Blood Flow of Au-Nanofluid Using Sisko Model in Stenotic Artery with Porous Walls and Viscous Dissipation Effect
Abstract
:1. Introduction
2. Mathematical Formulation
3. Numerical Method
4. Results and Discussion
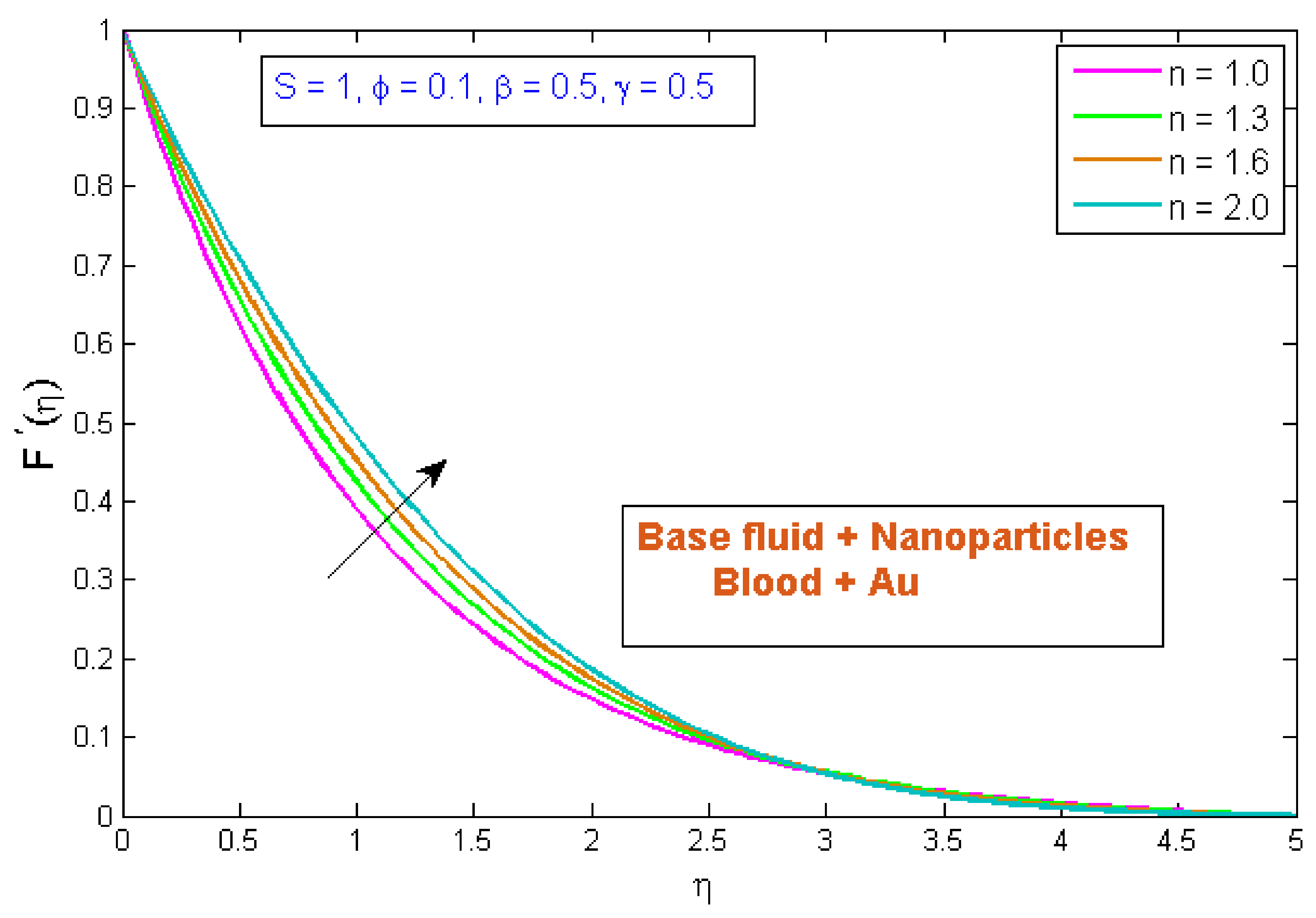
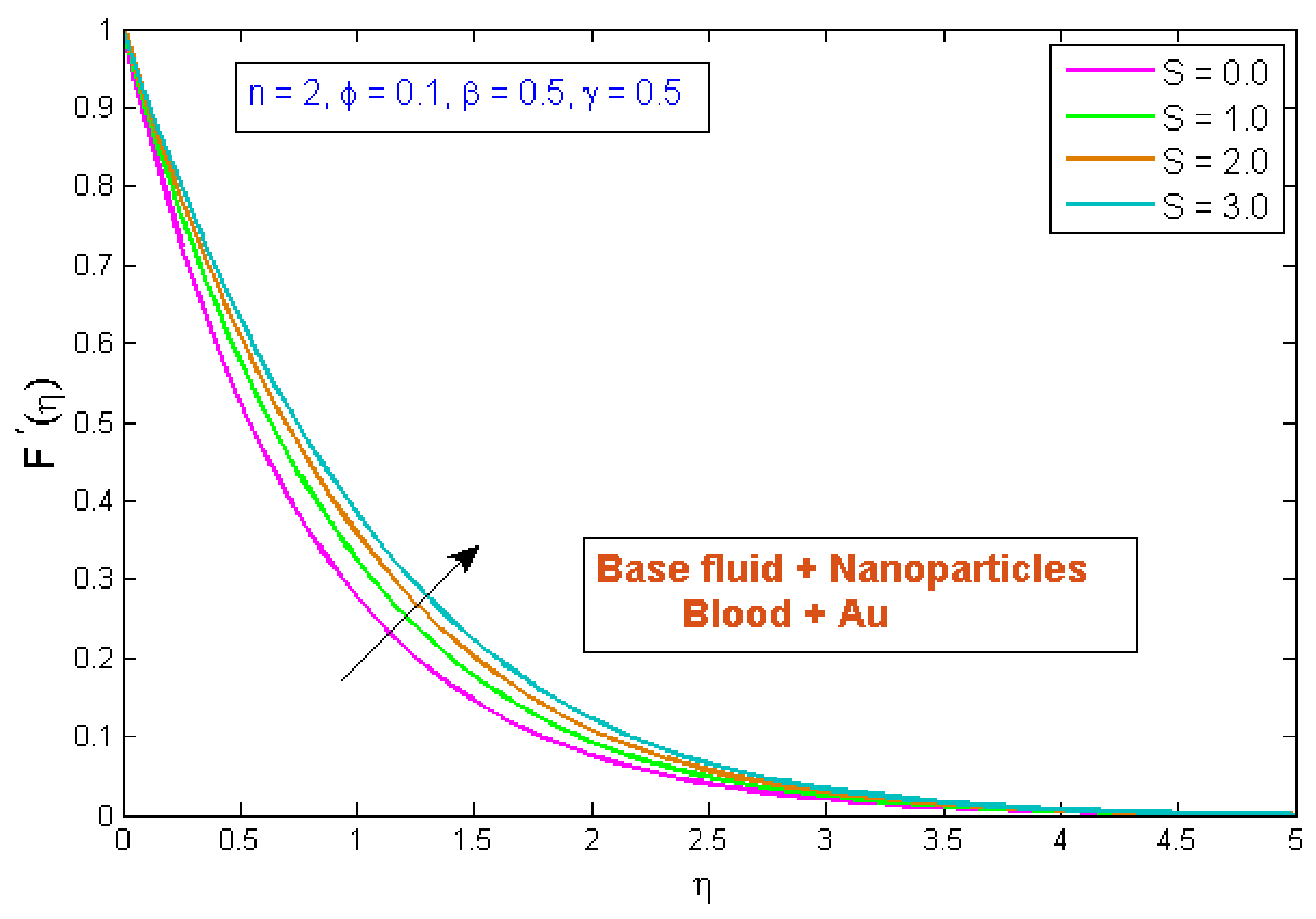
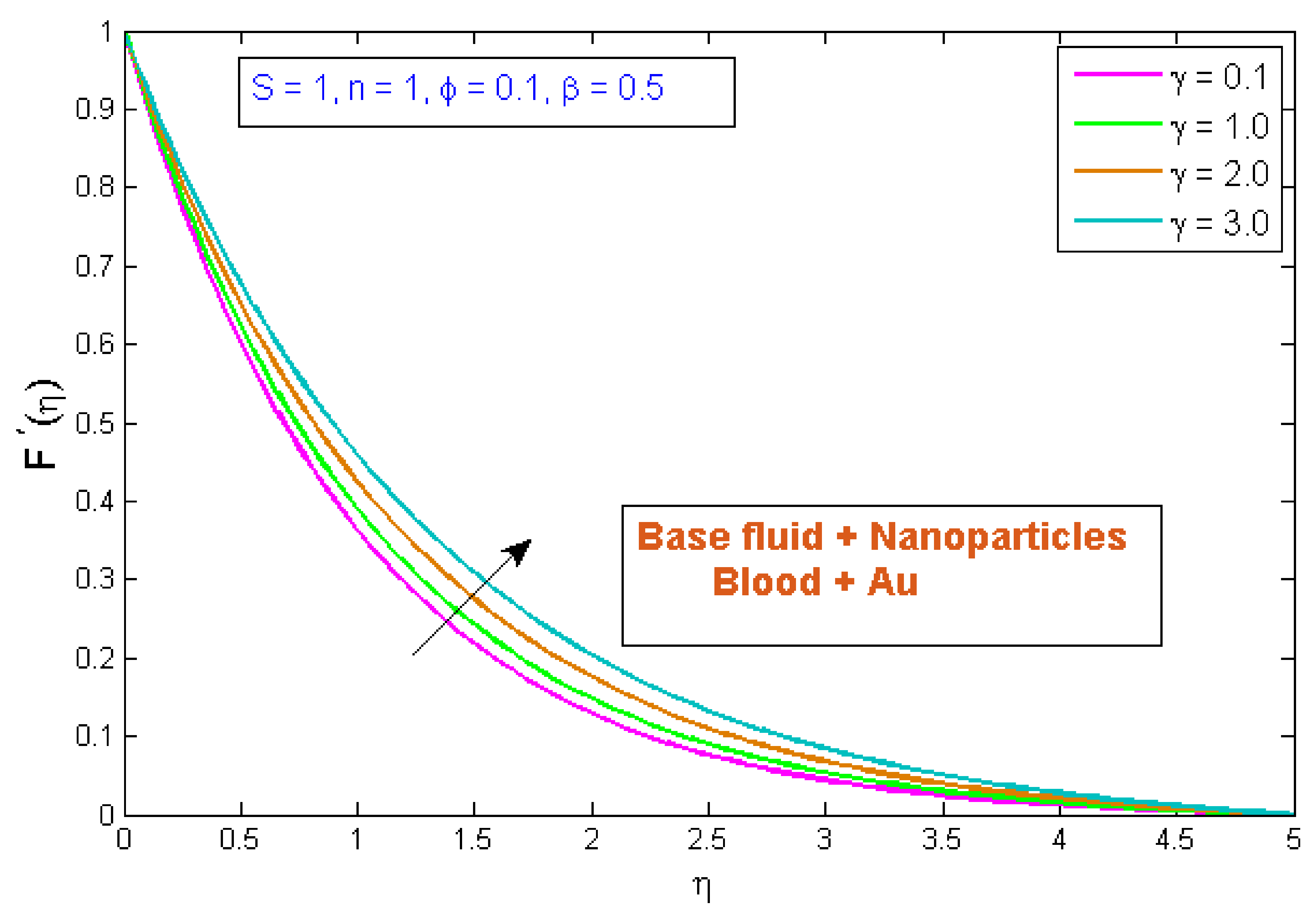
4.1. Velocity Profile
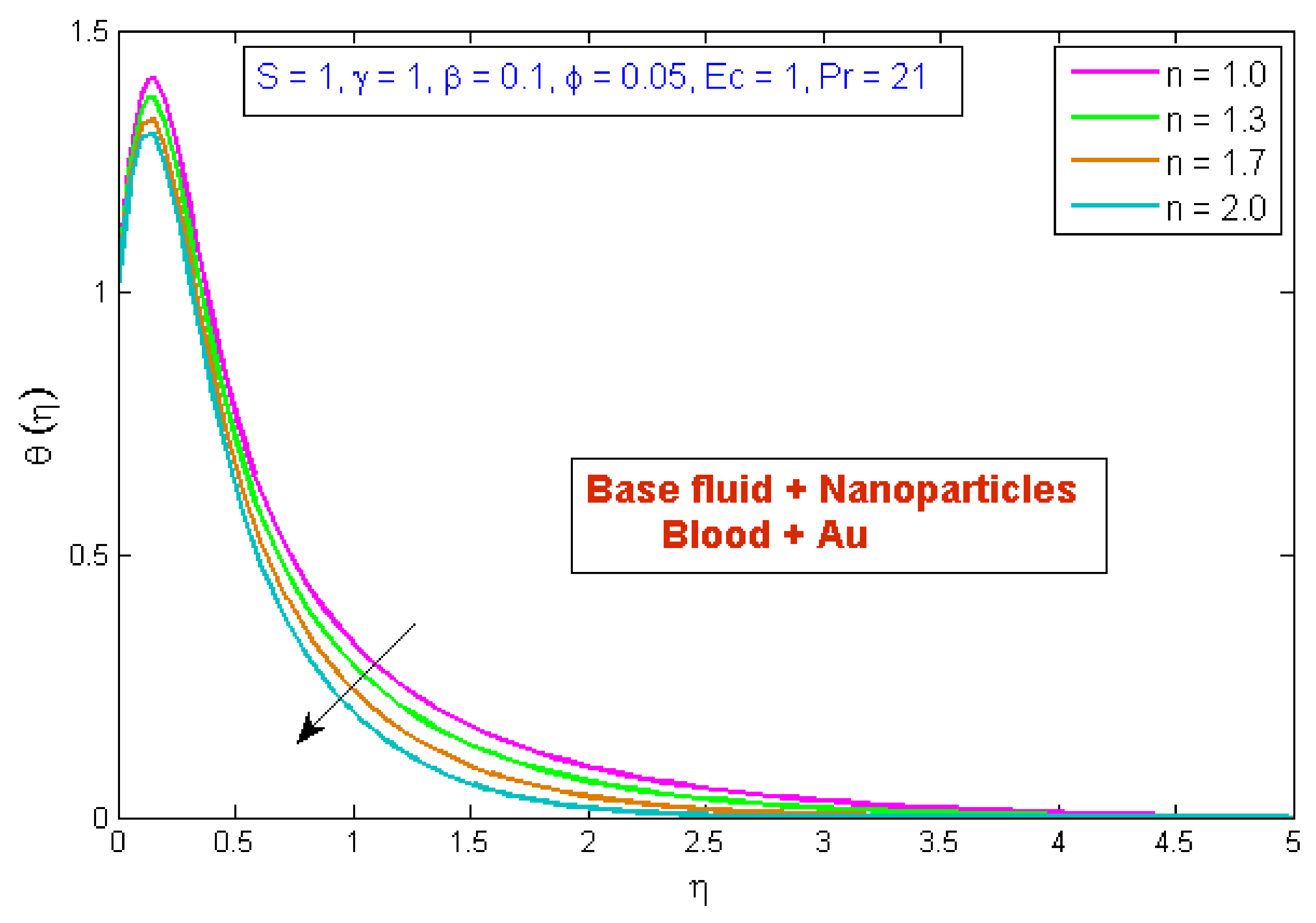
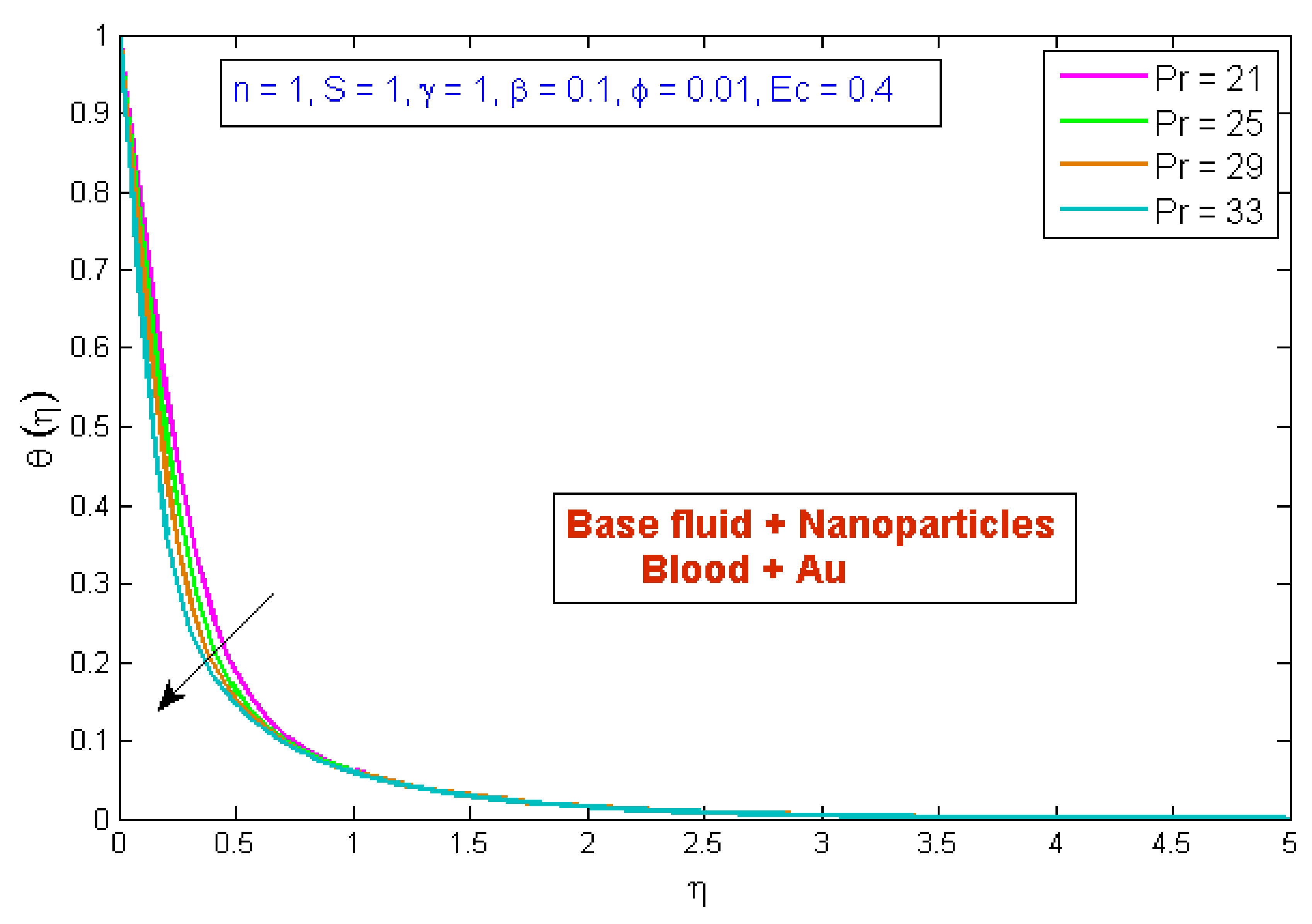
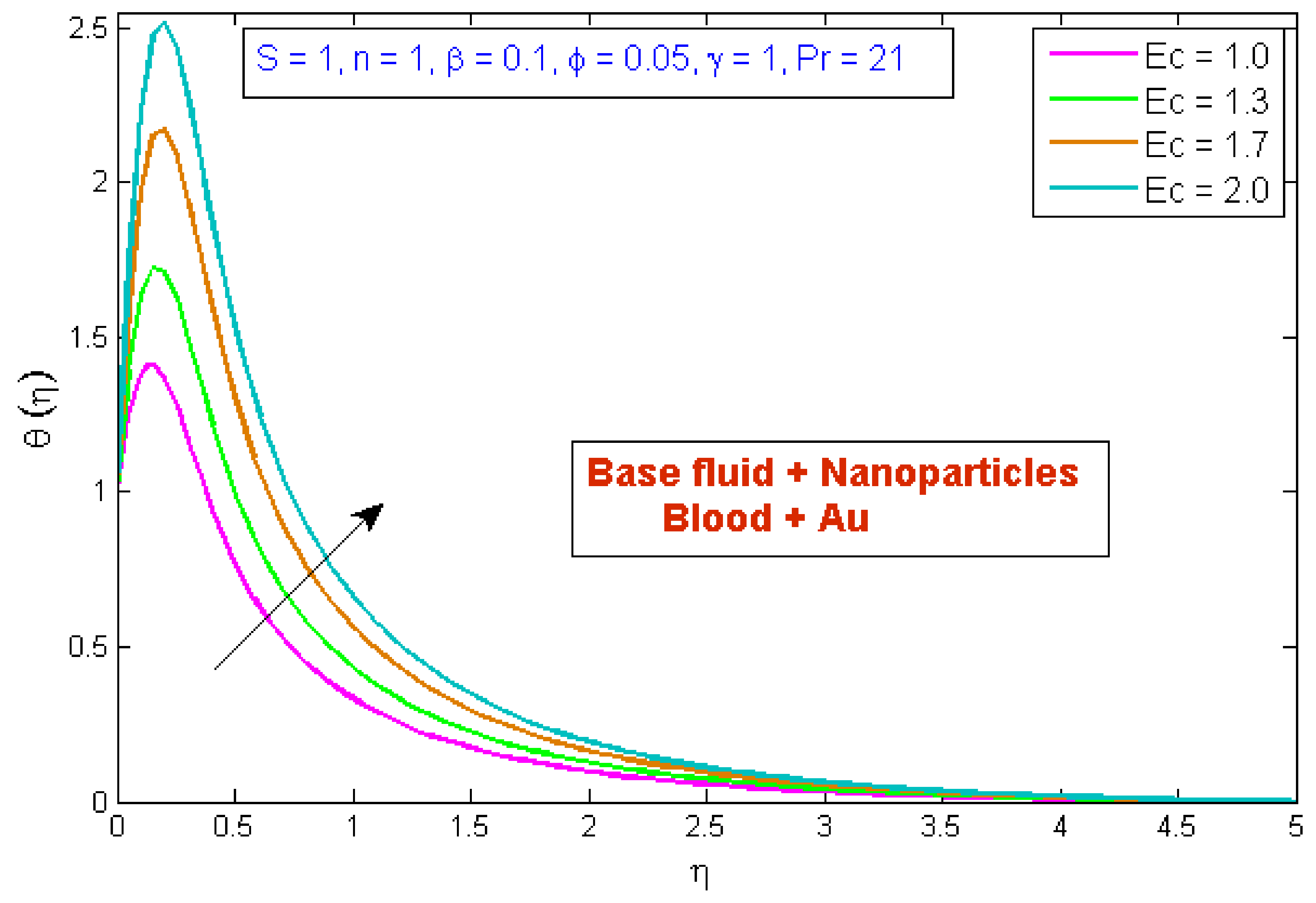
4.2. Temperature Profile
5. Physical Quantities
6. Conclusions
- When the volume fraction, fluid parameter, power law index, and curvature parameter were increased, the velocity profile decreased.
- When the porosity parameter was augmented, the velocity profile was diminished.
- The temperature profile was reduced as the volume fraction, power law index, and Prandtl number values were increased.
- The temperature profile was boosted as the curvature parameter and the Eckert number upsurged.
- The skin friction coefficient declined as the volume friction, Sisko fluid parameter, and porosity parameter increased, while the skin friction coefficient rose as the curvature parameter was enhanced.
- The Nusselt number grew when large value increases were attained in the volume fraction. Instead, as the values of the physical parameters and were boosted, the local Nusselt number declined.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Specific heat transfer | |
| Eckert number | |
| Thermal conduction | |
| Porousness medium | |
| Power law index | |
| Prandtl number | |
| Dimensionless Fluid parameter | |
| Temperature | |
| Components of velocity | |
| Coordinates axis | |
| Greek Letter | |
| Fluid parameter | |
| Porosity parameter | |
| Curvature parameter | |
| volume fraction parameter | |
| Independent coordinate | |
| Dynamic viscosity | |
| Kinematic viscosity | |
| Density | |
| Heat capacitance | |
| Subscripts | |
| Base fluid | |
| Solid nanoparticle | |
| Nanoparticle | |
| Wall | |
| Free-Stream | |
References
- Ellahi, R.; Rahman, S.U.; Nadeem, S.; Vafai, K. The blood flow of prandtl fluid through a tapered stenosed arteries in permeable walls with magnetic field. Commun. Theor. Phys. 2015, 63, 353–358. [Google Scholar] [CrossRef]
- Ardahaie, S.S.; Amiri, A.J.; Amouei, A.; Hosseinzadeh, K.; Ganji, D.D. Investigating the effect of adding nanoparticles to the blood flow in presence of magnetic field in a porous blood arterial. Inform. Med. Unlocked 2018, 10, 71–81. [Google Scholar] [CrossRef]
- Haghighi, A.R.; Asl, M.S. Mathematical modeling of micropolar fluid flow through an overlapping arterial stenosis. Int. J. Biomath. 2015, 8, 1550056. [Google Scholar] [CrossRef]
- Kanai, H.; Iizuka, M.; Sakamoto, K. One of the problems in the measurement of blood pressure by catheter-insertion: Wave reflection at the tip of the catheter. Med. Biol. Eng. 1970, 8, 483–496. [Google Scholar] [CrossRef]
- Leimgruber, P.P.; Roubin, G.S.; Anderson, H.V.; Bredlau, C.E.; Whitworth, H.B.; Douglas, J.S., Jr.; King, S.B., III; Greuntzig, A.R. Influence of intimal dissection on restenosis after successful coronary angioplasty. Circulation 1985, 72, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Back, L.H.; Kwack, E.Y.; Back, M.R. Flow rate-pressure drop relation in coronary angioplasty: Catheter obstruction effect. J. Biomech. Eng. 1996, 118, 83–89. [Google Scholar] [CrossRef]
- Back, L.H. Estimated mean flow resistance increase during coronary artery catheterization. J. Biomech. 1994, 27, 169–175. [Google Scholar] [CrossRef]
- Sarkar, A.; Jayaraman, G. Correction to flow rate—Pressure drop relation in coronary angioplasty: Steady streaming effect. J. Biomech. 1998, 31, 781–791. [Google Scholar] [CrossRef]
- Dash, R.K.; Jayaraman, G.; Mehta, K.N. Flow in a catheterized curved artery with stenosis. J. Biomech. 1999, 32, 49–61. [Google Scholar] [CrossRef]
- Sarwar, L.; Hussain, A. Flow characteristics of Au-blood nanofluid in stenotic artery. Int. Commun. Heat Mass Transf. 2021, 127, 105486. [Google Scholar] [CrossRef]
- Sisko, A.W. The flow of lubricating greases. Ind. Eng. Chem. 2002, 5, 1789–1792. [Google Scholar] [CrossRef]
- Khan, M.; Munawar, S.; Abbasbandy, S. Steady flow and heat transfer of a sisko fluid in annular pipe. Int. J. Heat Mass Transf. 2010, 53, 1290–1297. [Google Scholar] [CrossRef]
- Nadeem, S.; Akbar, N.S. Peristaltic flow of sisko fluid in a uniform inclined tube. Acta Mech. Sin. 2010, 26, 675–683. [Google Scholar] [CrossRef]
- Munir, A.; Shahzad, A.; Khan, M. Mixed convection heat transfer in sisko fluid with viscous dissipation: Effects of assisting and opposing buoyancy. Chem. Eng. Res. Des. 2015, 97, 120–127. [Google Scholar] [CrossRef]
- Hayat, T.; Moitsheki, R.J.; Abelman, S. Stokes’ first problem for sisko fluid over a porous wall. Appl. Math. Comput. 2010, 217, 622–628. [Google Scholar] [CrossRef]
- Sari, G.; Pakdemirli, M.; Hayat, T.; Aksoy, Y. Boundary layer equations and lie group analysis of a sisko fluid. J. Appl. Math. 2012, 2012, 259608. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995. [Google Scholar]
- Hatami, M.; Hatami, J.; Ganji, D.D. Computer simulation of MHD blood conveying gold nanoparticles as a third grade non-Newtonian nanofluid in a hollow porous vessel. Comput. Methods Programs Biomed. 2014, 113, 632–641. [Google Scholar] [CrossRef]
- Akbari, N.; Ganji, D.D.; Gholinia, M.; Gholinia, S. Computer simulation of blood flow with nanoparticles in a magnetic field as a third grade non-Newtonian through porous vessels by flex PDE software. Innov. Energy Res. 2017, 6, 1000154. [Google Scholar] [CrossRef]
- Srinivas, S.; Vijayalakshmi, A.; Subramanyam Reddy, A. Flow and heat transfer of gold-blood nanofluid in a porous channel with moving/stationary walls. J. Mech. 2016, 33, 395–404. [Google Scholar] [CrossRef]
- Hady, F.M.; Ibrahim, F.S.; Abdel-Gaied, S.M.; Eid, M.R. Radiation effect on viscous flow of a nanofluid and heat transfer over a nonlinearly stretching sheet. Nanoscale Res. Lett. 2012, 7, 229. [Google Scholar] [CrossRef]
- Buongiorno, J. Convective transport in nanofluids. J. Heat Transf. 2006, 128, 240–250. [Google Scholar] [CrossRef]
- Hady, F.; Eid, M.R.; Abdelhafez, M. A nanofluid flow in a non-linear stretching surface saturated in a porous medium with yield stress effect. Appl. Math. Inf. Sci. Lett. 2014, 2, 43–51. [Google Scholar] [CrossRef]
- Rehman, K.U.; Shatanawi, W.; Malik, M.Y. Heat transfer and double sampling of stratification phenomena in non-Newtonian liquid suspension: A comparative thermal analysis. Case Stud. Therm. Eng. 2022, 33, 101934. [Google Scholar] [CrossRef]
- Rehman, K.U.; Shatanawi, W.; Ashraf, S.; Kousar, N. Numerical analysis of Newtonian heating convective flow by way of two different surfaces. Appl. Sci. 2022, 12, 2383. [Google Scholar] [CrossRef]
- Rehman, K.U.; Shatanawi, W.; Abodayeh, K.; Shatanawi, T.A.M. A group theoretic analysis of mutual interactions of heat and mass transfer in a thermally slip semi-infinite domain. Appl. Sci. 2022, 12, 2000. [Google Scholar] [CrossRef]
- Dawar, A.; Islam, S.; Tassaddiq, A.; Shah, Z.; Deebani, W.; Rashid, A. Mixed convective flow of blood biofluids containing magnetite ferroparticles past a vertical flat plate: Shapes-based analysis. Waves Random Complex Media 2022, 1–25. [Google Scholar] [CrossRef]
- Alsagri, A.S.; Nasir, S.; Gul, T.; Islam, S.; Nisar, K.S.; Shah, Z.; Khan, I. MHD thin film flow and thermal analysis of blood with CNTs nanofluid. Coatings 2019, 9, 175. [Google Scholar] [CrossRef]
- Awais, M.; Malik, M.Y.; Bilal, S.; Salahuddin, T.; Hussain, A. Magnetohydrodynamic (MHD) flow of sisko fluid near the axisymmetric stagnation point towards a stretching cylinder. Results Phys. 2017, 7, 49–56. [Google Scholar] [CrossRef]





| Material | Symbol | |||
|---|---|---|---|---|
| Blood | -- | 1050 | 3617 | 0.52 |
| Gold | 19,300 | 129 | 318 |
| 0.01 | 0.1 | 0.1 | 0.1 | −1.053197 |
| 0.05 | −1.094823 | |||
| 0.1 | −1.155339 | |||
| 0.01 | 0.1 | −1.053197 | ||
| 0.3 | −1.16986 | |||
| 0.5 | −1.278185 | |||
| 0.1 | 0.1 | −1.053197 | ||
| 0.2 | −0.9924828 | |||
| 0.3 | −0.9448766 | |||
| 0.1 | 0.1 | −1.053197 | ||
| 0.3 | −1.138746 | |||
| 0.5 | −1.218283 |
| 0.01 | 0.1 | 0.1 | 3.928399 |
| 0.05 | 7.487721 | ||
| 0.1 | 10.59055 | ||
| 0.01 | 0.1 | 3.928399 | |
| 0.2 | 3.744353 | ||
| 0.3 | 3.573028 | ||
| 0.1 | 0.1 | 3.928399 | |
| 0.2 | 3.183704 | ||
| 0.3 | 2.43899 |
| Present Paper | L. Sarwar and A. Hussain [10] | ||
|---|---|---|---|
| 0.1 | 0.0 | −0.93947 | −0.939968 |
| 0.12 | −0.9295648 | −0.924794 | |
| 0.14 | −0.9180573 | −0.911311 | |
| 0.1 | 0.0 | −0.93947 | −0.939968 |
| 0.05 | −1.323752 | −1.329552 | |
| 0.1 | −1.714007 | −1.715985 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.-Q.; Rooman, M.; Vrinceanu, N.; Shah, Z.; Alshehri, A. Blood Flow of Au-Nanofluid Using Sisko Model in Stenotic Artery with Porous Walls and Viscous Dissipation Effect. Micromachines 2022, 13, 1303. https://doi.org/10.3390/mi13081303
Tang T-Q, Rooman M, Vrinceanu N, Shah Z, Alshehri A. Blood Flow of Au-Nanofluid Using Sisko Model in Stenotic Artery with Porous Walls and Viscous Dissipation Effect. Micromachines. 2022; 13(8):1303. https://doi.org/10.3390/mi13081303
Chicago/Turabian StyleTang, Tao-Qian, Muhammad Rooman, Narcisa Vrinceanu, Zahir Shah, and Ahmed Alshehri. 2022. "Blood Flow of Au-Nanofluid Using Sisko Model in Stenotic Artery with Porous Walls and Viscous Dissipation Effect" Micromachines 13, no. 8: 1303. https://doi.org/10.3390/mi13081303
APA StyleTang, T.-Q., Rooman, M., Vrinceanu, N., Shah, Z., & Alshehri, A. (2022). Blood Flow of Au-Nanofluid Using Sisko Model in Stenotic Artery with Porous Walls and Viscous Dissipation Effect. Micromachines, 13(8), 1303. https://doi.org/10.3390/mi13081303








