1. Introduction
Oxidative stress is encountered in human bodies whenever the production of reactive oxygen species (ROS) is high [
1]. Normally, they are promptly detoxified by antioxidant enzymes as the body is equipped with an efficient antioxidant system [
2]. Nearly one-fourth of oxygen we inhale is converted into ROS [
3]. Despite the heavy burden of the ROS, humans have further worsened the situation by depending more on synthetic/processed foods which further give rise to other free radicals for which the body does not have sufficient antioxidant enzymes to detoxify [
4]. This condition leads to a number of health complications, such as: diabetes; neurodegenerative diseases, such as Alzheimer’s; cardiovascular diseases; and even cancer [
5]. In Alzheimer’s disease, along with other complications, there is a deficit in acetylcholine for which the cholinesterase inhibitors are used as an antagonist [
6]. Oxidative stress is the key factor in aging, diabetes, Alzheimer’s, and dementia, etc. [
7]. There is a dire need to scavenge the mediators of stress. Related to this, efforts are being made from almost all research angles to lessen the complications of the mentioned diseases on human [
8]. Nanotechnology is the emerging research field, the science of reducing the material particle sizes to nanometer scale range (below 100 nm), that has shown applications in a range of different fields of science, and especially in the medical sciences [
9]. A number of approaches are being used in fabricating the nanoparticles, broadly categorized into chemical, biological, and physical approaches [
10]. The nanoparticles have also been reported to have other areas of biological potential, such as antimicrobial, anti-inflammatory, larvicidal, etc. [
11].
Several methods have been reported for the synthesis of nanoparticles as mentioned above [
10]. The same element nanoparticles have different sizes if produced by two or more than two different methods because the particles precipitated quickly have smaller sizes rather than in a method where they are precipitated slowly [
12]. The traditional NPs fabrication protocols, such as hydrothermal, sol-gel, and sonochemical, have disadvantages, such as high manufacturing costs, low production rate, and a high energy need [
13]. Chemical approaches (e.g., precipitation, hydrothermal, sonochemical, etc.) include the use of noxious chemicals, the generation of perilous side-products, and pollution from noxious substances [
14]. As a result, there is a need to develop NPs with greener routes, involving plants/microbes waste biomass as a reductant, that are clean, nontoxic, and ecologically friendly [
15]. Some of the major benefits include the fact that they may be created in short time, whereas micro-organism-assisted techniques require longer time due to microbial culture and growth [
16].
The biologically inspired methodologies in fabricating the NPs have shown enhanced structural/morphological and biological properties [
17]. Several natural materials have been employed as reducing/capping agents in the fabrication of NPs, namely plant/bacterial extracts, and biomaterials [
18]. The biologically generated FeNPs have shown diverse applications in different fields, including sensors, chemical reaction catalysts, bio-labeling, photo catalysts, and as antimicrobial agents [
19]. Several researchers have used micro-organisms, most notably
Fusarium oxyporum, as a bio-reductant for the synthesis of FeNPs [
20], believing that the growth of micro-organisms is high in comparison to plants [
21]. The biomass of
F. oxyporum and the extracted constituents from this fungus have converted silver ions to stable AgNPs [
22]. Moreover, the biomass of bacteria and fungus, or the extracted bio-compounds from these microbes, have also been employed for the synthesis of FeNPs [
23].
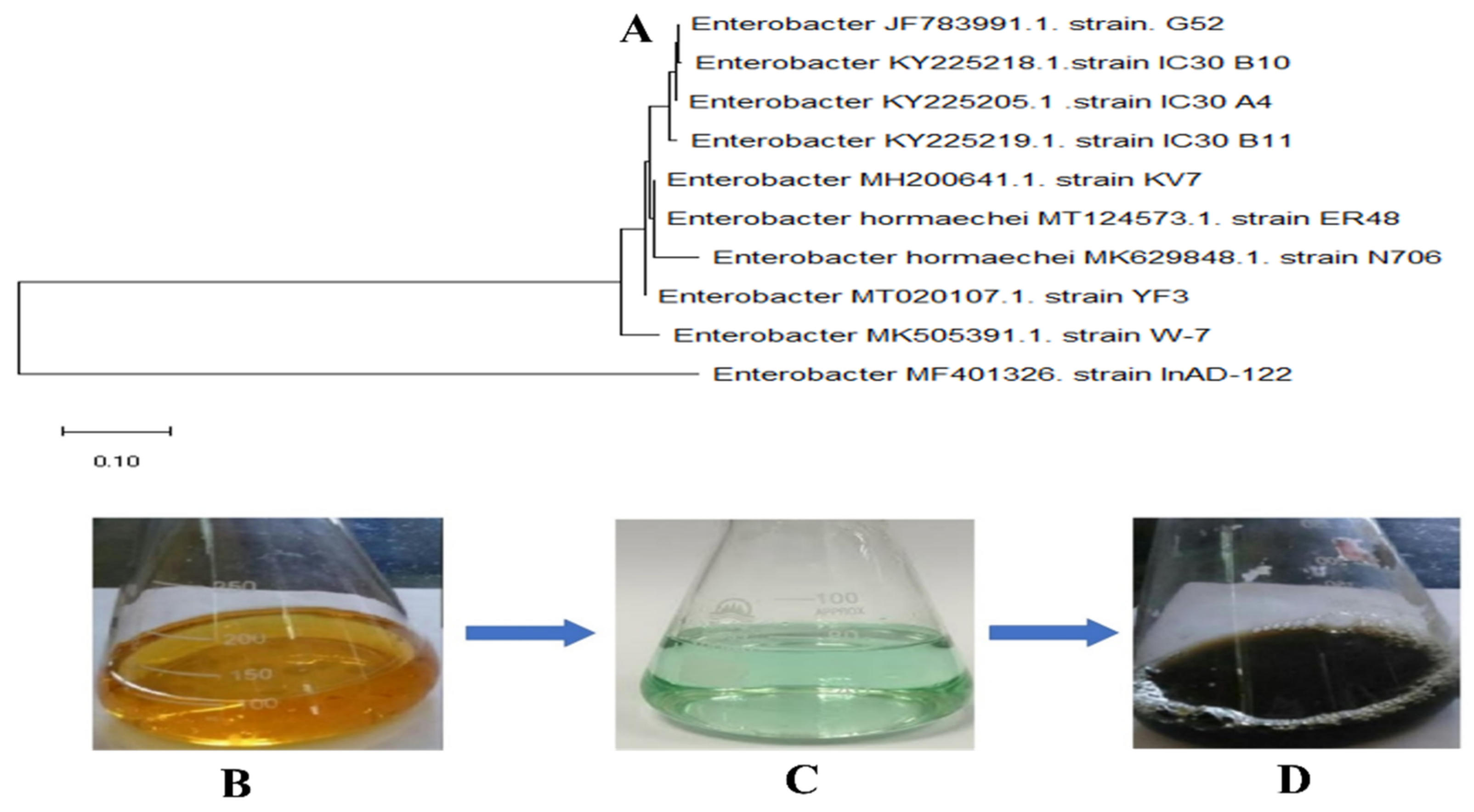
To the best of our knowledge, Enterobacter strain G52 biomass has not been used in the fabrication of iron nanoparticles. The aim of the present study was to synthesize FeNPs using Enterobacter strain G52 biomass as a reductant. The synthesized FeNPs were characterized by UV-spectroscopy, XRD, SEM, TGA, EDX, and FTIR techniques to visualize their morphology, involvement of bioactive compounds, stability, crystallinity of the particles, etc. Furthermore, antibacterial, antifungal, antioxidant, anti-inflammatory, anti-leishmanial, anti-Alzheimer’s, anti-larvicidal, protein kinase, anti-diabetic, and biocompatibility were also evaluated following standard protocols.
4. Discussion
Because of its simplicity of use and cost efficiency, as well as its potential for large-scale manufacturing, the NPs synthesis approach has piqued the scientific community’s attention in recent years. We used a biosynthesis approach to make FeNPs from
Enterobacter strain G52, and we tested the NPs against urinary tract infection (UTI) isolates. Furthermore, there is no evidence in the literature to support the anti-Alzheimer’s effects of bacterial biomass produced FeNPs. In the first steps, the FeNPs were explored for their morphological features employing diverse analytical tools, including UV-spectroscopy, FTIR, TGA, XRD, SEM, and EDX. According to UV-spectroscopy, the sample absorbed energy at 284 nm, which is a sign for typical peak value for FeNPs. The results were validated by X-ray spectroscopy [
25]. Aside from that, an absorption peak at 284 nm with no other peak demonstrated the NPs exceptional purity. Many investigations revealed a significant absorption peak of FeNPs below 374 nm wavelengths, related to the sample red shift at 500 °C and 700 °C [
39].
The vibrations of alkanes, phenol, alcohols, aromatics, alkenes, alkyl-halides, and aliphatic amines were revealed by FTIR analysis of iron nanoparticles which are in accordance with previous results [
40]. Furthermore, –C=O–, C–O–C, and C–O stretching vibrations were shown to generate maxima in carboxylic acid, polysaccharide, and amino acid, respectively [
41]. The produced FeNPs has crystalline sizes in the range of 23 nm, as estimated by Nano-measurer and ImageJ analysis, as confirmed by SEM micrographs. The size of NPs was larger in this work than in [
42], which might be attributed to changes in synthesis settings, such as temperature, incubation period, bacterial extract type, and handling applications.
Furthermore, an EDX analysis showed pure FeNPs phases and a strong peak in the EDX spectrum, showing that the test sample contained pure iron. The EDX spectra of FeNPs were obtained using a simple precipitation process using iron as the starting material. Pure FeNPs with substantial peaks have been successfully synthesized, according to the EDX spectrum. However, additional peaks in the spectrum were detected, suggesting that bacterial biomolecules were involved in nanoparticle synthesis. Throughout, in correlation with other reports, we found the same EDX pattern of FeNPs with great purity [
43]. The XRD profile was employed to assess the size and crystallinity of the bio-fabricated FeNPs. The XRD spectrum demonstrated the planar alignment and crystalline structure of FeNPs as 56.44, 53.49, 45.45, 38.98, 36.59, 31.71, and 20.75 degrees at 2Theta. Numerous XRD reflection planes indicate that the centered cubic structure, as attested by JCPDS Card No. 36–1451. According to Scherer’s equation, the average crystal size is 20.7 nm [
44].
After thorough morphological and chemical analysis, the produced NPs were tested for a range of biological applications against human pathogenic microbes and several enzymes which are involved in the prognosis of several fatal diseases. UTIs are one of the most prevalent bacterial illnesses, affecting around 150 million individuals worldwide each year. Even though both men and women are susceptible to UTIs, women are more likely to become infected, with up to half of all women being ill at some time in their life. Due to the increasing prevalence of multidrug-resistant (MDR), which render antibiotic therapy for acute infection ineffective [
45], current therapeutic options are inadequate. In our experiments, FeNPs showed remarkable bactericidal action against UTI isolates, such as
P. stuarti, P. aeruginosa, S. aureus, and
E. coli. As a result of our findings, we may assume that biosynthesized FeNPs have significant bactericidal action in their natural state and that coating pharmaceuticals can boost their effectiveness against MDR bacteria. Our results are incongruent with earlier reports [
34]. The antifungal efficacy of bacterial biomass mediated NPs was also studied, with the maximum zones of inhibition (ZOI) recorded against
A. niger, F. solani, and
A. flavus (12 ± 2.1 mm, 15.4 ± 0.3 mm, and 13.5 ± 0.13 mm), respectively.
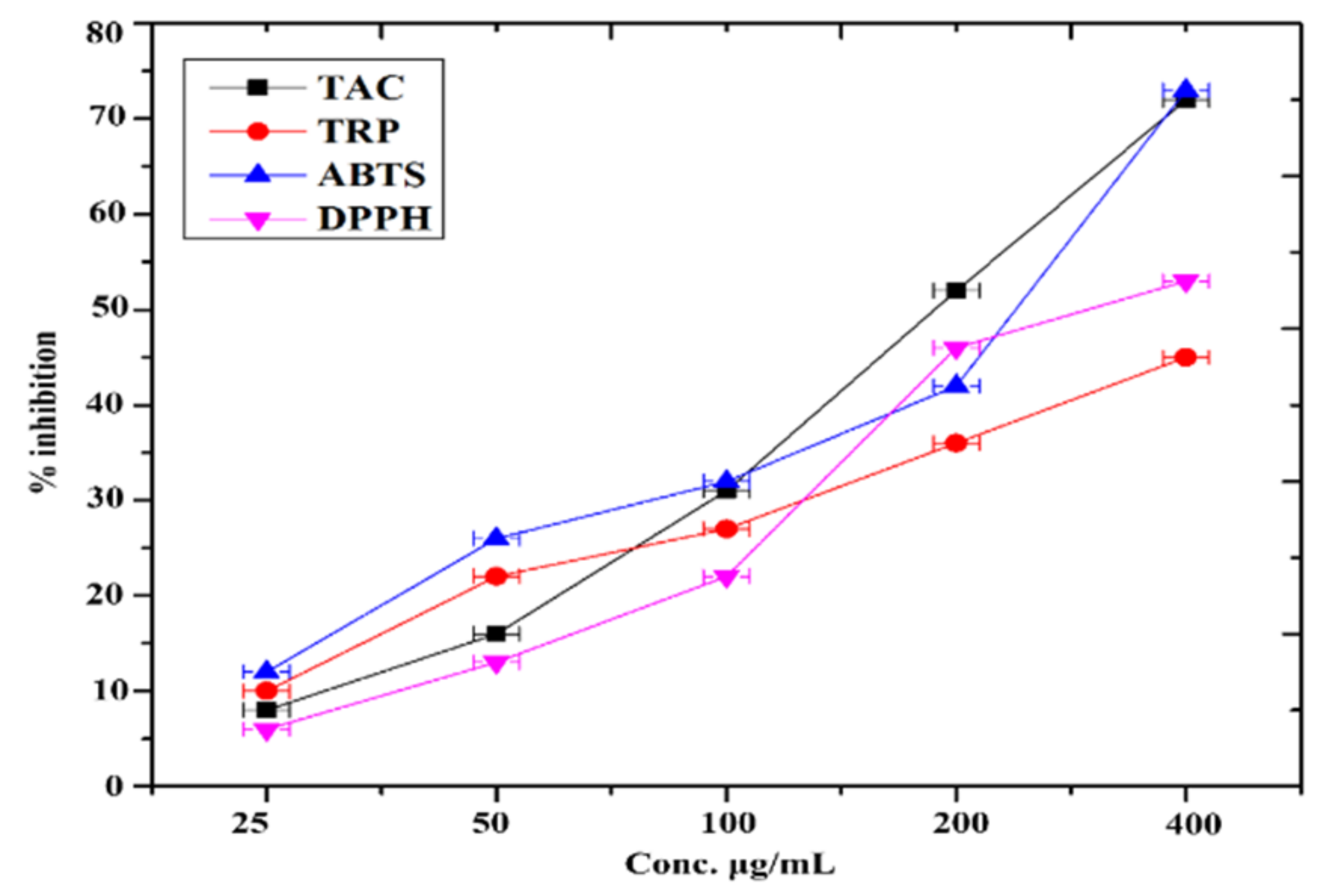
We also checked the inhibition of COX-1, COX-2, sPLA2, and 15-LOX by in vitro to confirm the anti-inflammatory action of the FeNPs. Tested NPs showed moderate inhibition potential against sPLA2 (49.5 ± 0.6), 15-LOX (62.3 ± 0.5); COX-1 (36.2 ± 0.4) and COX-2 (49.6 ± 0.3) had the best anti-inflammatory efficacy among all samples. Similar results were reported by [
41]. A variety of concentrations of synthesized NPs (25, 50, 100, 150, and 200 ppm) were evaluated against
Aedes aegypti second and fourth instars in this work. At 200 ppm and 25 ppm percent mortality of 45.2 ± 0.3% and 12.6 ± 0.4% were found, respectively, in accordance with previous results [
46].
Leishmaniasis is a severe worldwide health burden, according to the World Health Organization (WHO), with a wide range of clinical symptoms and possibly deadly consequences. According to the study, 1.5 to 2 million cases occur yearly, placing 350 million individuals at risk [
47]. We have tested our synthesized products against both forms of leishmania parasite to provide a framework for proper NPs based anti-leishmanial remedies. At 400 μg/mL, the NPs exposed to promastigote and amastigote parasite types had significant death rates of 62.4 ± 1.19% and 57.5 ± 1.09%, respectively. In accordance with these results, we can believe that FeNPs might be used as a future treatment for cutaneous leishmaniasis. Cholinesterase inhibitors are one of the most effective Alzheimer’s disease (AD) treatments now available, and they may be taken at any stage of the disease. Several synthesized and natural substances were shown to inhibit cholinesterase enzymes efficiently [
44]. The FeNPs were plenty efficient at 1000 μg/mL against AChE (43.41 ± 0.32) and BChE (57.57 ± 0.63).
Protein kinase are enzymes that aid cell interaction and progression through the cell cycle by allowing cells to communicate across the nuclear membrane. Tyrosine and protein kinases phosphorylate serine-threonine residues, which is significant in cancer therapy. These residues function in metabolism, cell apoptosis, and cellular proliferation and differentiation regulation and control. Out of control phosphorylation may induce and encourage genomic alterations that lead to cancer [
48]. A dose-dependent inhibitory effect for NPs formulations was discovered. At 5 mg/mL, the largest ZOI was found to be 12.52 ± 0.31, while the lowest ZOI was found to be 2.24 ± 0.28 at 0.5 mg/mL. A similar approach was shown in a previous study [
49]. Diabetes Mellitus (DM) is a term applied to explain a group of metabolic illnesses characterized by persistent hyperglycemia [
50]. When insulin comes into contact with body cells, its synthesis is minimal or inactive, leading to a malfunction. One of the essential tactics for treating diabetes is to reduce postprandial hyperglycemia, which may be performed by blocking the two most essential carbohydrate hydrolyzing enzymes in the digestive tract, α-amylase and α-glucosidase [
51]. The effective inhibition of α-amylase and α-glucosidase was investigated at diverse concentration of FeNPs ranging from 400 μg/mL to 25 μg/mL. The inhibitory efficacy of α-amylase and α-glucosidase was observed to be relatively high. At 400 μg/mL, maximum α-amylase inhibition was determined to be 51.32 ± 0.39 and 58.37 ± 0.68 for α-glucosidase, respectively, as previously reported by [
52].
Biocompatibility is one of the most crucial aspects of the therapeutic usability of nano-systems. The degree of biocompatibility is determined by physico-chemical properties and the environment to which NPs are exposed [
53]. We tested the FeNPs in vitro hemocompatibility because of this importance. Even at the maximal concentration of 400 μg/mL, the NPs exhibited excellent biocompatibility against collected hRBCs. Our findings show that FeNPs generated by
Enterobacter strain G52 are very biocompatible and may be employed in diverse biological applications. Our results revealed that FeNPs mediated by the
Enterobacter strain G52 are stable in vitro and might be used for practical medicinal applications.