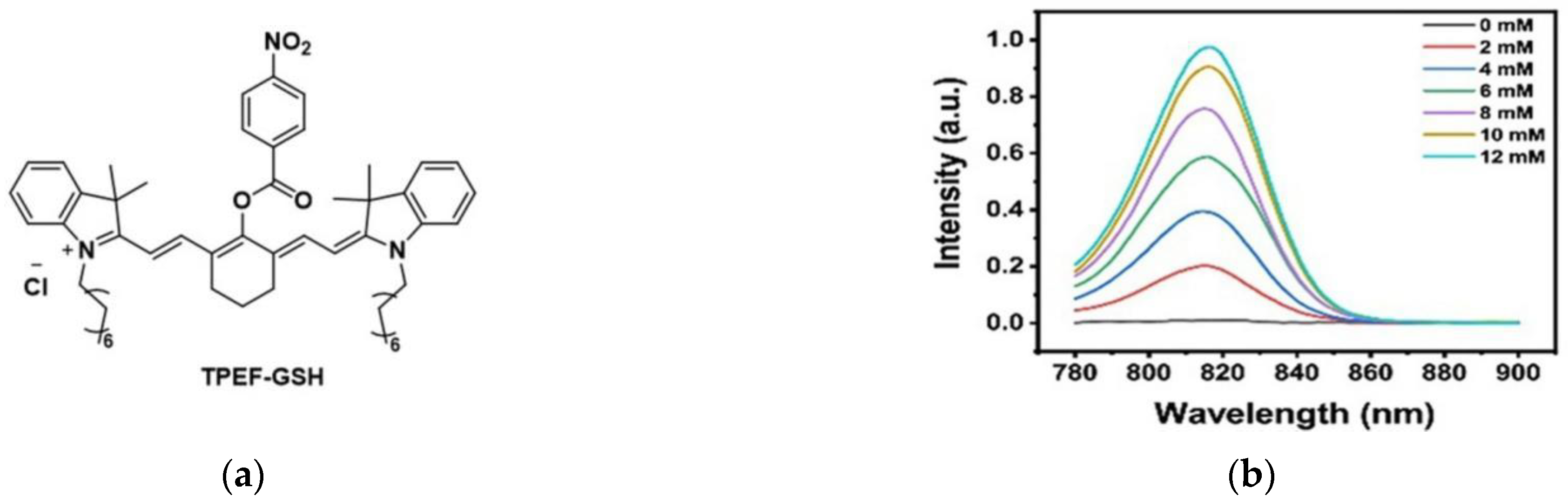
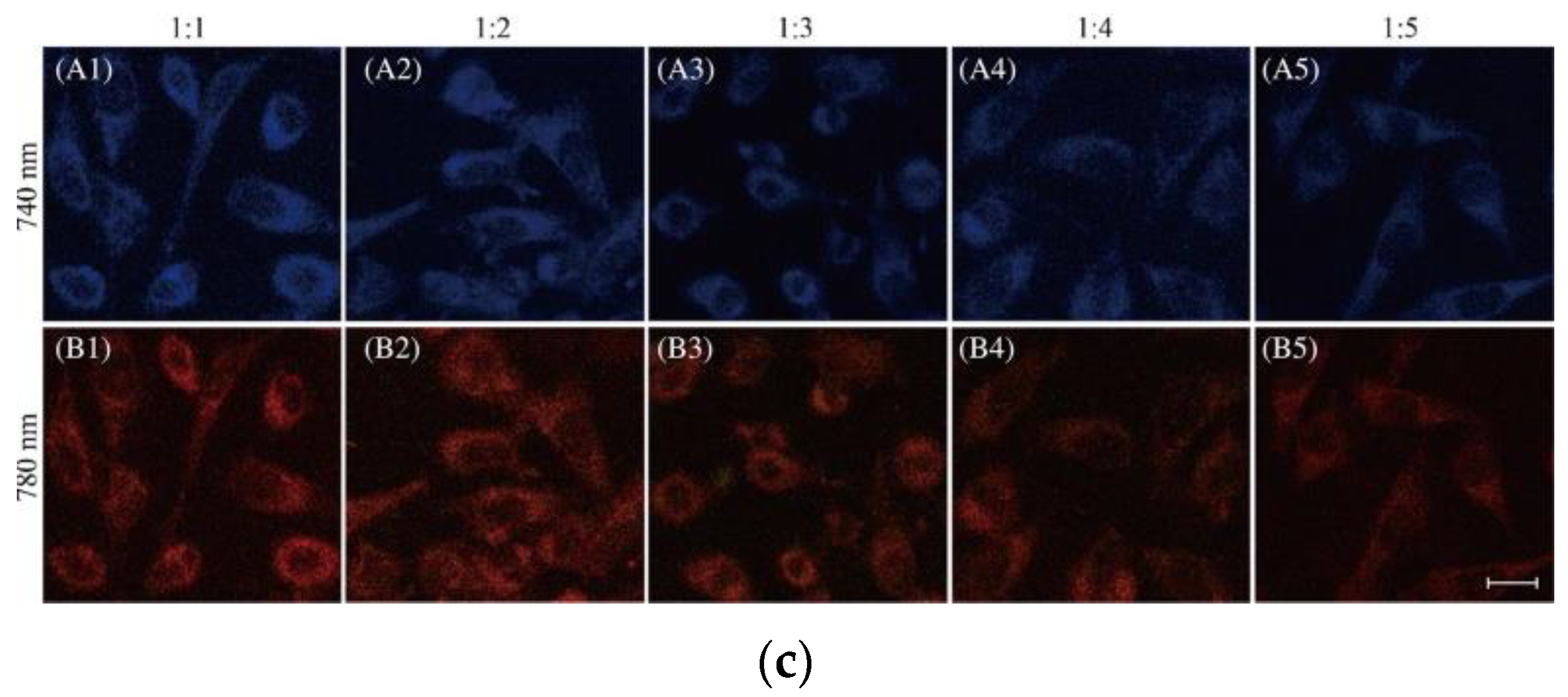
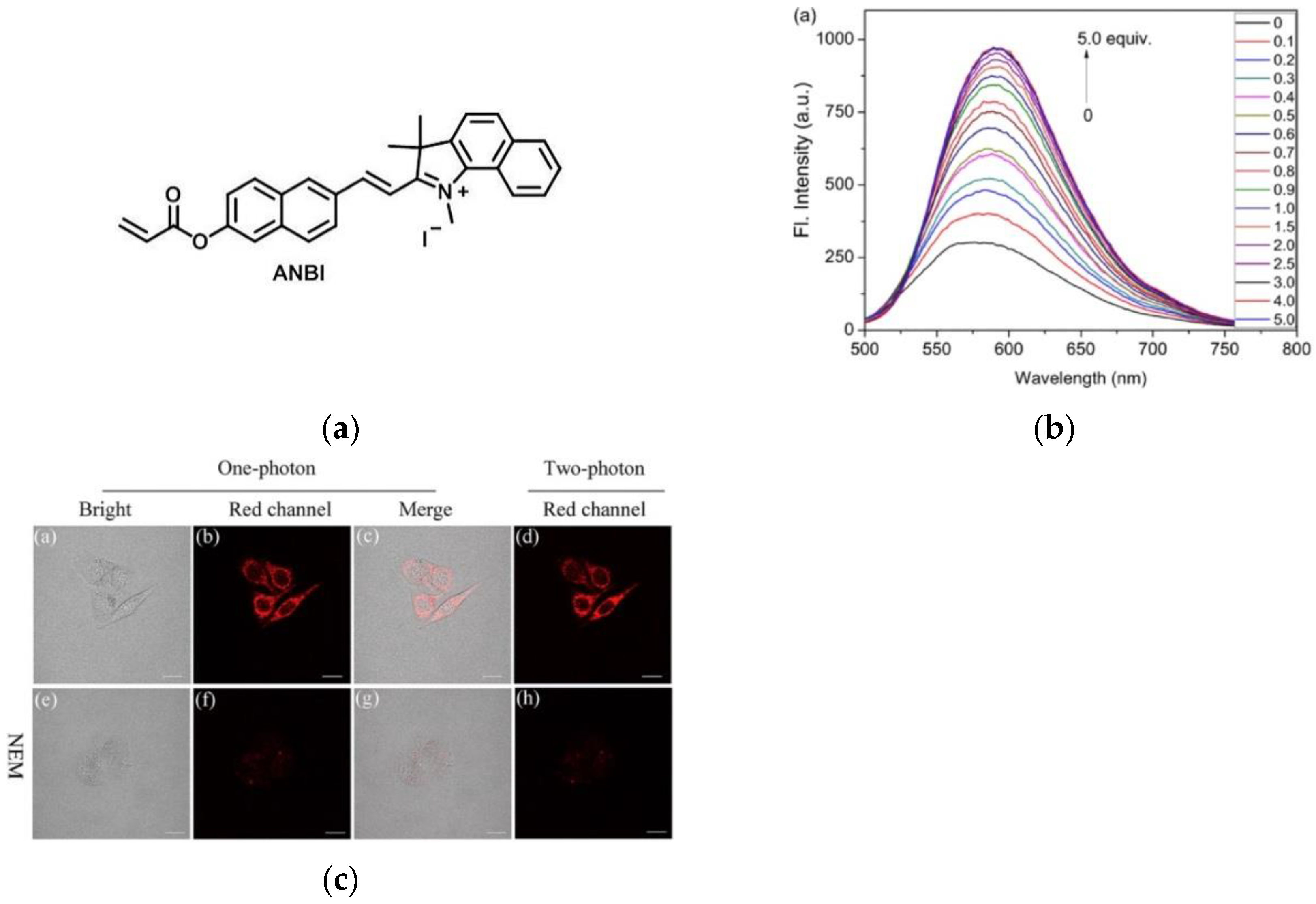
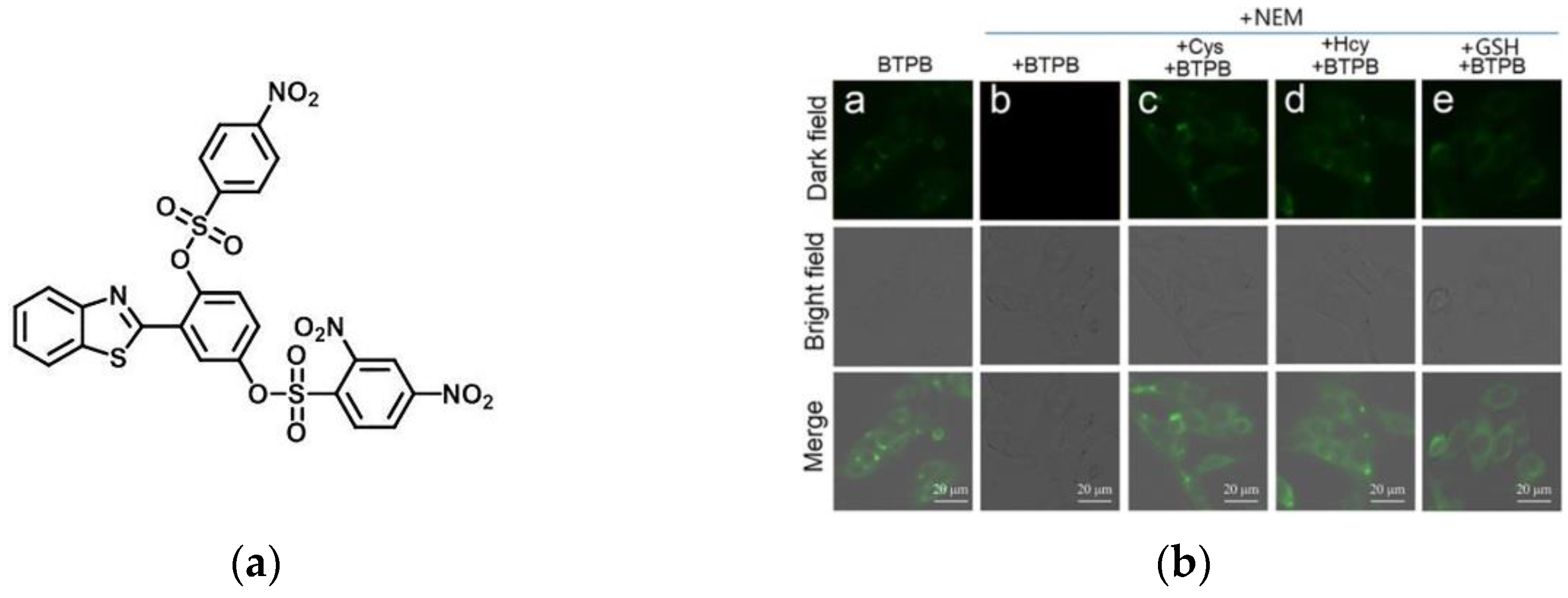
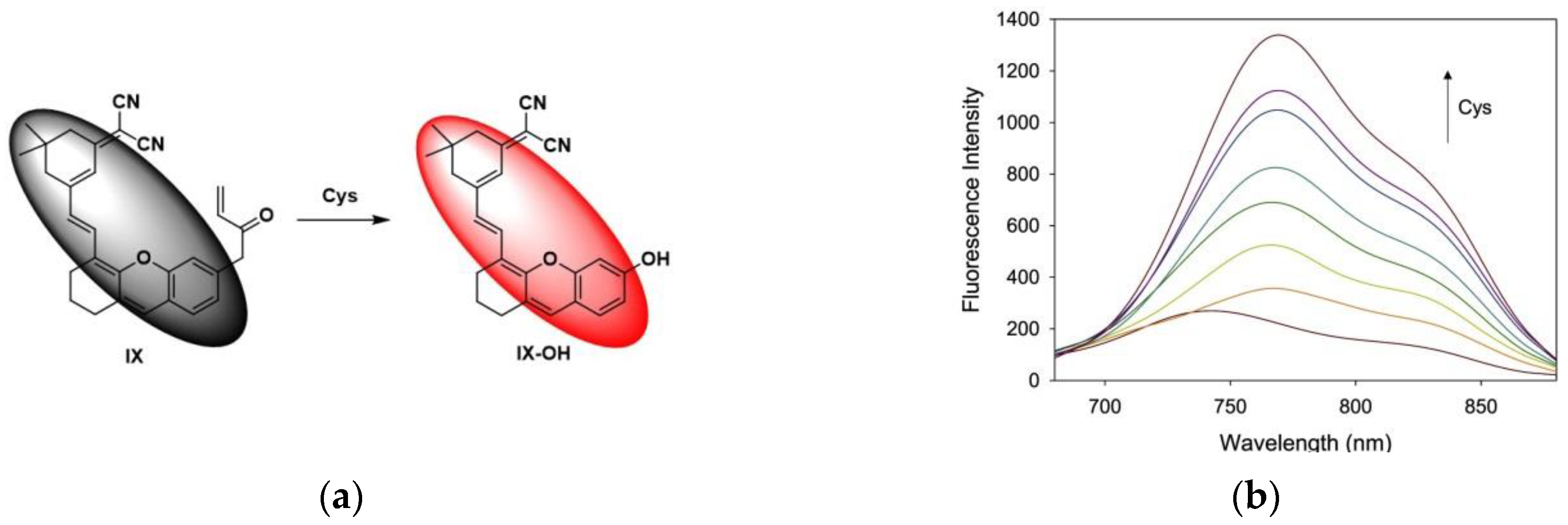
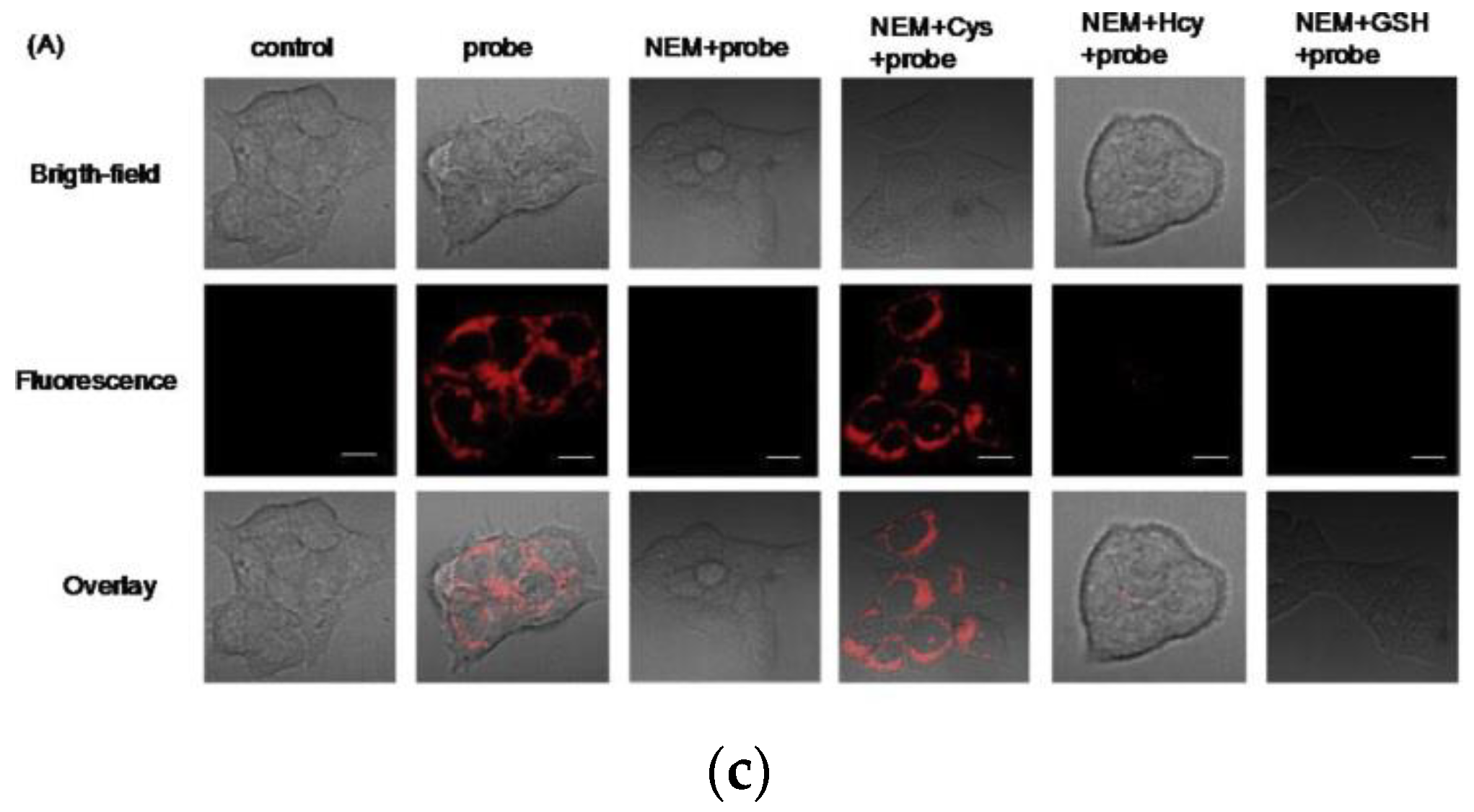
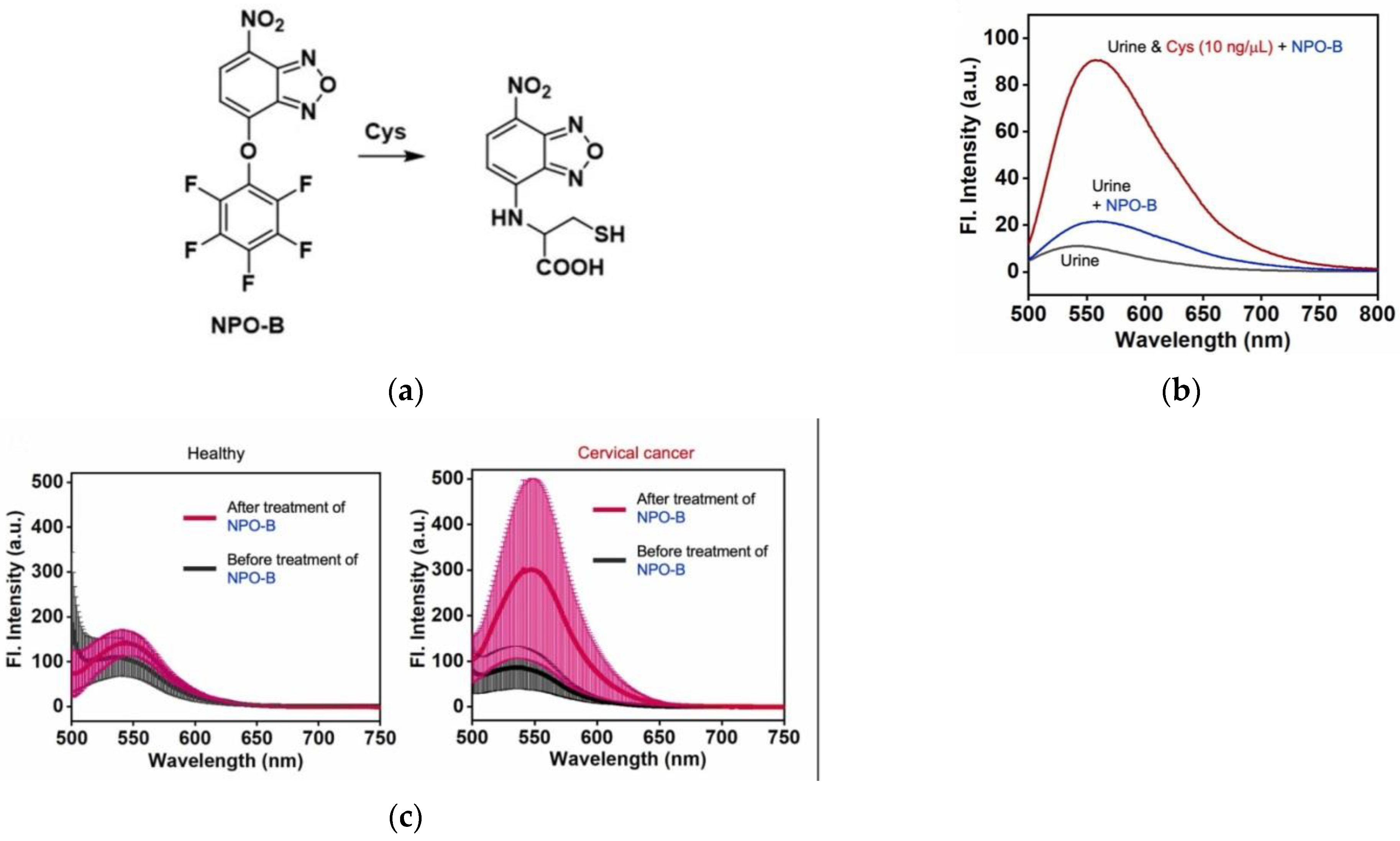
6. Fluorescent Probe for Detecting Other Biothiols
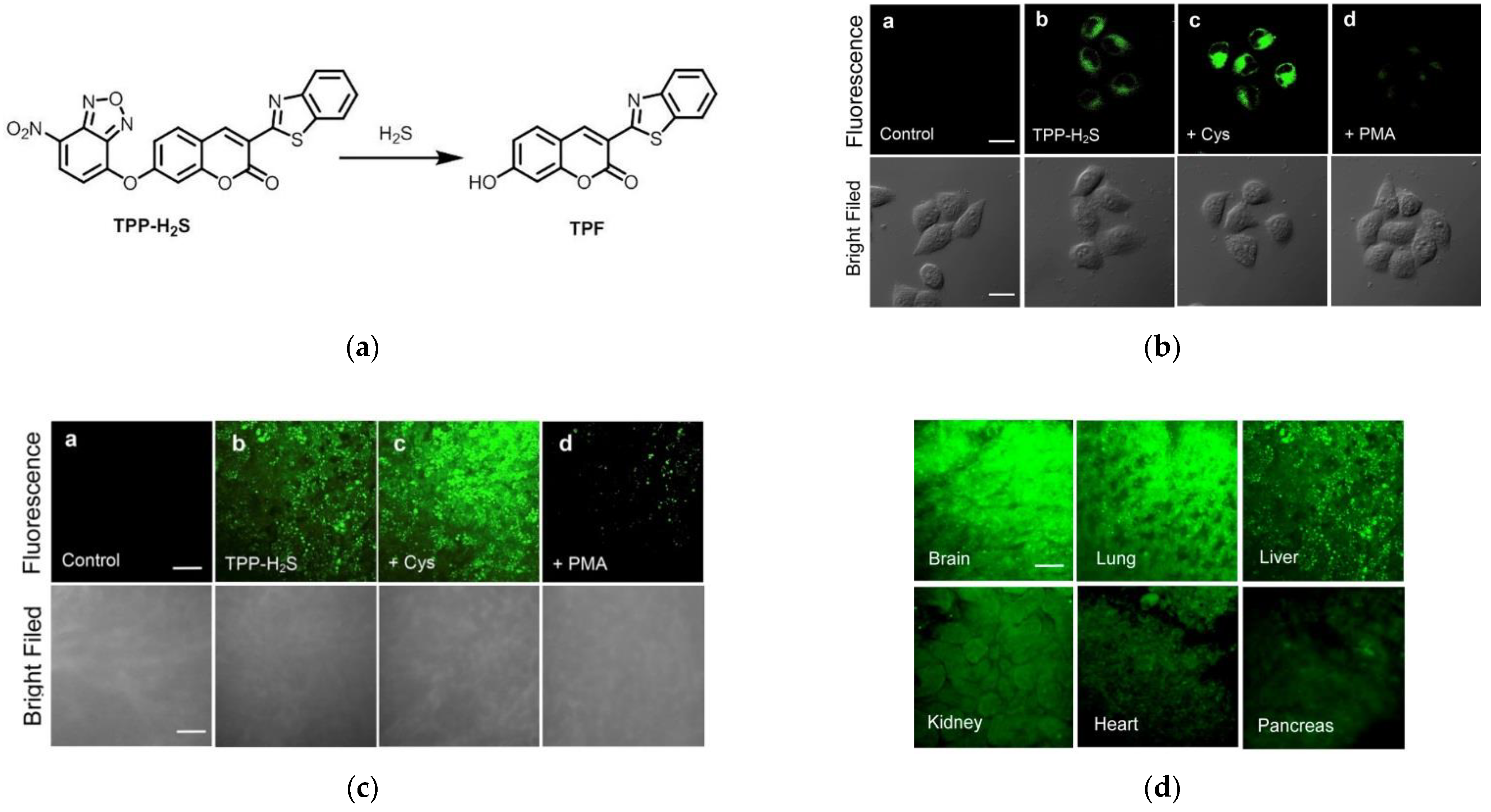
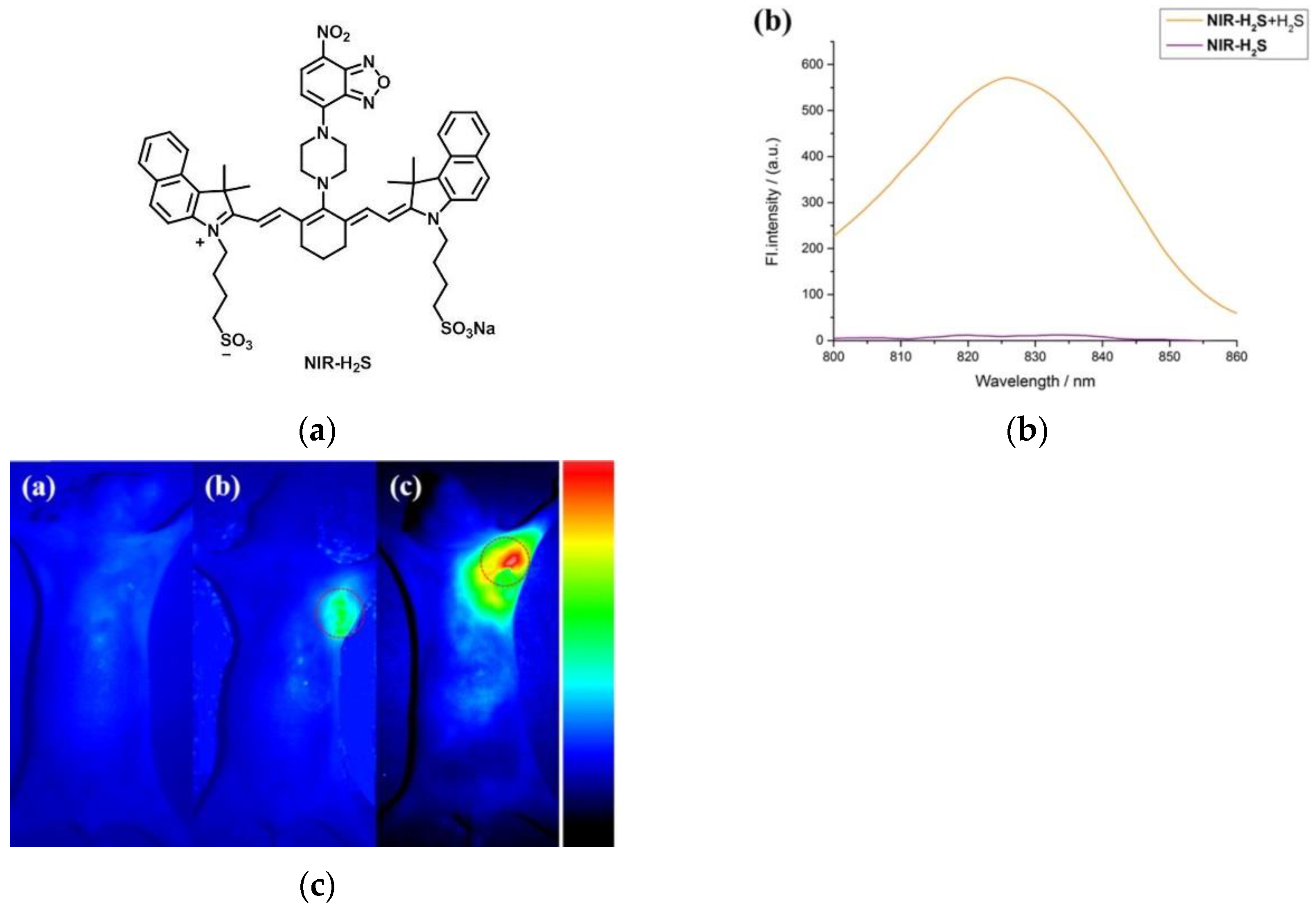
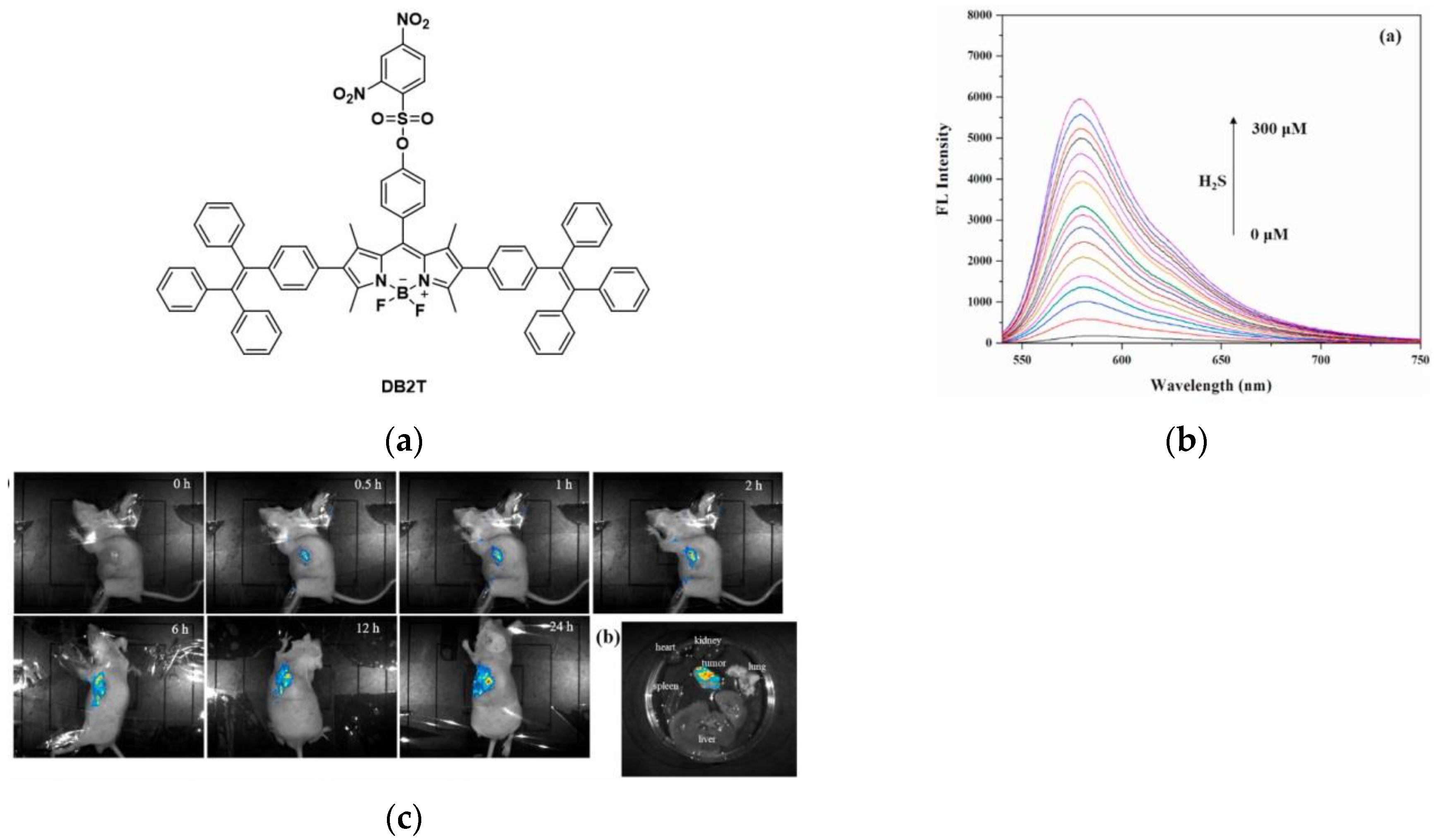
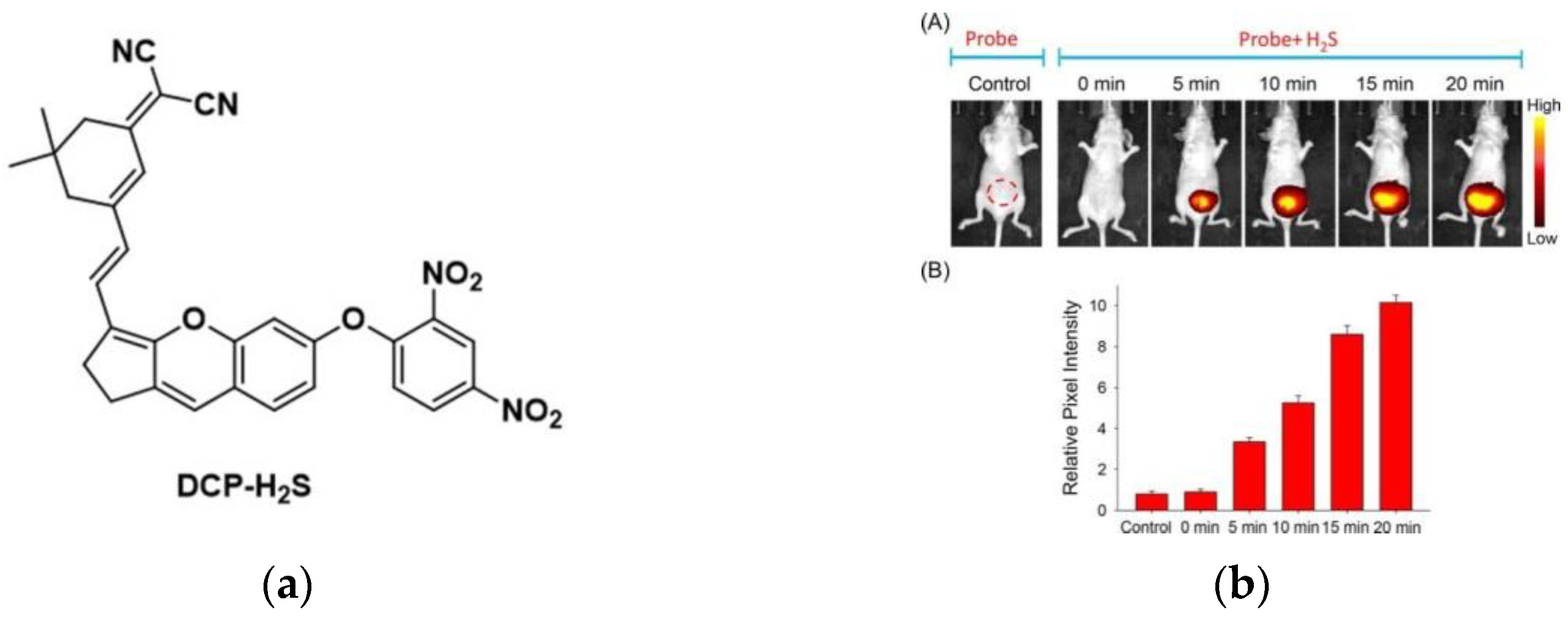
7. Fluorescent Probe for Detecting Hydrogen Sulfide (H2S)
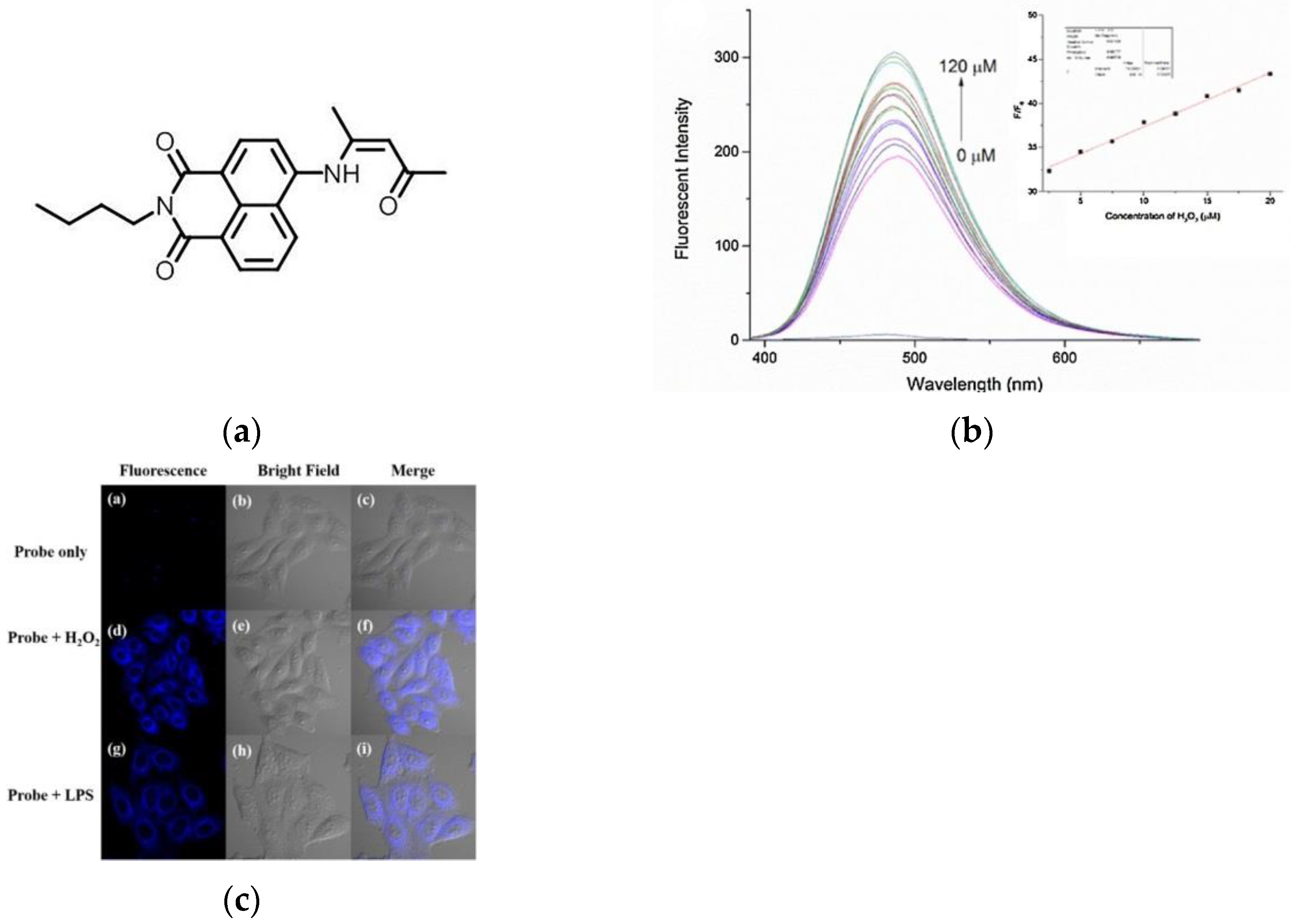
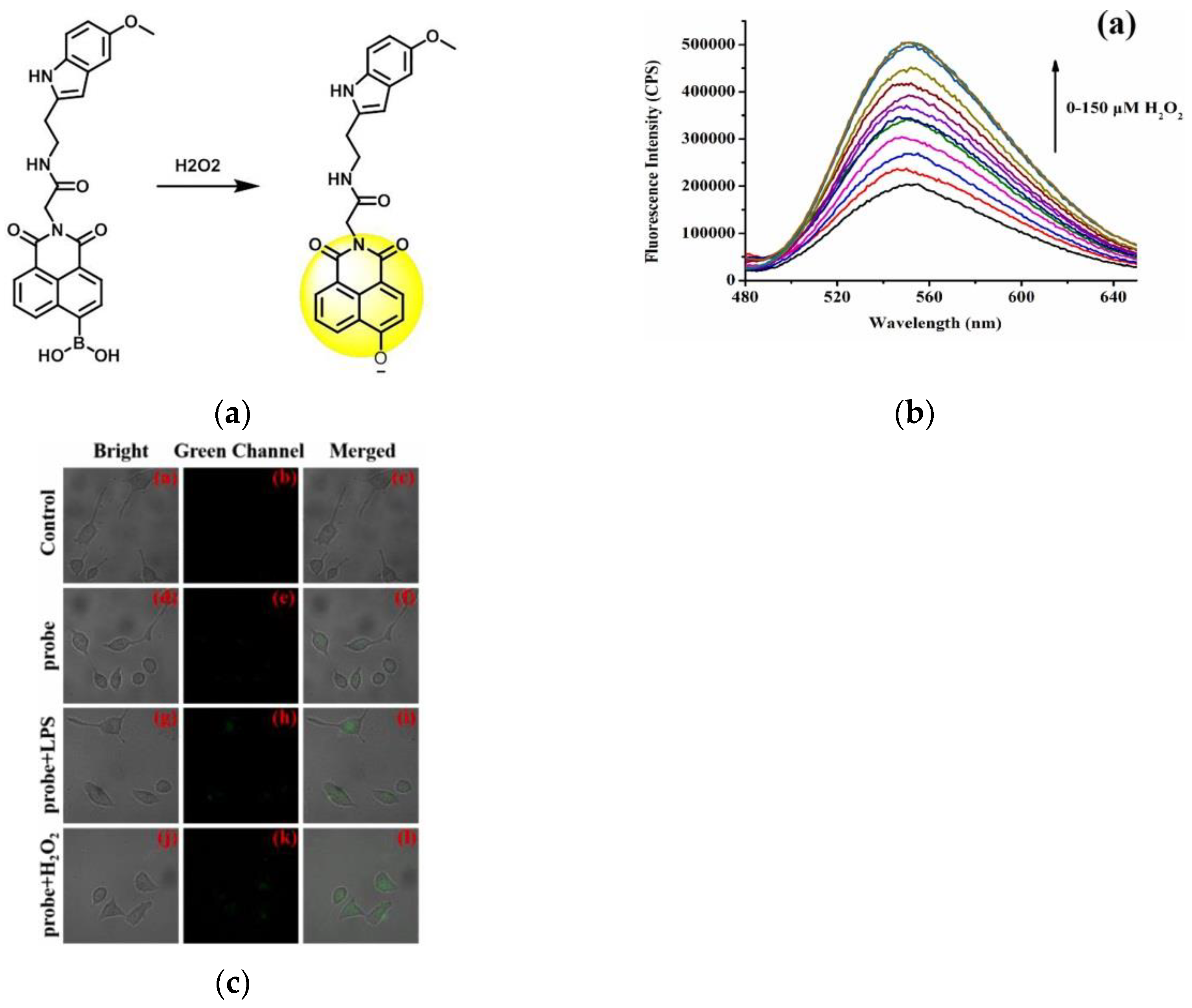
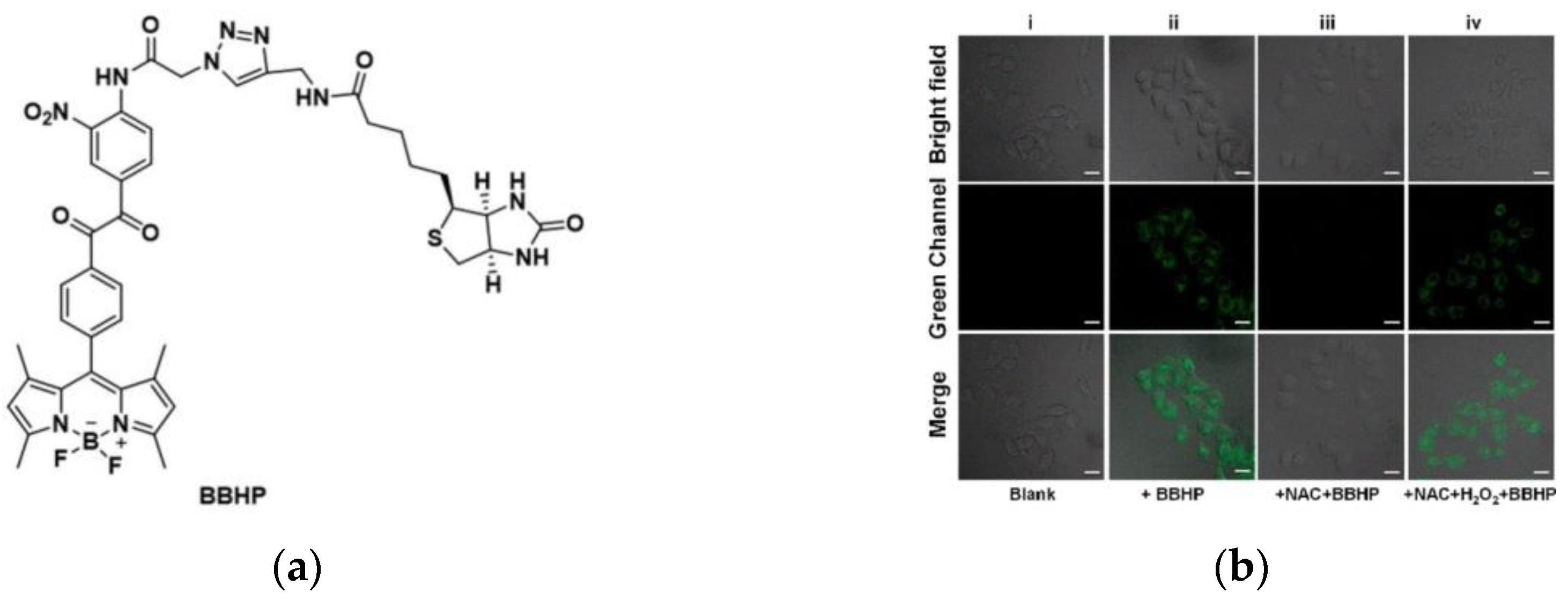
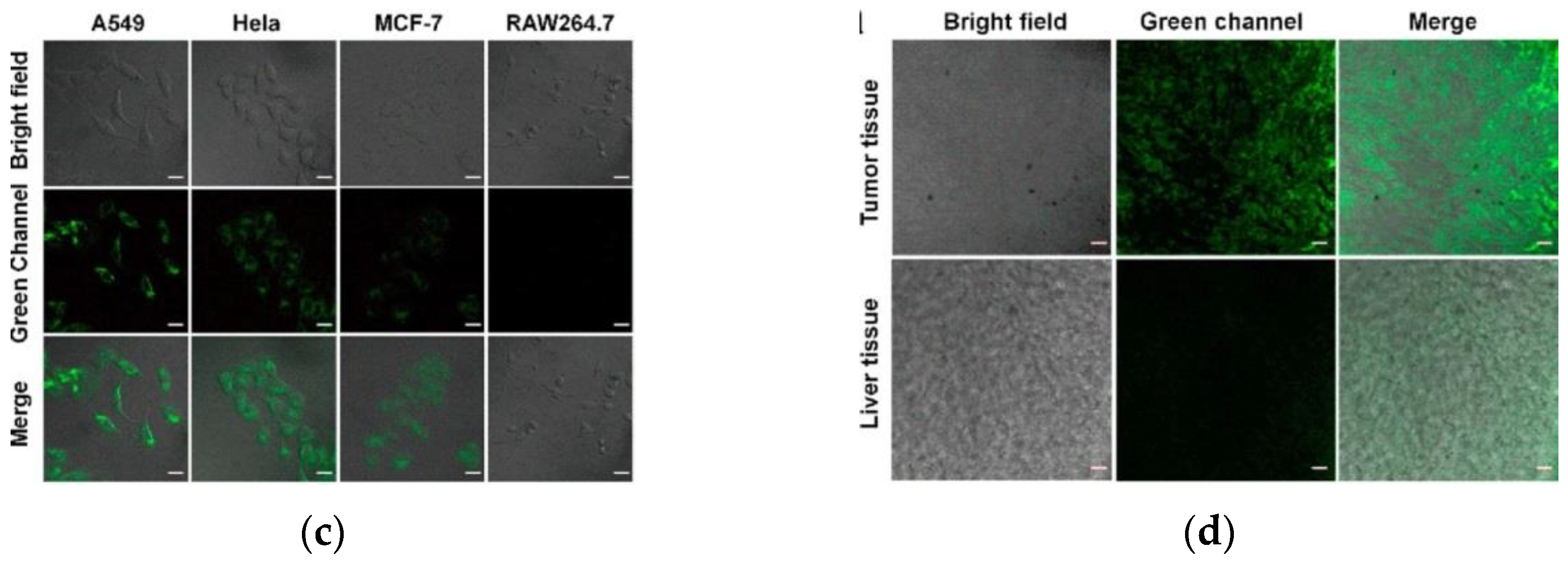
8. Fluorescent Probe for Detecting Hydrogen Peroxide (H2O2)
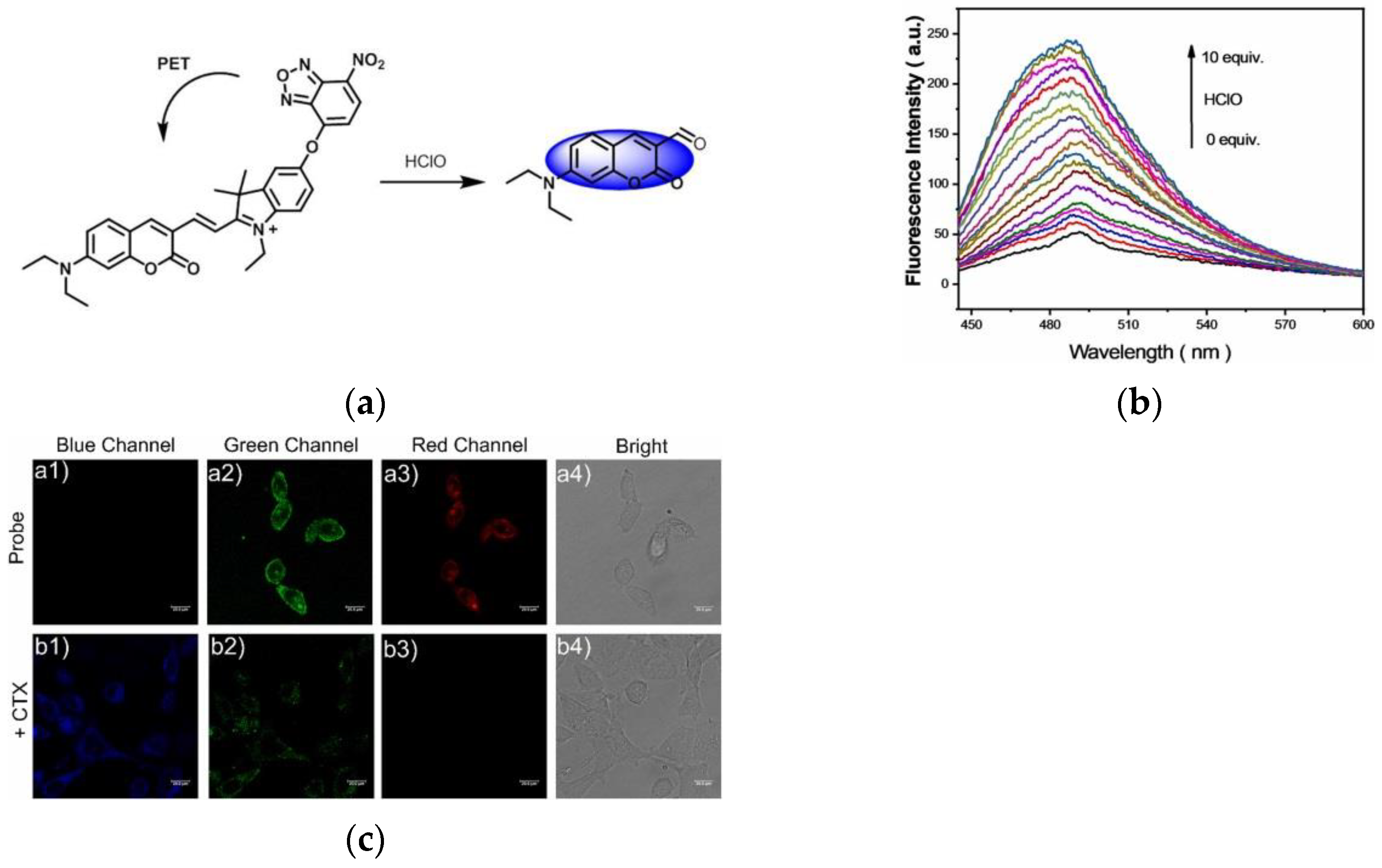
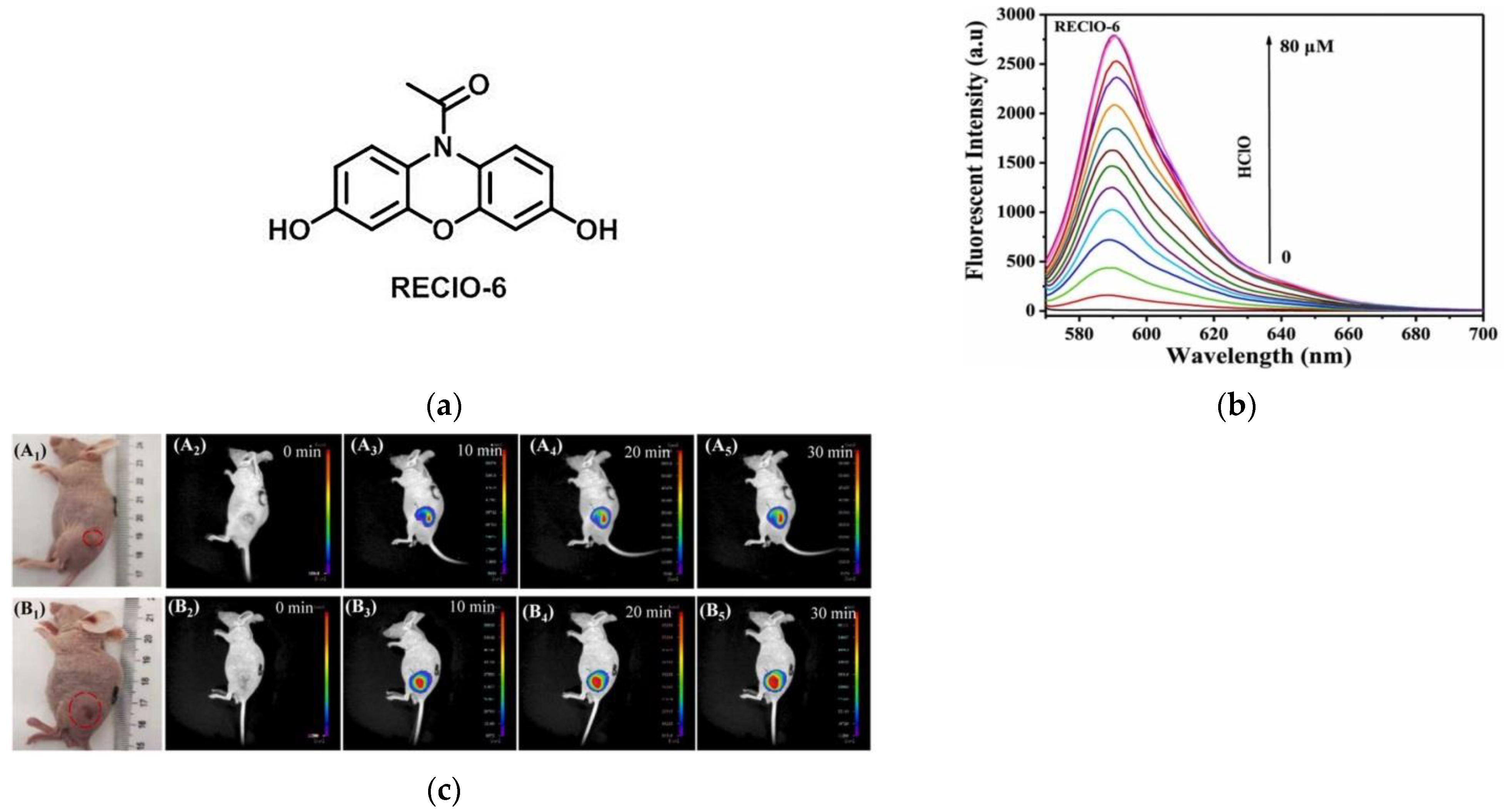
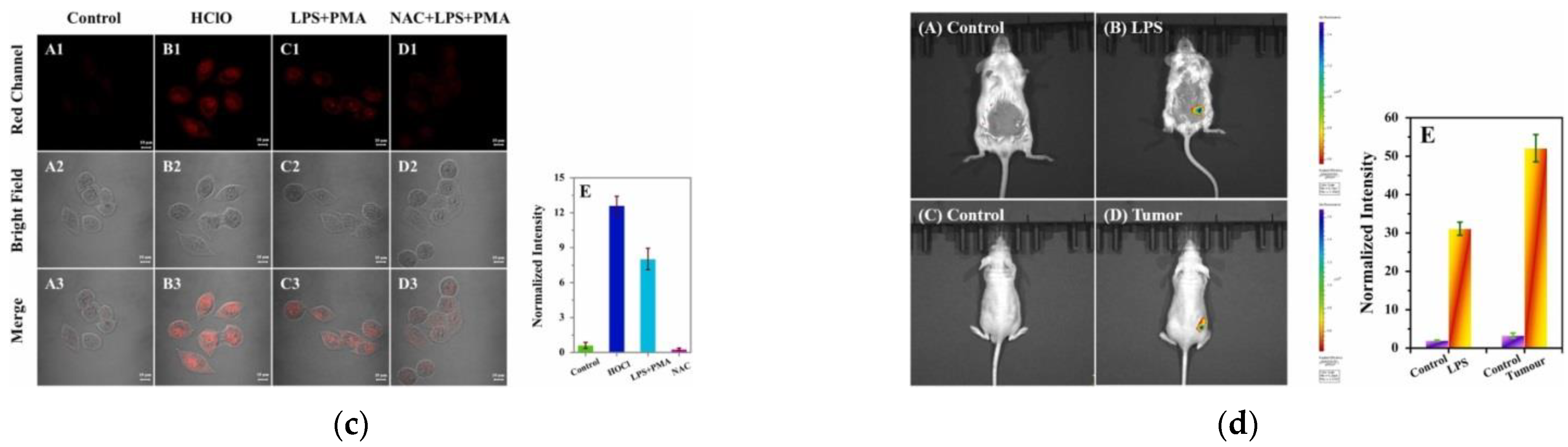
9. Fluorescent Probe for Detecting Hypochlorous Acid (HOCl)
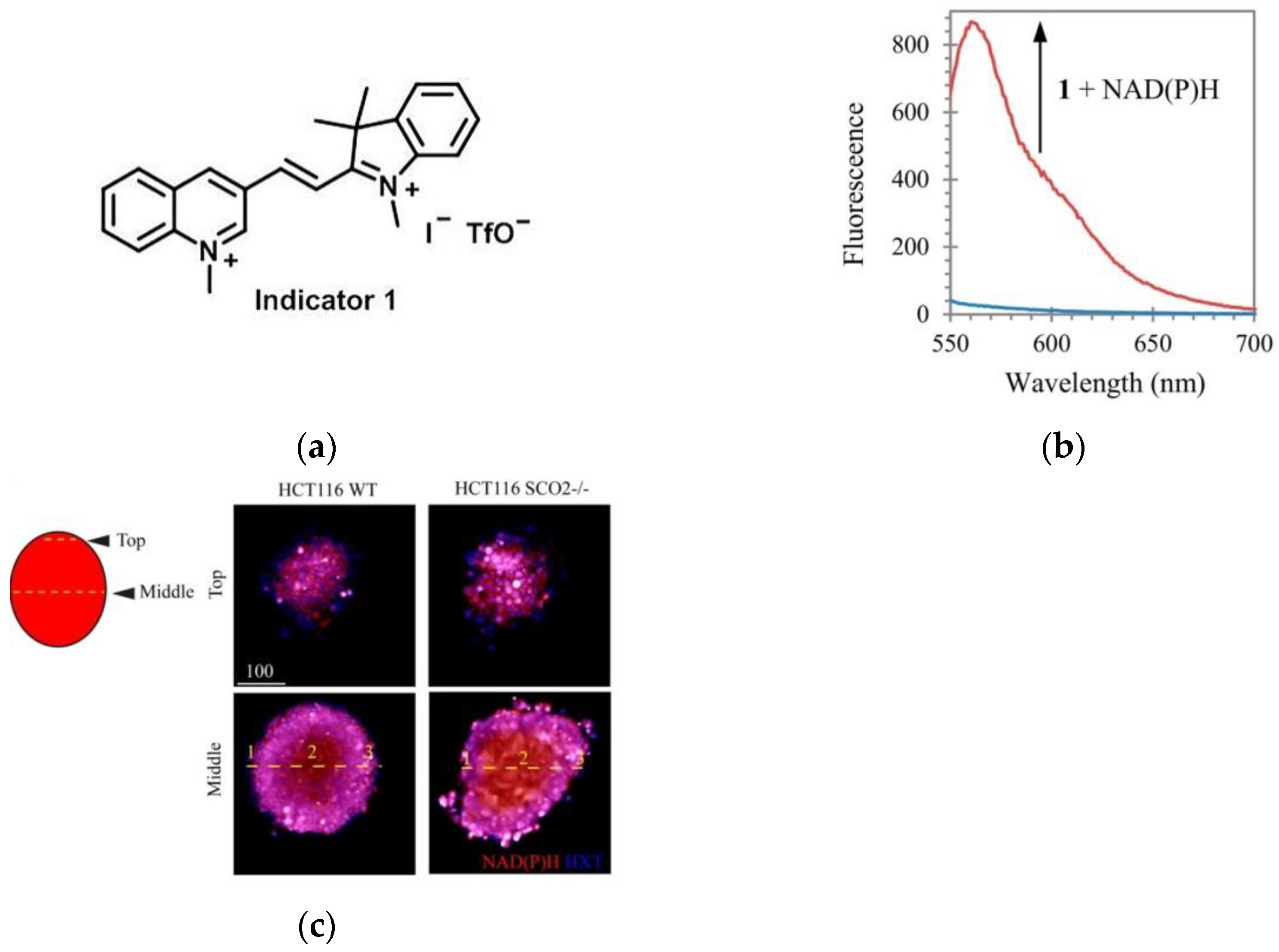
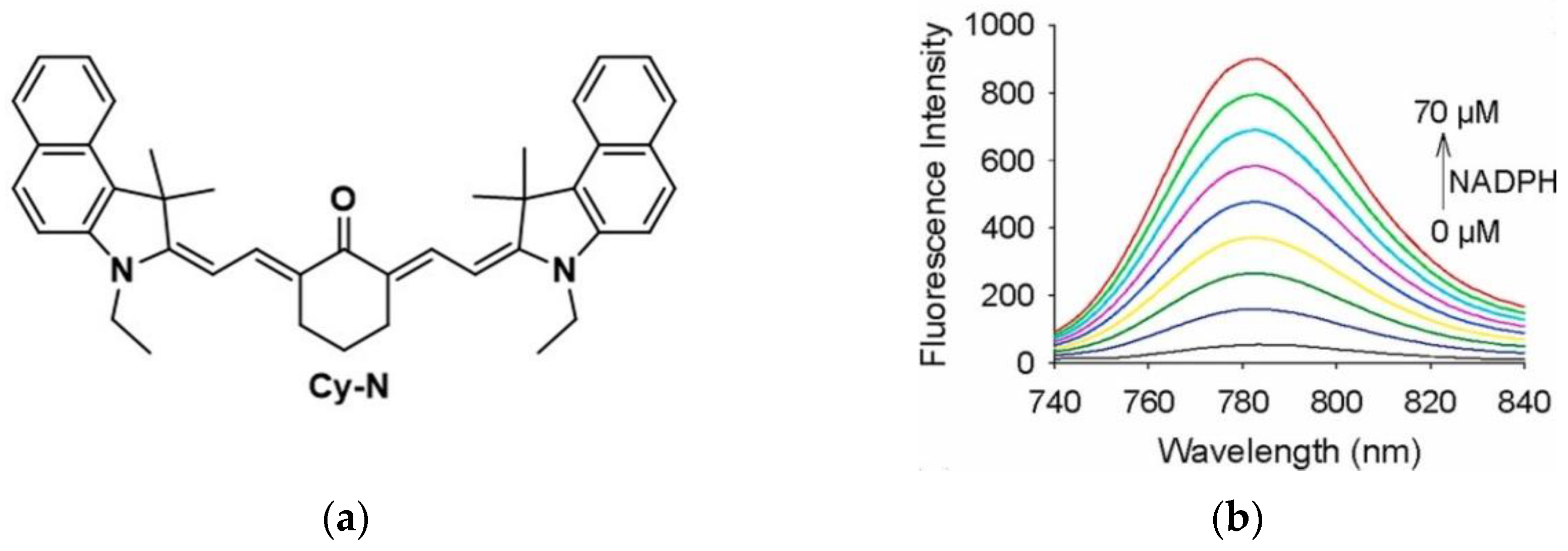
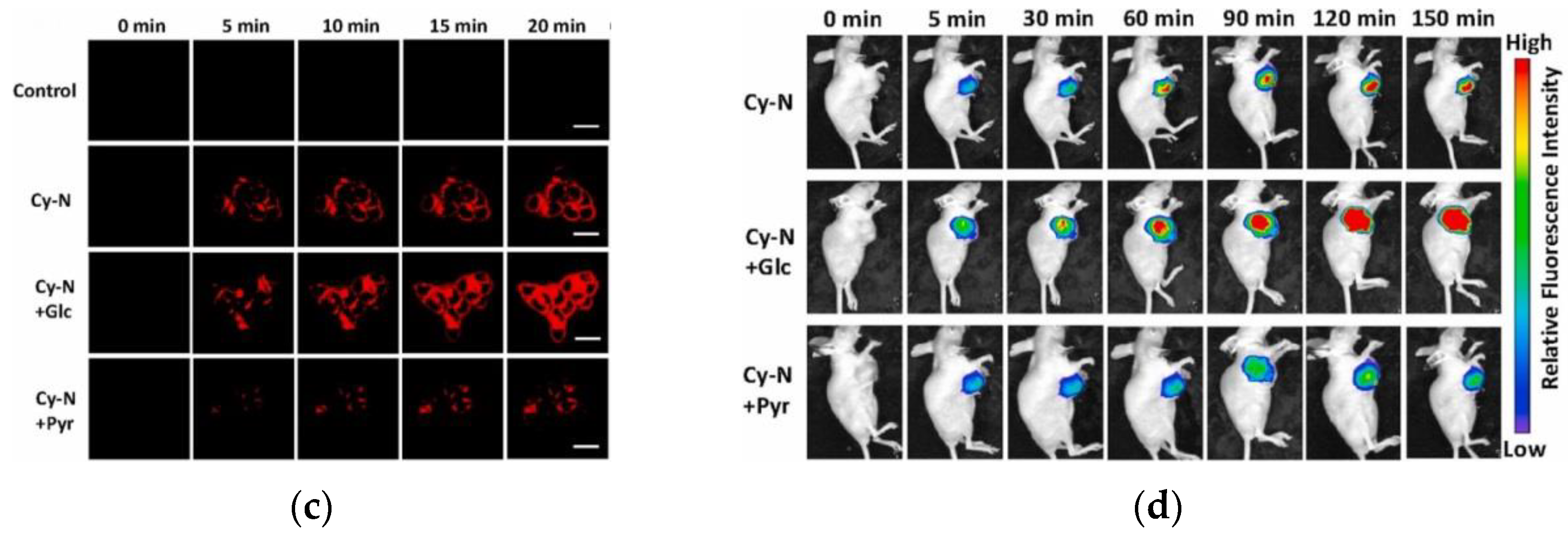
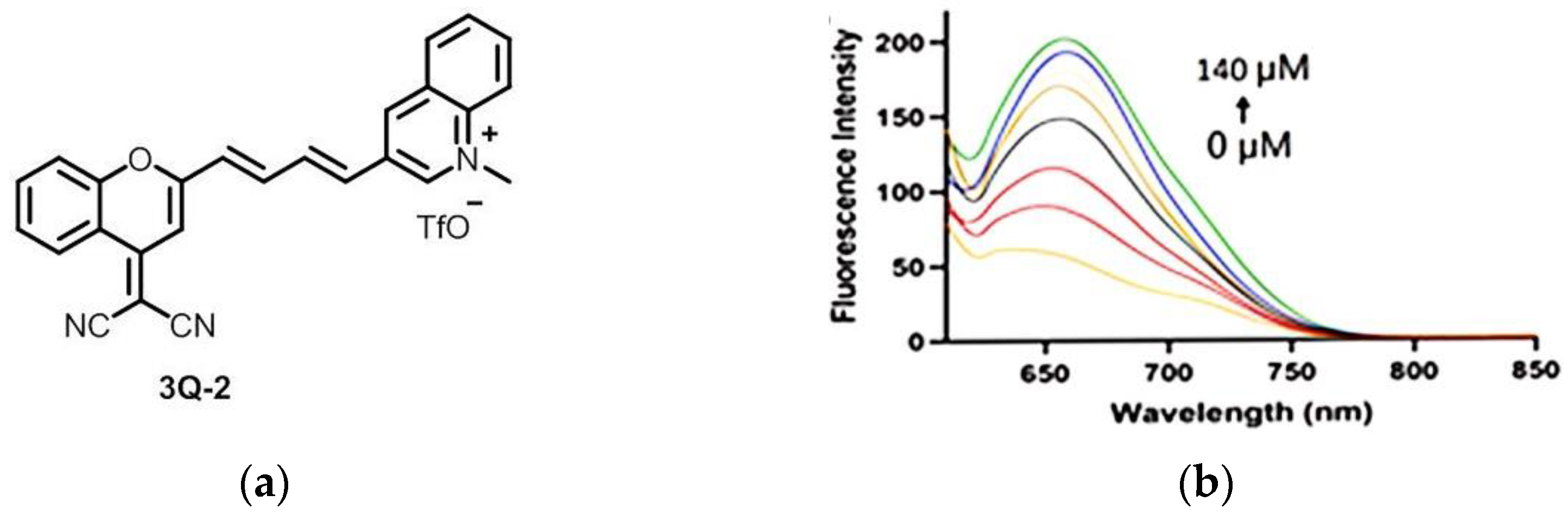
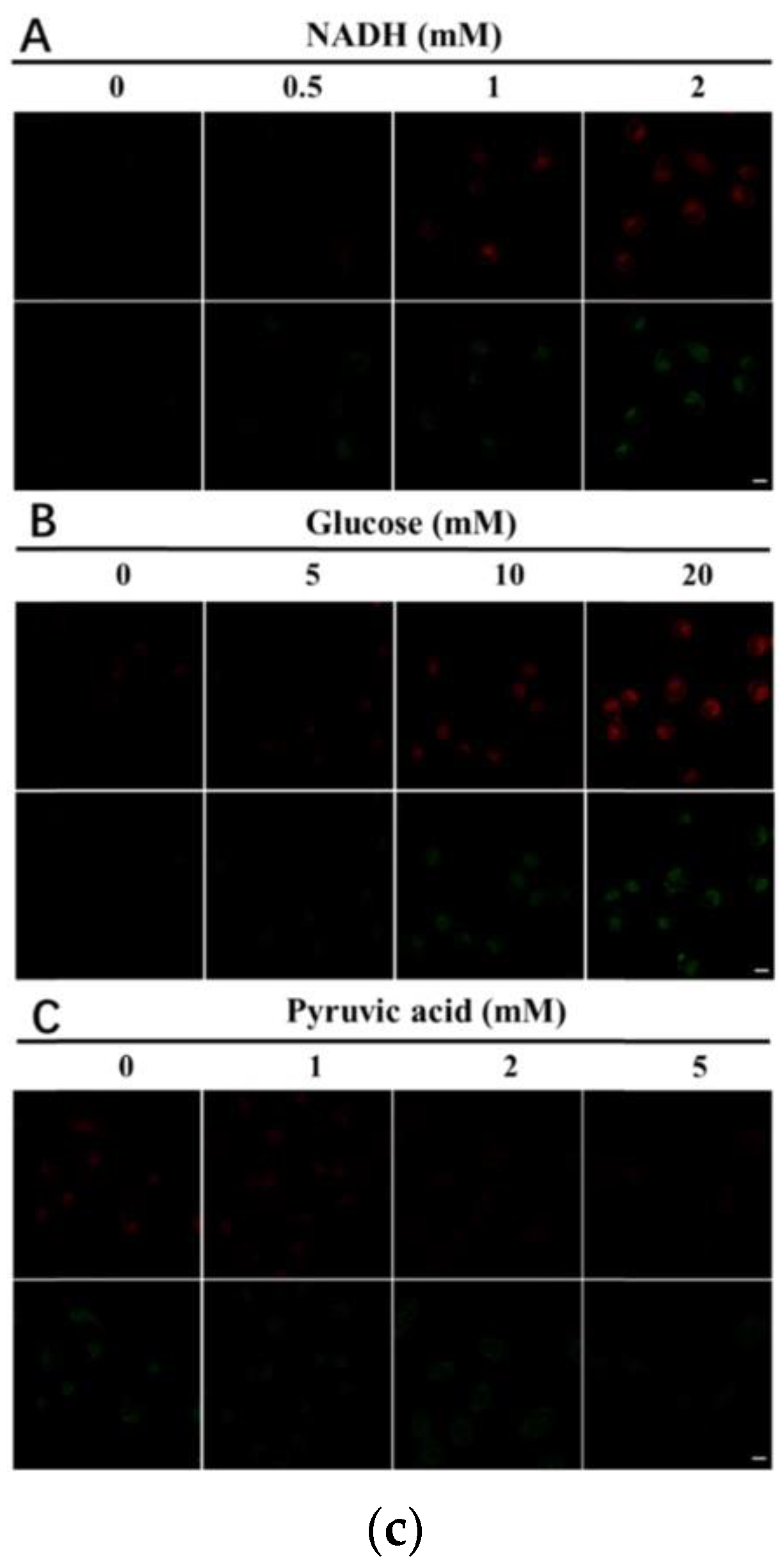
10. Fluorescent Probe for Detecting NADH
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buys, S.S.; Partridge, E.; Black, A.; Johnson, C.; Lamerato, L.; Isaacs, C.; Reding, D.; Greenlee, R.; Kessel, B.; Fouad, M.; et al. Effect of screening on ovarian cancer mortality in the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer randomized screening trial. J. Clin. Oncol. 2011, 29, 5001. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Wang, L.; Qin, X.; Jiang, B.-P.; Ji, S.-C.; Shen, X.-C.; Liang, H. A general approach to design dual ratiometric fluorescent and fhotoacoustic frobes for quantitatively visualizing tumor hypoxia levels in vivo. Angew. Chem. Int. Ed. 2022, 61, e202107076. [Google Scholar]
- Li, J.; Liu, F.; Shao, Q.; Min, Y.; Costa, M.; Yeow, E.K.L.; Xing, B. Enzyme-responsive cell-penetrating peptide ponjugated mesoporous silica quantum dot nanocarriers for controlled release of nucleus-targeted drug molecules and real-time intracellular fluorescence imaging of tumor cells. Adv. Healthc. Mater. 2014, 3, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Wang, T.; Wang, H.; Zheng, Y. Microfluidic techniques for tumor cell detection. Electrophoresis 2019, 40, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef]
- Jaffer, F.A.; Weissleder, R. Molecular imaging in the clinical arena. JAMA 2005, 293, 855–862. [Google Scholar] [CrossRef]
- Weissleder, R. Scaling down imaging: Molecular mapping of cancer in mice. Nat. Rev. Cancer 2002, 2, 11–18. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, J.; Li, Y.; Zheng, J.; Yang, R. Sensitive and rapid detection of endogenous hydrogen sulfide distributing in different mouse viscera via a two-photon fluorescent probe. Anal. Chim. Acta 2015, 896, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotech. 2007, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, W.; Guo, Z. Metal coordination in photoluminescent sensing. Chem. Soc. Rev. 2013, 42, 1568–1600. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.F.P.; Brand, L.M.; de Figueiredo, J.A.P.; Steier, L.; Lamers, M.L. Use of autofluorescence and fluorescent probes as a potential diagnostic tool for oral cancer: A systematic review. Photodiagn. Photodyn. Ther. 2021, 33, 102073. [Google Scholar] [CrossRef]
- Thong, P.S.P.; Olivo, M.; Chin, W.W.L.; Bhuvaneswari, R.; Mancer, K.; Soo, K.C. Clinical application of fluorescence endoscopic imaging using hypericin for the diagnosis of human oral cavity lesions. Br. J. Cancer 2009, 101, 1580–1584. [Google Scholar] [CrossRef]
- Paganin-Gioanni, A.; Bellard, E.; Paquereau, L.; Ecochard, V.; Golzio, M.; Teissié, J. Fluorescence imaging agents in cancerology. Radiol. Oncol. 2010, 44, 142–148. [Google Scholar] [CrossRef]
- Singh, A.K.; Nair, A.V.; Singh, N.D.P. Small Two-photon organic fluorogenic probes: Sensing and bioimaging of cancer relevant biomarkers. Anal. Chem. 2022, 94, 177–192. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Q. Understanding the global cancer statistics 2018: Implications for cancer control. Sci. China Life Sci. 2021, 64, 1017–1020. [Google Scholar] [CrossRef]
- Cao, W.; Chen, H.-D.; Yu, Y.-W.; Li, N.; Chen, W.-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Y.; Guo, Z. Recent progress on molecularly near-infrared fluorescent probes for chemotherapy and phototherapy. Coord. Chem. Rev. 2021, 427, 213556. [Google Scholar] [CrossRef]
- Youy, L.; Lvy, Z.; Liy, C.; Ye, W.; Zhou, Y.; Jin, J.; Han, Q. Worldwide cancer statistics of adolescents and young adults in 2019: A systematic analysis of the Global Burden of Disease Study 2019. ESMO Open 2021, 6, 100255. [Google Scholar] [CrossRef]
- Kulasingam, V.; Prassas, I.; Diamandis, E.P. Towards personalized tumor markers. NPJ Precis. Onc. 2017, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Design and construction of COX-2 specific fluorescent probes. Mol. Cell. Probes 2019, 48, 101472. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Yeh, C.-N.; Lee, W.-Y.; Huang, Y.-C.; Chang, K.-W.; Lin, K.-J.; Tien, S.-F.; Su, W.-C.; Yang, C.-H.; Chen, J.-T.; et al. [123I] Iodooctyl fenbufen amide as a SPECT tracer for imaging tumors that over-express COX enzymes. Biomaterials 2013, 34, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Winzer, K.-J.; Müller, B.-M.; Weichert, W.; Pest, S.; Köbel, M.; Kristiansen, G.; Reles, A.; Siegert, A.; Guski, H.; et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer 2003, 97, 2978–2987. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Lu, W.; Huang, J.; Zhu, W.; Li, H.; Xu, Y.; Qian, X. A highly selective space-folded photo-induced electron transfer fluorescent probe for carbonic anhydrase isozymes IX and its applications for biological imaging. Chem. Commun. 2011, 47, 8301–8303. [Google Scholar] [CrossRef]
- Banerjee, J.; Haldar, M.K.; Manokaran, S.; Mallik, S.; Srivastava, D.K. New fluorescent probes for carbonic anhydrases. Chem. Commun. 2007, 2723–2725. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Wang, J.; Dou, B.; Zhou, F.; Cao, J.; Qu, J.; Cao, Z.; Zhao, W.; Peng, X. Fluorescence discrimination of cancer from inflammation by molecular response to COX-2 enzymes. J. Am. Chem. Soc. 2013, 135, 17469–17475. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387. [Google Scholar] [CrossRef] [PubMed]
- Kam, P.C.A.; See, A.U.L. Cyclo-oxygenase isoenzymes: Physiological and pharmacological role. Anaesthesia 2000, 55, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Samad, T.A.; Moore, K.A.; Sapirstein, A.; Billet, S.; Allchorne, A.; Poole, S.; Bonventre, J.V.; Woolf, C.J. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001, 410, 471–475. [Google Scholar] [CrossRef]
- Rizzo, M.T. Cyclooxygenase-2 in oncogenesis. Clin. Chim. Acta 2011, 412, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Schmedtje, J.F.; Ji, Y.-S.; Liu, W.L.; DuBois, R.N.; Runge, M.S. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 1997, 272, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M.; Kumar, V.; Mohan, C.G. Synthesis, biological evaluation and molecular docking studies of stellatin derivatives as cyclooxygenase (COX-1, COX-2) inhibitors and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2011, 21, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Vitale, P.; Tacconelli, S.; Perrone, M.G.; Malerba, P.; Simone, L.; Scilimati, A.; Lavecchia, A.; Dovizio, M.; Marcantoni, E.; Bruno, A.; et al. Synthesis, pharmacological characterization, and docking analysis of a novel family of diarylisoxazoles as highly selective cyclooxygenase-1 (COX-1) inhibitors. J. Med. Chem. 2013, 56, 4277–4299. [Google Scholar] [CrossRef]
- Wang, B.; Fan, J.; Wang, X.; Zhu, H.; Wang, J.; Mu, H.; Peng, X. A Nile blue based infrared fluorescent probe: Imaging tumors that over-express cyclooxygenase-2. Chem. Commun. 2015, 51, 792–795. [Google Scholar] [CrossRef]
- Gurram, B.; Zhang, S.; Li, M.; Li, H.; Xie, Y.; Cui, H.; Du, J.; Fan, J.; Wang, J.; Peng, X. Celecoxib conjugated fluorescent pobe for identification and discrimination of cyclooxygenase-2 enzyme in cancer cells. Anal. Chem. 2018, 90, 5187–5193. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, M.K.; Lee, D.J.; Song, D.H.; Lim, C.S.; Noh, C.-K.; Lee, D.M.; Choi, K.S.; Shin, S.J.; Kim, H.M. Development of two-photon fluorescence probe for detecting cyclooxygenase-2 level in human colorectal cancer tissue. Sens. Actuators B Chem. 2021, 330, 129329. [Google Scholar] [CrossRef]
- Uddin, M.J.; Lo, J.H.; Oltman, C.G.; Crews, B.C.; Huda, T.; Liu, J.; Kingsley, P.J.; Lin, S.; Milad, M.; Aleem, A.M.; et al. Discovery of a redox-activatable chemical probe for detection of cyclooxygenase-2 in cells and animals. ACS Chem. Biol. 2022, 17, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, L. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 2009, 29, 29–64. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xu, C.; Zhao, Y.; Yu, C.; Shen, S.; Li, L.; Huang, W. Recent progress in small molecule fluorescent probes for nitroreductase. Chin. Chem. Lett. 2018, 29, 1451–1455. [Google Scholar] [CrossRef]
- Cui, L.; Zhong, Y.; Zhu, W.; Xu, Y.; Du, Q.; Wang, X.; Qian, X.; Xiao, Y. A new prodrug-derived ratiometric fluorescent probe for hypoxia: High selectivity of nitroreductase and imaging in tumor cell. Org. Lett. 2011, 13, 928–931. [Google Scholar] [CrossRef]
- Tian, X.; Li, Z.; Sun, Y.; Wang, P.; Ma, H. Near-infrared fluorescent probes for hypoxia detection via ioint regulated enzymes: Design, synthesis, and application in living cells and mice. Anal. Chem. 2018, 90, 13759–13766. [Google Scholar] [CrossRef] [PubMed]
- Kiyose, K.; Hanaoka, K.; Oushiki, D.; Nakamura, T.; Kajimura, M.; Suematsu, M.; Nishimatsu, H.; Yamane, T.; Terai, T.; Hirata, Y.; et al. Hypoxia-sensitive fluorescent probes for in vivo real-time gluorescence imaging of acute ischemia. J. Am. Chem. Soc. 2010, 132, 15846–15848. [Google Scholar] [CrossRef]
- Takahashi, S.; Piao, W.; Matsumura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kamachi, T.; Kohno, M.; Nagano, T.; Hanaoka, K. Reversible off–on fluorescence probe for hypoxia and imaging of hypoxia–normoxia cycles in live cells. J. Am. Chem. Soc. 2012, 134, 19588–19591. [Google Scholar] [CrossRef]
- Li, L.; Ding, L.; Zhang, X.; Wen, D.; Zhang, M.; Liu, W.; Wang, H.; Wang, B.; Yan, L.; Guo, L.; et al. A nitroreductase-responsive near-infrared phototheranostic probe for in vivo imaging of tiny tumor and photodynamic therapy. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 267, 120579. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Liu, J.; Fu, J.; Sun, Y.; Ouyang, R.; Miao, Y. A near-infrared frequency upconversion probe for nitroreductase detection and hypoxia tumor in vivo imaging. Sens. Actuators B Chem. 2019, 286, 337–345. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Long, R.C.; Papas, K.K.; Sambanis, A.; Constantinidis, I. In vitro monitoring of total choline levels in a bioartificial pancreas: 1H NMR spectroscopic studies of the effects of oxygen level. J. Magn. Reson. 2000, 146, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Povich, M.J. Measurement of dissolved oxygen concentrations and diffusion coefficients by electron spin resonance. Anal. Chem. 1975, 47, 346–347. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Song, H.; Liu, X.; Xie, X.; Pang, X.; Zhou, Y. Red-emitting fluorescent probe for selective and sensitive determination hypochlorite in living cells. J. Lumin. 2019, 210, 472–478. [Google Scholar] [CrossRef]
- Bai, J.; Yang, X.; Qian, Y. A ratiometric and near-infrared fluorescent probe for discrimination of Cys/Hcy/GSH in aqueous solution and its intracellular imaging in living cells. J. Lumin. 2020, 221, 117055. [Google Scholar] [CrossRef]
- Guo, T.; Cui, L.; Shen, J.; Zhu, W.; Xu, Y.; Qian, X. A highly sensitive long-wavelength fluorescence probe for nitroreductase and hypoxia: Selective detection and quantification. Chem. Commun. 2013, 49, 10820–10822. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Gao, X.; Zhang, Y.; Shi, W.; Ma, H. Nitroreductase detection and hypoxic tumor cell imaging by a designed sensitive and selective fluorescent probe, 7-[(5-nitrofuran-2-yl)methoxy]-3H-phenoxazin-3-one. Anal. Chem. 2013, 85, 3926–3932. [Google Scholar] [CrossRef]
- Wei, C.; Shen, Y.; Xu, Z.; Peng, S.; Yuan, Z.; He, Y.; Yin, J.; Chen, H. A novel off-on fluorescent probe for imaging of hypoxia in tumor cell. J. Photochem. Photobiol. A Chem. 2018, 353, 292–298. [Google Scholar] [CrossRef]
- Zheng, J.; Shen, Y.; Xu, Z.; Yuan, Z.; He, Y.; Wei, C.; Er, M.; Yin, J.; Chen, H. Near-infrared off-on fluorescence probe activated by NTR for in vivo hypoxia imaging. Biosens. Bioelectron. 2018, 119, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, C.; Yu, H.; Zhang, Y.; Ji, M. A highly sensitive and selective “turn on” fluorescent probe based on fused four-ring quinoxaline skeleton for endogenous detection of NTR. J. Lumin. 2021, 238, 118313. [Google Scholar] [CrossRef]
- Li, M.; Kong, X.; Yin, Y.; Zhang, Y.; Dai, X.; Wang, J.; Lin, W. A novel red-emitting two-photon fluorescent probe for imaging nitroreductases in cancer cells and tumor tissues with hypoxia conditions. J. Photochem. Photobiol. A Chem. 2022, 424, 113657. [Google Scholar] [CrossRef]
- Wang, S.; Tan, W.; Lang, W.; Qian, H.; Guo, S.; Zhu, L.; Ge, J. Fluorogenic and mitochondria-localizable probe enables selective labeling and imaging of nitroreductase. Anal. Chem. 2022, 94, 7272–7277. [Google Scholar] [CrossRef]
- He, D.-D.; Liu, W.; Sun, R.; Fan, C.; Xu, Y.-J.; Ge, J.-F. N-pyridineium-2-yl darrow red analogue: Unique near-infrared lysosome-biomarker for the detection of cancer cells. Anal. Chem. 2015, 87, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, E.S.; Ebersold, M.; Garrett, W.; Pypaert, M.; Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 2003, 299, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lv, Y.; Jie, X.; Meng, Z.; Wang, X.; Huang, J.; Peng, A.; Tian, Z. A super-sensitive ratiometric fluorescent probe for monitoring intracellular subtle pH fluctuation. Sens. Actuators B Chem. 2018, 273, 167–175. [Google Scholar]
- Li, X.; Yue, Y.; Wen, Y.; Yin, C.; Huo, F. Hemicyanine based fluorimetric and colorimetric pH probe and its application in bioimaging. Dyes Pigm. 2016, 134, 291–296. [Google Scholar] [CrossRef]
- Xiong, H.; Kos, P.; Yan, Y.; Zhou, K.; Miller, J.B.; Elkassih, S.; Siegwart, D.J. Activatable water-soluble probes enhance tumor imaging by responding to dysregulated pH and exhibiting high tumor-to-liver fluorescence emission contrast. Bioconjug.Chem. 2016, 27, 1737–1744. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Lee, S.; Yin, C.; Yoon, J. A review: The trend of progress about pH probes in cell application in recent years. Analyst 2016, 142, 30–41. [Google Scholar] [CrossRef]
- Ma, J.; Li, W.; Li, J.; Shi, R.; Yin, G.; Wang, R. A small molecular pH-dependent fluorescent probe for cancer cell imaging in living cell. Talanta 2018, 182, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, X.; Wang, Y.; Du, Q.; Xu, T.; Du, B.; Yao, H. The ratiometric fluorescence nanoparticle based on SiRB for pH detection of tumor. Eur. J. Pharm. Sci. 2018, 118, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Zhang, T.; Shen, S.-L.; Miao, J.-Y.; Zhao, B.-X. A ratiometric lysosomal pH probe based on the naphthalimide–rhodamine system. J. Mater. Chem. B 2015, 3, 3260–3266. [Google Scholar] [CrossRef]
- Wen, J.; Xia, P.; Zheng, Z.; Xu, Y.; Li, H.; Liu, F.; Sun, S. Naphthalimide-rhodamine based fluorescent probe for ratiometric sensing of cellular pH. Chin. Chem. Lett. 2017, 28, 2005–2008. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Zhang, K.; Ou, T.; Li, Y.; Zhang, C.; Dong, C.; Shuang, S.; Wong, M.S. A two-photon ratiometric fluorescent probe for effective monitoring of lysosomal pH in live cells and cancer tissues. Sens. Actuators B Chem. 2018, 262, 913–921. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Ge, H.; Zhu, X.; Zhao, Z.; Qi, Z.-q.; Wang, M.; Wang, J. Dual hepatocyte-targeting fluorescent probe with high sensitivity to tumorous pH: Precise detection of hepatocellular carcinoma cells. Sens. Actuators B Chem. 2019, 285, 584–589. [Google Scholar] [CrossRef]
- Zhang, T.; Huo, F.; Zhang, W.; Chao, J.; Yin, C. Ultra-pH-sensitive sensor for visualization of lysosomal autophagy, drug-induced pH alteration and malignant tumors microenvironment. Sens. Actuators B Chem. 2021, 345, 130393. [Google Scholar] [CrossRef]
- Feng, Z.; Wu, J.; Jiang, M.; Sha, J.; Liu, W.; Ren, H.; Zhang, W.; Lee, C.-S.; Wang, P. A rhodamine derivative-based fluorescent probe for visual monitoring of pH changes in the Golgi apparatus. Sens. Actuators B Chem. 2022, 366, 131963. [Google Scholar] [CrossRef]
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in cancer cell death. Cancers 2011, 3, 1285–1310. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, F.; Yin, C. Photocontrolled single-/dual-site alternative fluorescence probes distinguishing detection of H2S/SO2 in vivo. Org. Lett. 2019, 21, 5277–5280. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. A highly sensitive, cell-membrane-permeable fluorescent probe for glutathione. Bioorg. Med. Chem. Lett. 2014, 24, 4363–4366. [Google Scholar] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Zhao, H.; Wen, X.; Li, W.; Li, Y.; Yin, C. A copper-mediated on–off–on gold nanocluster for endogenous GSH sensing to drive cancer cell recognition. J. Mater. Chem. B 2019, 7, 2169–2176. [Google Scholar] [CrossRef]
- Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Rational design of reversible fluorescent probes for live-cell imaging and quantification of fast glutathione dynamics. Nat. Chem. 2017, 9, 279–286. [Google Scholar] [CrossRef]
- Schnelldorfer, T.; Gansauge, S.; Gansauge, F.; Schlosser, S.; Beger, H.G.; Nussler, A.K. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer 2000, 89, 1440–1447. [Google Scholar] [CrossRef]
- Bui, Q.N.; Li, Y.; Jang, M.-S.; Huynh, D.P.; Lee, J.H.; Lee, D.S. Redox- and pH-sensitive polymeric micelles based on poly(β-amino ester)-grafted disulfide methylene oxide poly(ethylene glycol) for anticancer drug delivery. Macromolecules 2015, 48, 4046–4054. [Google Scholar] [CrossRef]
- Yuan, Z.; Gui, L.; Zheng, J.; Chen, Y.; Qu, S.; Shen, Y.; Wang, F.; Er, M.; Gu, Y.; Chen, H. GSH-activated light-up near-infrared fluorescent probe with high affinity to αvβ3 integrin for precise early tumor identification. ACS Appl. Mater. Interfaces 2018, 10, 30994–31007. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar]
- Wang, Z.-G.; Ding, X.-J.; Huang, Y.-Y.; Yan, X.-J.; Ding, B.; Li, Q.-Z.; Xie, C.-Z.; Xu, J.-Y. The development of coumarin Schiff base system applied as highly selective fluorescent/colorimetric probes for Cu2+ and tumor biomarker glutathione detection. Dyes Pigm. 2020, 175, 108156. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhang, Y.; Cao, Q.; Chen, Y. A GSH-responsive PET-based fluorescent probe for cancer cells imaging. Chin. Chem. Lett. 2021, 32, 1541–1544. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Liu, G.; Jin, L.; Lin, Z.; Lin, L.; Wu, Y.; Chen, J.; Lin, H.; Song, J. In-vivo two-photon visualization and quantitative detection of redox state of cancer. J. Biophotonics 2022, 15, e202100357. [Google Scholar] [CrossRef]
- Pastore, A.; Alisi, A.; Di Giovamberardino, G.; Crudele, A.; Ceccarelli, S.; Panera, N.; Dionisi-Vici, C.; Nobili, V. Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int. J. Mol. Sci. 2014, 15, 21202–21214. [Google Scholar] [CrossRef] [PubMed]
- Koning, A.M.; Meijers, W.C.; Pasch, A.; Leuvenink, H.G.D.; Frenay, A.-R.S.; Dekker, M.M.; Feelisch, M.; de Boer, R.A.; van Goor, H. Serum free thiols in chronic heart failure. Pharmacol. Res. 2016, 111, 452–458. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Jiang, E.; Zhu, M.; Wang, Z.; Fan, S.; Gao, Q.; Liu, S.; Li, Q.X.; Hua, R. A colorimetric and ratiometric dual-site fluorescent probe with 2,4-dinitrobenzenesulfonyl and aldehyde groups for imaging of aminothiols in living cells and zebrafish. Dyes Pigm. 2018, 156, 338–347. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, Y.; Qiu, F.; Iqbal, K.; Wang, Y.; Song, X.; Wang, Y.; Zhang, G.; Liu, W. Dual-site and dual-excitation fluorescent probe that can be tuned for discriminative detection of cysteine, homocystein, and thiophenols. Anal. Chem. 2018, 90, 14048–14055. [Google Scholar] [CrossRef]
- Zhu, D.; Miao, Z.Y.; Hu, Y.; Zhang, X.J. Single-step, homogeneous and sensitive detection for microRNAs with dual-recognition steps based on luminescence resonance energy transfer (LRET) using upconversion nanoparticles. Biosens. Bioelectron. 2018, 100, 475–481. [Google Scholar] [CrossRef]
- Han, C.; Yang, H.; Chen, M.; Su, Q.; Feng, W.; Li, F. Mitochondria-targeted near-infrared fluorescent off–on probe for selective detection of cysteine in living cells and in vivo. ACS Appl. Mater. Interfaces 2015, 7, 27968–27975. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, S.; Gu, K.; Guo, Z.; Huang, X.; Wang, M.; Amin, H.M.; Zhu, W.; Shi, P. GSH-activated NIR fluorescent prodrug for podophyllotoxin delivery. ACS Appl. Mater. Interfaces 2017, 9, 29496–29504. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, L.; Chen, L.; Ma, H.; Liu, H.-W.; Zhang, X.-B. Engineering of a near-infrared fluorescent probe for real-time simultaneous visualization of intracellular hypoxia and induced mitophagy. Chem. Sci. 2018, 9, 5347–5353. [Google Scholar] [CrossRef]
- Combs, J.A.; DeNicola, G.M. The non-essential amino acid cysteine becomes essential for tumor proliferation and survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, N.; Huang, Y.; Yu, F.; Li, B.; Lv, C.; Chen, L. Mitochondria-targeting near-infrared ratiometric fluorescent probe for selective imaging of cysteine in orthotopic lung cancer mice. Sens. Actuators B Chem. 2019, 282, 69–77. [Google Scholar] [CrossRef]
- Dai, X.; Kong, X.; Lin, W. A novel fluorescent probe with large Stokes shift for two-photon imaging of biothiols in living cells, liver tissues and tumor tissues. Dyes Pigm. 2017, 142, 306–314. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Yang, Y.; Wang, L.; Wu, X.; Fan, S.; Wang, Z.; Hua, R.; Wang, Y.; Li, Q.X. A ratiometric fluorescence probe with large stokes based on excited-stated intramolecular proton transfer (ESIPT) for rapid detection and imaging of biothiols in human liver cancer HepG2 cells and zebrafish. J. Mol. Liq. 2019, 287, 111016. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.Q.; Mao, G.J.; Jiang, W.L.; Tan, M.; Xu, F.; Li, C.Y. A near-infrared fluorescent probe with large Stokes shift for imaging Cys in tumor mice. Anal. Chim. Acta 2021, 1171, 338655. [Google Scholar] [CrossRef] [PubMed]
- An, J.M.; Suh, J.; Kim, J.; Kim, Y.; Chung, J.Y.; Kim, H.S.; Cho, S.Y.; Ku, J.H.; Kwak, C.; Kim, H.H.; et al. First-in-Class: Cervical cancer diagnosis based on a urine test with fluorescent cysteine probe. Sens. Actuators B Chem. 2022, 360, 131646. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Ge, Y.; Li, W.; Shu, Q.; Guan, H.; Yang, K.; Myatt, L.; Sun, K. Cortisol induces aromatase expression in human placental syncytiotrophoblasts through the cAMP/Sp1 pathway. Endocrinology 2012, 153, 2012–2022. [Google Scholar] [CrossRef]
- Kashfi, K.; Olson, K.R. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem. Pharmacol. 2013, 85, 689–703. [Google Scholar] [CrossRef]
- Kajimura, M.; Fukuda, R.; Bateman, R.M.; Yamamoto, T.; Suematsu, M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 2009, 13, 157–192. [Google Scholar] [CrossRef]
- Kang, J.; Huo, F.; Ning, P.; Meng, X.; Chao, J.; Yin, C. Two red-emission single and double ‘arms’ fluorescent materials stemed from ‘one-pot’ reaction for hydrogen sulfide vivo imaging. Sens. Actuators B Chem. 2017, 250, 342–350. [Google Scholar] [CrossRef]
- Blackstone, E.; Morrison, M.; Roth Mark, B. H2S induces a suspended animation-like state in mice. Science 2005, 308, 518. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Fan, J.; Hu, C.; Cao, J.; Zhang, H.; Xiong, X.; Wang, J.; Cui, S.; Sun, S.; Peng, X. A two-photon fluorescent probe with near-infrared emission for hydrogen sulfide imaging in biosystems. Chem. Commun. 2013, 49, 3890–3892. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Huo, F.; Yin, C. A novel ratiometric fluorescent H2S probe based on tandem nucleophilic substitution/cyclization reaction and its bioimaging. Dyes Pigm. 2017, 146, 287–292. [Google Scholar] [CrossRef]
- Zhao, Q.; Huo, F.; Kang, J.; Zhang, Y.; Yin, C. A novel FRET-based fluorescent probe for the selective detection of hydrogen sulfide (H2S) and its application for bioimaging. J. Mater. Chem. B 2018, 6, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Kang, J.; Wen, Y.; Huo, F.; Zhang, Y.; Yin, C. “Turn-on” fluorescent probe for detection of H2S and its applications in bioimaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 189, 8–12. [Google Scholar] [CrossRef]
- Luo, W.; Xue, H.; Ma, J.; Wang, L.; Liu, W. Molecular engineering of a colorimetric two-photon fluorescent probe for visualizing H2S level in lysosome and tumor. Anal. Chim. Acta 2019, 1077, 273–280. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Modis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef]
- Li, H.; Fang, Y.; Yan, J.; Ren, X.; Zheng, C.; Wu, B.; Wang, S.; Li, Z.; Hua, H.; Wang, P.; et al. Small-molecule fluorescent probes for H2S detection: Advances and perspectives. Trends Anal. Chem. 2021, 134, 116117. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Xiong, J.; Xia, L.; Huang, Q.; Huang, J.; Gu, Y.; Wang, P. Cyanine-based NIR fluorescent probe for monitoring H2S and imaging in living cells and in vivo. Talanta 2018, 184, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.-Y.; Fan, L.; Shen, H.; Wu, B.; Kong, S.; Luo, Y.; Huang, Z.-S.; Ye, X. A multifunctional BODIPY based fluorescent probe for hydrogen sulfide detection and photodynamic anticancer therapy in HCT116 colon cancer cell. Dyes Pigm. 2022, 197, 109897. [Google Scholar] [CrossRef]
- Yan, L.; Gu, Q.S.; Jiang, W.L.; Tan, M.; Tan, Z.K.; Mao, G.J.; Xu, F.; Li, C.Y. Near-infrared fluorescent probe with large stokes shift for imaging of hydrogen sulfide in tumor-bearing mice. Anal. Chem. 2022, 94, 5514–5520. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Yue, Y.; Guo, Y.; Shao, S. A water-soluble BODIPY derivative as a highly selective “Turn-On” fluorescent sensor for H2O2 sensing in vivo. Biosens. Bioelectron. 2014, 56, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Rhee Sue, G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; Van de Bittner, G.C.; Chang, C.J. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, J.; Han, G.; Liu, Z.; Liu, C.; Zhang, C.; Liu, B.; Jiang, C.; Liu, R.; Zhao, T.; et al. Real-time discrimination and versatile profiling of spontaneous reactive oxygen species in living organisms with a single fluorescent probe. J. Am. Chem. Soc. 2016, 138, 3769–3778. [Google Scholar] [CrossRef]
- Kim, D.-S.; Jeon, S.-E.; Jeong, Y.-M.; Kim, S.-Y.; Kwon, S.-B.; Park, K.-C. Hydrogen peroxide is a mediator of indole-3-acetic acid/horseradish peroxidase-induced apoptosis. FEBS Lett. 2006, 580, 1439–1446. [Google Scholar] [CrossRef]
- Setsukinai, K.-i.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, K.; Gu, Y. A highly selective fluorescent turn-on NIR probe for the bioimaging of hydrogen peroxide in vitro and in vivo. Sens. Actuators B Chem. 2016, 228, 174–179. [Google Scholar] [CrossRef]
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef]
- Jin, H.; Heller, D.A.; Kalbacova, M.; Kim, J.-H.; Zhang, J.; Boghossian, A.A.; Maheshri, N.; Strano, M.S. Detection of single-molecule H2O2 signalling from epidermal growth factor receptor using fluorescent single-walled carbon nanotubes. Nat. Nanotechnol. 2010, 5, 302–309. [Google Scholar] [CrossRef]
- Li, N.; Huang, J.; Wang, Q.; Gu, Y.; Wang, P. A reaction based one- and two-photon fluorescent probe for selective imaging H2O2 in living cells and tissues. Sens. Actuators B Chem. 2018, 254, 411–416. [Google Scholar] [CrossRef]
- Li, X.; Gao, N.; Liu, C.; Yu, M.; Rong, X.; Zhang, Y.; Su, M.; Wang, X.; Zhu, H.; Wang, K.; et al. A melatonin-based targetable fluorescent probe activated by hydrogen peroxide for tumor cells screening. Sens. Actuators B Chem. 2022, 353, 131051. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Wang, C.Q.; Yin, N.; Wang, Y.; Qin, G.; Xu, Q.; Gong, J.; Liu, H.; Duan, X. Target-binding accelerated response for sensitive detection of basal H2O2 in tumor cells and tissues via a dual-functional fluorescence probe. Anal. Chem. 2022, 94, 5962–5969. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, L.; Agrawalla, B.K.; Park, S.-J.; Zhu, H.; Sivaraman, B.; Peng, J.; Xu, Q.-H.; Chang, Y.-T. Development of targetable two-photon fluorescent probes to image hypochlorous acid in mitochondria and lysosome in live cell and inflamed mouse model. J. Am. Chem. Soc. 2015, 137, 5930–5938. [Google Scholar] [CrossRef]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Wang, B.; Cao, X.; Zhu, L.; Hou, J.-T.; Zeng, L. Revealing HOCl burst from endoplasmic reticulum in cisplatin-treated cells via a ratiometric fluorescent probe. Chin. Chem. Lett. 2021, 32, 1795–1798. [Google Scholar] [CrossRef]
- Deng, B.; Ren, M.; Kong, X.; Zhou, K.; Lin, W. Development of an enhanced turn-on fluorescent HOCl probe with a large Stokes shift and its use for imaging HOCl in cells and zebrafish. Sens. Actuators B Chem. 2018, 255, 963–969. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radical Bio. Med. 2000, 29, 403–409. [Google Scholar] [CrossRef]
- Best, Q.A.; Sattenapally, N.; Dyer, D.J.; Scott, C.N.; McCarroll, M.E. pH-Dependent Si-fluorescein hypochlorous acid fluorescent probe: Spirocycle ring-opening and excess hypochlorous acid-induced chlorination. J. Am. Chem. Soc. 2013, 135, 13365–13370. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.W.; Whiteman, M.; Cheung, N.S. Chlorinative stress: An under appreciated mediator of neurodegeneration? Cell. Signal. 2007, 19, 219–228. [Google Scholar] [CrossRef]
- Li, K.; Hou, J.-T.; Yang, J.; Yu, X.-Q. A tumor-specific and mitochondria-targeted fluorescent probe for real-time sensing of hypochlorite in living cells. Chem. Commun. 2017, 53, 5539–5541. [Google Scholar] [CrossRef]
- Gungor, N.; Knaapen, A.M.; Munnia, A.; Peluso, M.; Haenen, G.R.; Chiu, R.K.; Godschalk, R.W.; van Schooten, F.J. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis 2010, 25, 149–154. [Google Scholar] [CrossRef]
- Bauer, G. HOCl and the control of oncogenesis. J. Inorg. Biochem. 2018, 179, 10–23. [Google Scholar] [CrossRef]
- Liu Tracy, W.; Gammon Seth, T.; Yang, P.; Fuentes, D.; Piwnica-Worms, D. Myeloid cell–derived HOCl is a paracrine effector that trans-inhibits IKK/NF-κB in melanoma cells and limits early tumor progression. Sci. Signal. 2021, 14, eaax5971. [Google Scholar]
- Zhu, N.; Guo, X.; Pang, S.; Chang, Y.; Liu, X.; Shi, Z.; Feng, S. Mitochondria-immobilized unimolecular fluorescent probe for multiplexing imaging of living cancer cells. Anal. Chem. 2020, 92, 11103–11110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Z.; Long, S.; Du, J.; Fan, J.; Peng, X. Synthesis of an ultrasensitive BODIPY-derived fluorescent probe for detecting HOCl in live cells. Nat. Protoc. 2018, 13, 2348–2361. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; Xie, P.; Han, X.; Zhang, D.; Ye, Y.; Zhao, Y. Visualization of biothiols and HClO in cancer therapy via a multi-responsive fluorescent probe. Sens. Actuators B Chem. 2021, 347, 130620. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, H.J.; Li, Q.; Ding, Y.; Hu, C.; Wu, Y.C. A specifically triggered turn-on fluorescent probe platform and its visual imaging of HClO in cells, arthritis and tumors. J. Hazard. Mater. 2022, 427, 127874. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ye, M.; Li, B.; Wu, T.; Lu, C.; Liu, P.; Li, H.; Zhou, X.; Wang, Y.; Liang, T.; et al. Bioimaging of hypochlorous acid using a near-infrared fluorescent probe derived from rhodamine dye with a large Stokes shift. Sens. Actuators B Chem. 2022, 364, 131868. [Google Scholar] [CrossRef]
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research applications of proteolytic enzymes in molecular biology. Biomolecules 2013, 3, 923–942. [Google Scholar] [CrossRef]
- Cui, X.; Li, L.; Yan, G.; Meng, K.; Lin, Z.; Nan, Y.; Jin, G.; Li, C. High expression of NQO1 is associated with poor prognosis in serous ovarian carcinoma. BMC Cancer 2015, 15, 244. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Ross, D.; Kepa, J.K.; Winski, S.L.; Beall, H.D.; Anwar, A.; Siegel, D. NAD(P)H:quinone oxidoreductase 1 (NQO1): Chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem. Biol. Interact. 2000, 129, 77–97. [Google Scholar] [CrossRef]
- Antonio, F.S.; Akemi, M.-Y.; Melissa, R.; Byoung, B.S.; Sarah, E.L.; Laurie, J.G.; Takao, Y.; Brunhilde, F.-H. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J. Clin. Investig. 2013, 123, 1068–1081. [Google Scholar]
- Zhang, Q.; Piston David, W.; Goodman Richard, H. Regulation of corepressor function by nuclear NADH. Science 2002, 295, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Lemire, J.; Appanna, V.D. Metabolic networks to combat oxidative stress in Pseudomonas fluorescens. Antonie van Leeuwenhoek 2011, 99, 433–442. [Google Scholar] [CrossRef]
- Mayevsky, A.; Rogatsky, G.G. Mitochondrial function in vivo evaluated by NADH fluorescence: From animal models to human studies. J. Appl. Physiol. Cell Physiol. 2007, 292, C615–C640. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, J.M.; Kurdys, J.G.; Muntean, D.M.; Rosca, M.G. Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxid. Redox Signal. 2017, 30, 375–398. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, H.; Sun, Y.; Liu, J. The recent development of fluorescent probes for the detection of NADH and NADPH in living cells and in vivo. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 245, 118919. [Google Scholar] [CrossRef]
- Podder, A.; Thirumalaivasan, N.; Chao, Y.K.; Kukutla, P.; Wu, S.-P.; Bhuniya, S. Two-photon active fluorescent indicator for detecting NADH dynamics in live cells and tumor tissue. Sens. Actuators B Chem. 2020, 324, 128637. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Kim, B.; Peng, J.; Berry, S.N.; Ni, Y.; Su, D.; Lee, J.; Yuan, L.; Chang, Y.T. Boronic acid: A bio-inspired strategy to increase the sensitivity and selectivity of fluorescent NADH probe. J. Am. Chem. Soc. 2016, 138, 10394–10397. [Google Scholar] [CrossRef]
- Fomin, M.A.; Dmitriev, R.I.; Jenkins, J.; Papkovsky, D.B.; Heindl, D.; König, B. Two-acceptor cyanine-based fluorescent indicator for NAD(P)H in tumor cell models. ACS Sens. 2016, 1, 702–709. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, W.L.; Wang, W.X.; Mao, G.J.; Li, Y.; Li, C.Y. NAD(P)H-triggered probe for dual-modal imaging during energy metabolism and novel strategy of enhanced photothermal therapy in tumor. Biomaterials 2021, 271, 120736. [Google Scholar] [CrossRef]
- Wei, H.; Yu, Y.; Wu, G.; Wang, Y.; Duan, S.; Han, J.; Cheng, W.; Li, C.; Tian, X.; Zhang, X. Dual-responsive fluorescent probe for imaging NAD(P)H and mitochondrial viscosity and its application in cancer cell ferroptosis. Sens. Actuators B Chem. 2022, 350, 130862. [Google Scholar] [CrossRef]
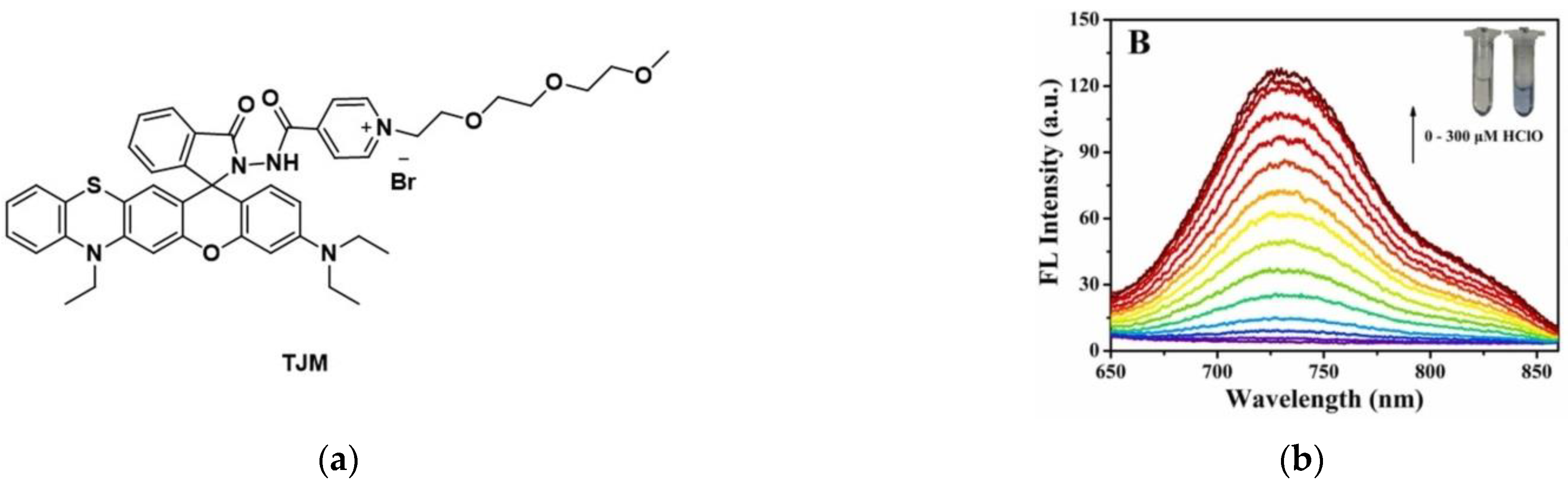
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Yin, C.; Huo, F. Small-Molecule Fluorescent Probes for Detecting Several Abnormally Expressed Substances in Tumors. Micromachines 2022, 13, 1328. https://doi.org/10.3390/mi13081328
Yao L, Yin C, Huo F. Small-Molecule Fluorescent Probes for Detecting Several Abnormally Expressed Substances in Tumors. Micromachines. 2022; 13(8):1328. https://doi.org/10.3390/mi13081328
Chicago/Turabian StyleYao, Leilei, Caixia Yin, and Fangjun Huo. 2022. "Small-Molecule Fluorescent Probes for Detecting Several Abnormally Expressed Substances in Tumors" Micromachines 13, no. 8: 1328. https://doi.org/10.3390/mi13081328
APA StyleYao, L., Yin, C., & Huo, F. (2022). Small-Molecule Fluorescent Probes for Detecting Several Abnormally Expressed Substances in Tumors. Micromachines, 13(8), 1328. https://doi.org/10.3390/mi13081328





