Droplet Microfluidic Device for Chemoenzymatic Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Basic and Starting Solutions
2.3. Microfluidic Chips
2.4. Numerical Simulations
2.5. Fluid Management
2.6. Registration of Luminescence
2.7. Imaging
3. Results and Discussion
3.1. Microfluidic Device for Droplet Generation
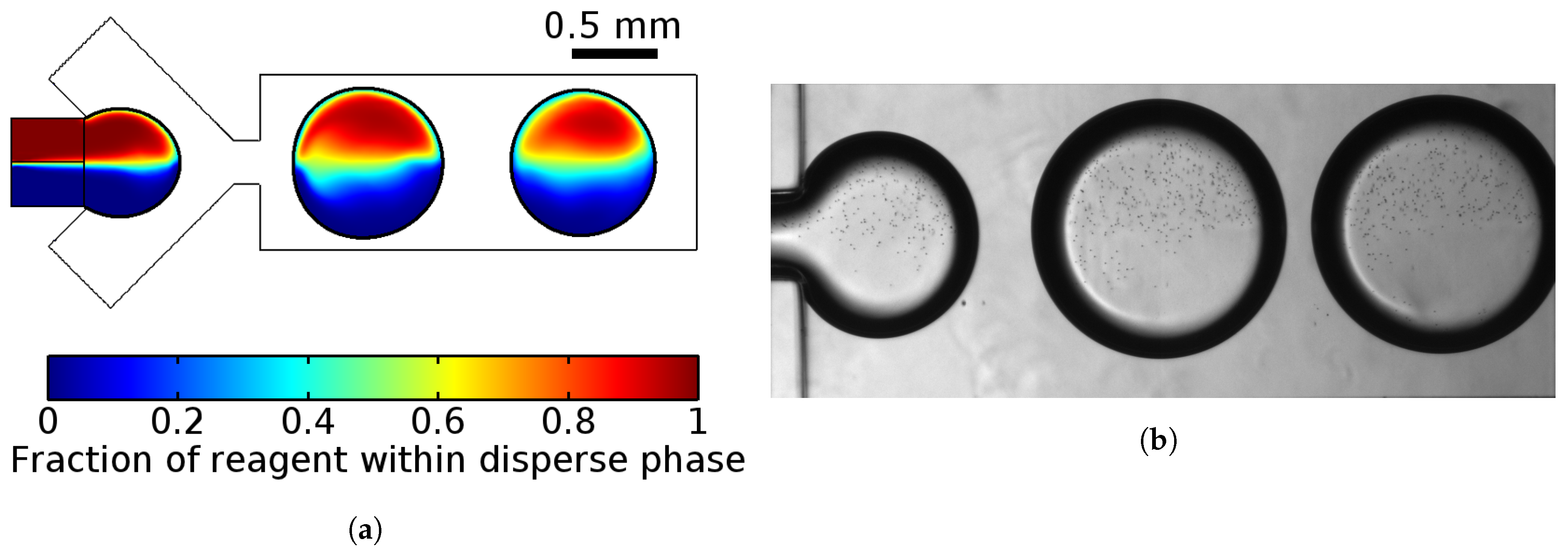
3.1.1. Droplet Generation
- Solutions did not mix in the droplets;
- Changing proportions of pressure of two confluencing liquids of the dispersed phase provide the opportunity to control the composition of droplets;
- Changing the ratio between the dispersed and carrier phases can possibly generate droplets from 0.5 mm (droplet volume ≈ 0.05 L) to 1 mm (droplet volume ≈ 0.2 L). Linear dependence between them was observed as in the case of smaller droplets [52].
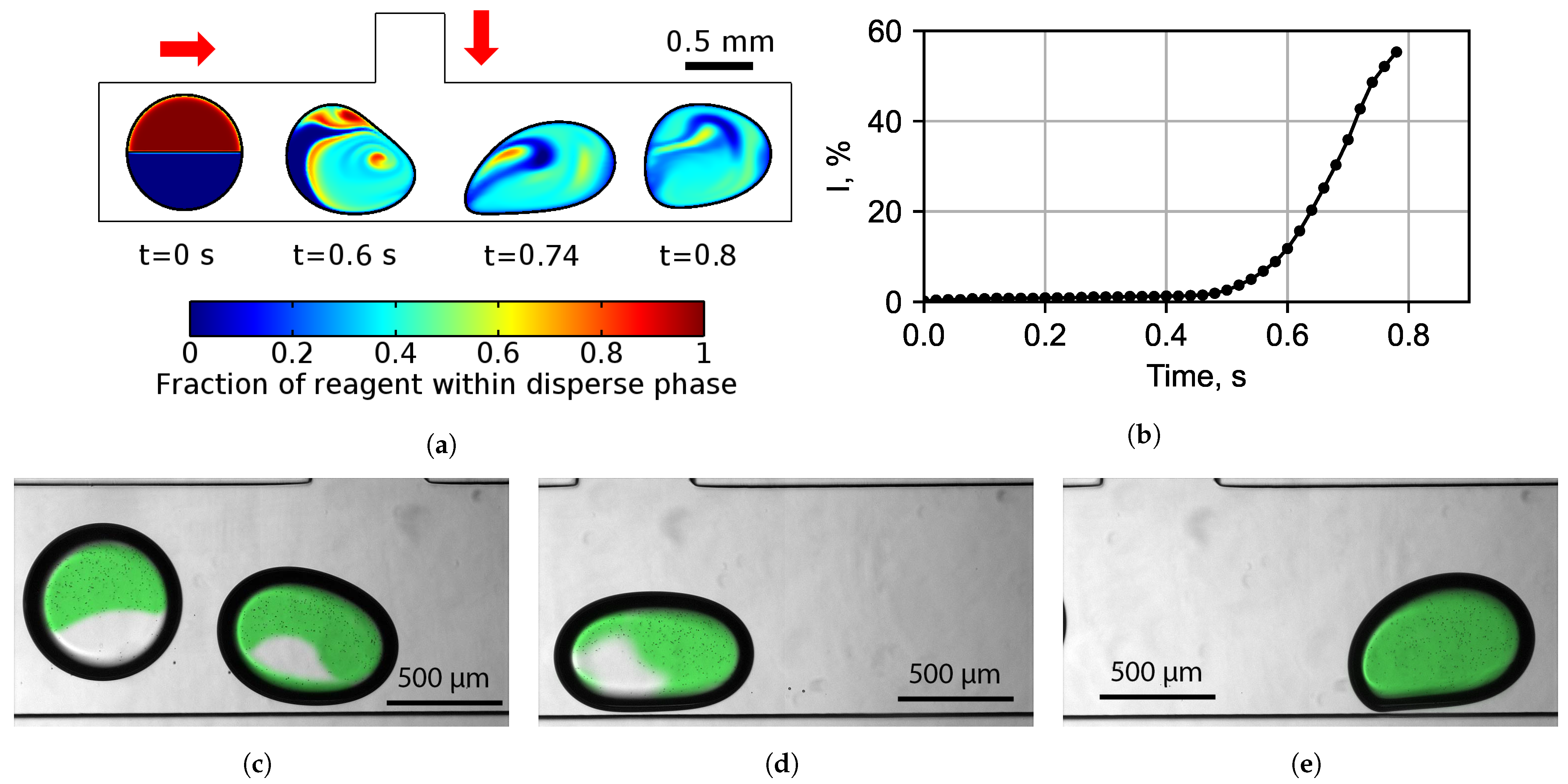
3.1.2. Improving Mixing Efficiency Inside Droplets
3.1.3. Final Chip Design
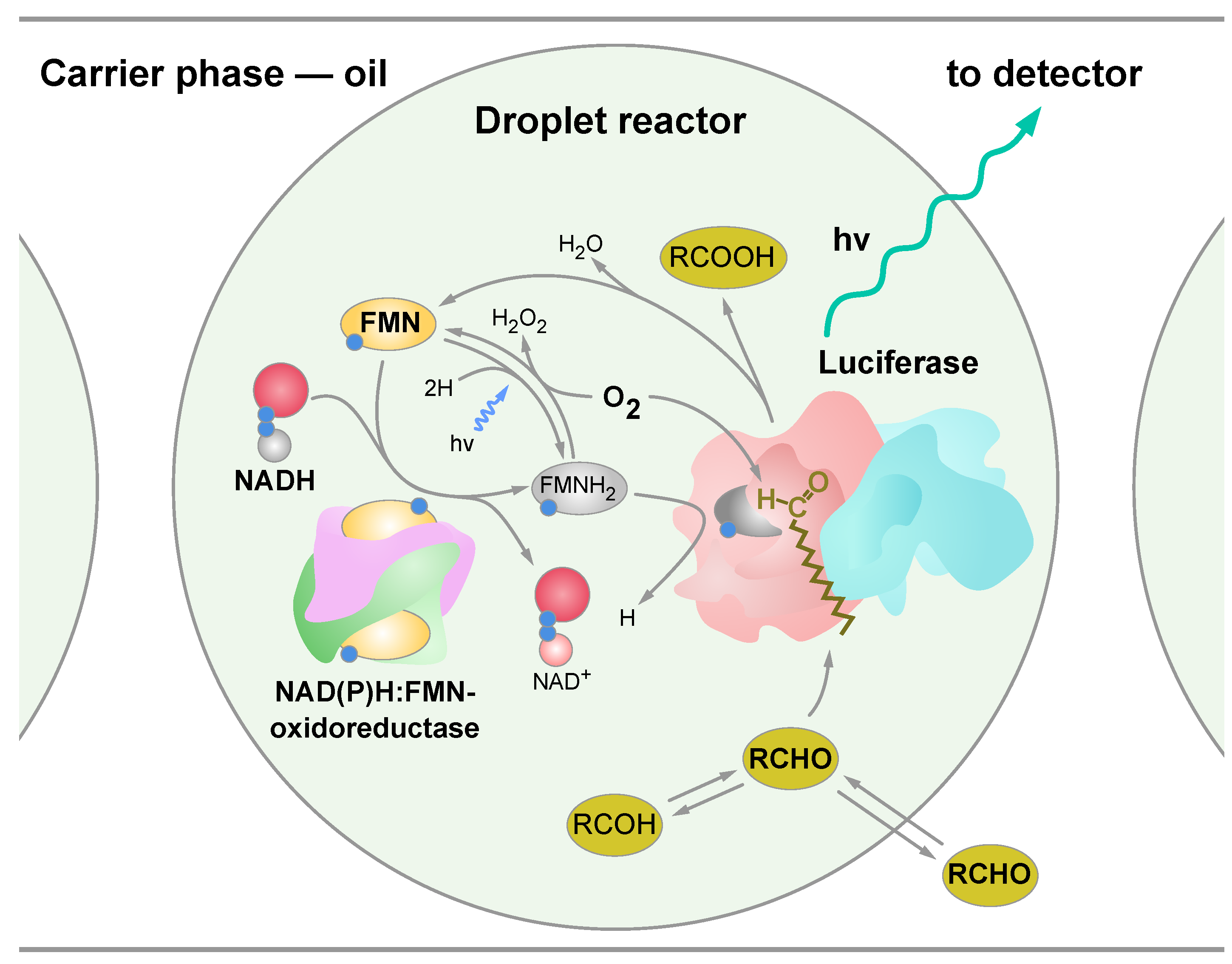
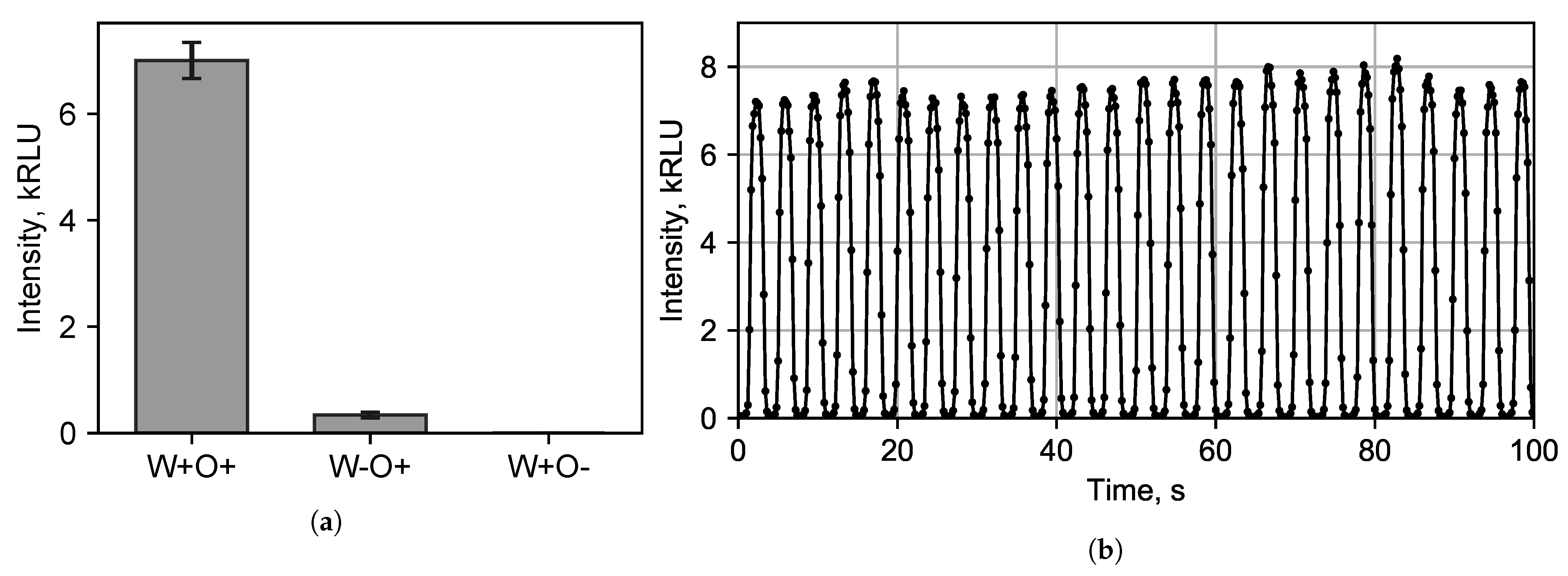
3.2. Bioluminescent Reaction Specificity in Droplets
- (1)
- Tetradecanal dissolved in the carrier phase and in the dispersed phase (W+O+). To dissolve tetradecanal in water, it was first dissolved in ethanol and only then an alcoholic solution of the tetradecanal was diluted with water;
- (2)
- Tetradecanal dissolved only in the carrier phase (W-O+), while ethanol was added to the dispersed phase without tetradecanal;
- (3)
- Tetradecanal only dissolved in the dispersed phase (W+O-).
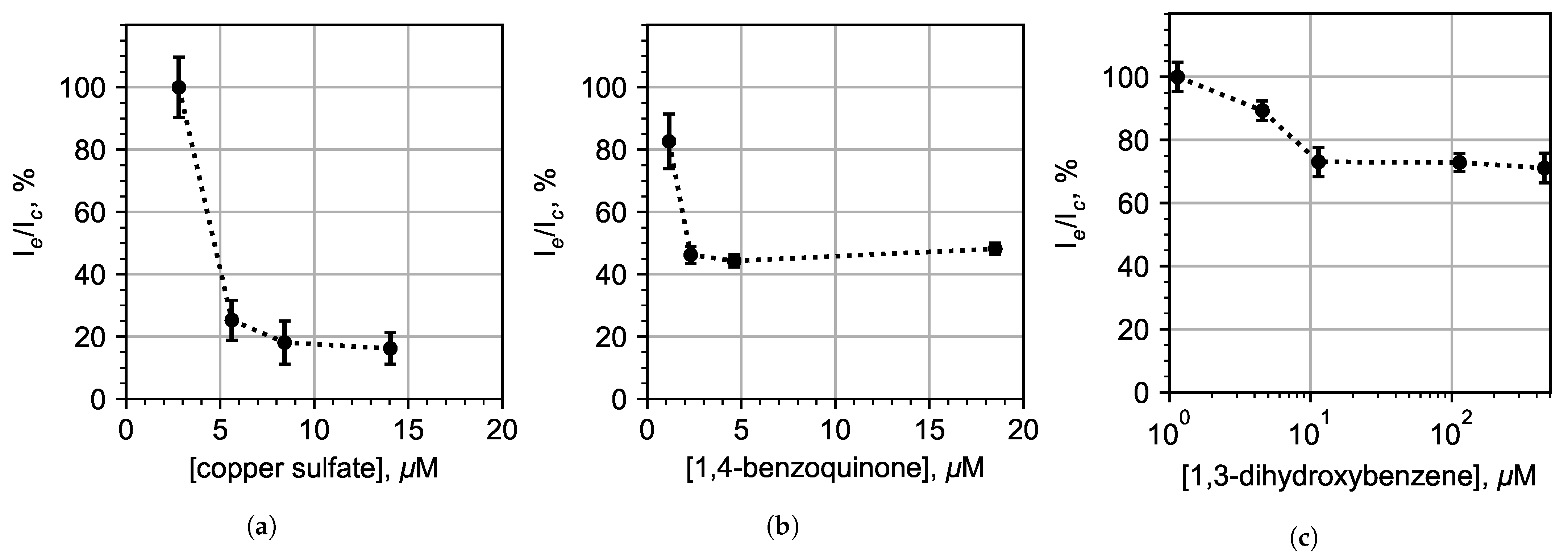
3.3. Chemoenzymatic Sensing for Water Pollution Monitoring
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Red&Luc | NAD(P)H:FMN–Oxidoreductase and Luciferase; |
| PMT | Photomultiplier Tube |
| FMN | Flavin Mononucleotide |
| NADH | Nicotinamide Adenine Dinucleotide |
| LLPS | Liquid–Liquid Phase Separation |
| RLU | Relative Light Unit |
| MPC | Maximum Permissible Concentration |
| MIERID | Microfluidic Immobilized Enzymes Reactor in Droplets |
References
- Dubey, N.C.; Tripathi, B.P. Nature Inspired Multienzyme Immobilization: Strategies and Concepts. ACS Appl. Bio Mater. 2021, 4, 1077–1114. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.; Yang, T.; Stavrakis, S. Droplet-based optofluidic systems for measuring enzyme kinetics. Anal. Bioanal. Chem. 2020, 412, 3265–3283. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.P. Overview of heavy metals and aquatic environment with notes on their recovery. Ecoscan 2010, 4, 235–240. [Google Scholar]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Javed, M.; Ahmad, M.I.; Usmani, N.; Ahmad, M. Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci. Rep. 2017, 7, 1675. [Google Scholar] [CrossRef]
- Triki, H.Z.; Laabir, M.; Lafabrie, C.; Malouche, D.; Bancon-Montigny, C.; Gonzalez, C.; Deidun, A.; Pringault, O.; Daly-Yahia, O.K. Do the levels of industrial pollutants influence the distribution and abundance of dinoflagellate cysts in the recently-deposited sediment of a Mediterranean coastal ecosystem? Sci. Total. Environ. 2017, 595, 380–392. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Smirnov, I.V.; Stepanova, A.V.; Bobik, T.V.; Mokrushina, Y.A.; Ponomarenko, N.A.; Belogurov, A.A.; Rubtsova, M.P.; Kartseva, O.V.; Gomzikova, M.O.; et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 2550–2555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Bai, Y.; Zhu, N.; Xu, J. Microfluidic reactor with immobilized enzyme-from construction to applications: A review. Chin. J. Chem. Eng. 2021, 30, 136–145. [Google Scholar] [CrossRef]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Hsiung, C.; Lajoie, C.A. Kinetic analysis of bacterial bioluminescence. Biotechnol. Bioeng. 2003, 81, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Leth, S.; Maltoni, S.; Simkus, R.; Mattiasson, B.; Corbisier, P.; Klimant, I.; Wolfbeis, O.S.; Csoregi, E. Engineered bacteria based biosensors for monitoring bioavailable heavy metals. Electroanalysis 2002, 14, 35. [Google Scholar] [CrossRef]
- Hakkila, K.; Green, T.; Leskinen, P.; Ivask, A.; Marks, R.; Virta, M. Detection of bioavailable heavy metals in EILATox-Oregon samples using whole-cell luminescent bacterial sensors in suspension or immobilized onto fibre-optic tips. J. Appl. Toxicol. 2004, 24, 333–342. [Google Scholar] [CrossRef]
- Medvedeva, S.E.; Tyulkova, N.A.; Kuznetsov, A.M.; Rodicheva, E.K. Bioluminescent Bioassays Based on Luminous Bacteria. J. Sib. Fed. Univ. Biol. 2009, 4, 418–452. [Google Scholar]
- Cevenini, L.; Calabretta, M.M.; Tarantino, G.; Michelini, E.; Roda, A. Smartphone-interfaced 3D printed toxicity biosensor integrating bioluminescent “sentinel cells”. Sens. Actuators B Chem. 2016, 225, 249–257. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Kondik, A.M.; Kratasyuk, V.A. Bioluminescent enzymatic rapid assay of water integral toxicity. Environ. Monit. Assess. 2013, 185, 5909–5916. [Google Scholar] [CrossRef]
- Esimbekova, E.; Kratasyuk, V.; Shimomura, O. Application of Enzyme Bioluminescence in Ecology. In Bioluminescence: Fundamentals and Applications in Biotechnology—Volume 1; Springer: Berlin/Heidelberg, Germany, 2014; Volume 144, pp. 67–109. [Google Scholar]
- Denisov, I.; Lukyanenko, K.; Yakimov, A.; Kukhtevich, I.; Esimbekova, E.; Belobrov, P. Disposable luciferase-based microfluidic chip for rapid assay of water pollution. Luminescence 2018, 33, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.W.; Riley, W.H.; Massa, J. The purification, properties, and chemiluminescent quantum yield of bacterial luciferase. J. Biol. Chem. 1965, 240, 1473–1481. [Google Scholar] [CrossRef]
- Petushkov, V.; Kratasyuk, G.; Rodionova, N.; Fish, A.; Belobrov, P. Two-enzyme NADH:FMN-oxidoreductase-luciferase system from luminescent bacteria. Biochem. Acad. Sci. USSR 1984, 49, 593–604. [Google Scholar]
- Kudryasheva, N.S.; Kudinova, I.Y.; Esimbekova, E.N.; Kratasyuk, V.A.; Stom, D.I. The influence of quinones and phenols on the triple NAD(H)-dependent enzyme systems. Chemosphere 1999, 38, 751–758. [Google Scholar] [CrossRef]
- Kratasyuk, V.A.; Esimbekova, E.N.; Gladyshev, M.I.; Khromichek, E.B.; Kuznetsov, A.M.; Ivanova, E.A. The use of bioluminescent biotests for study of natural and laboratory aquatic ecosystems. Chemosphere 2001, 42, 909–915. [Google Scholar] [CrossRef]
- Vetrova, E.; Esimbekova, E.; Remmel, N.; Kotova, S.; Beloskov, N.; Kratasyuk, V.; Gitelson, I. A bioluminescent signal system: Detection of chemical toxicants in water. Lumin. J. Biol. Chem. Lumin. 2007, 22, 206–214. [Google Scholar] [CrossRef]
- Esimbekova, E.N.; Kalyabina, V.P.; Kopylova, K.V.; Torgashina, I.G.; Kratasyuk, V.A. Design of bioluminescent biosensors for assessing contamination of complex matrices. Talanta 2021, 233, 122509. [Google Scholar] [CrossRef] [PubMed]
- Woutersen, M.; Belkin, S.; Brouwer, B.; van Wezel, A.P.; Heringa, M.B. Are luminescent bacteria suitable for online detection and monitoring of toxic compounds in drinking water and its sources? Anal. Bioanal. Chem. 2011, 400, 915–929. [Google Scholar] [CrossRef] [Green Version]
- Charrier, T.; Chapeau, C.; Bendria, L.; Picart, P.; Daniel, P.; Thouand, G. A multi-channel bioluminescent bacterial biosensor for the on-line detection of metals and toxicity. Part II: Technical development and proof of concept of the biosensor. Anal. Bioanal. Chem. 2011, 400, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Almog, R.; Yagur-Kroll, S.; Levkov, K.; Melamed, S.; Shacham-Diamand, Y.; Belkin, S. Online Monitoring of Water Toxicity by Use of Bioluminescent Reporter Bacterial Biochips. Environ. Sci. Technol. 2011, 45, 8536–8544. [Google Scholar] [CrossRef] [PubMed]
- Elad, T.; Belkin, S. Reporter Gene Assays in Ecotoxicology. In In Vitro Environmental Toxicology—Concepts, Application and Assessment; Reifferscheid, G., Buchinger, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 157, pp. 135–157. [Google Scholar]
- Zurita, J.L.; Jos, A.; Cameán, A.M.; Salguero, M.; López-Artíguez, M.; Repetto, G. Ecotoxicological evaluation of sodium fluoroacetate on aquatic organisms and investigation of the effects on two fish cell lines. Chemosphere 2007, 67, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Dong, M.; Zheng, W.; Sun, J.; Jiang, X. Double-Enzymes-Mediated Bioluminescent Sensor for Quantitative and Ultrasensitive Point-of-Care Testing. Anal. Chem. 2017, 89, 5422–5427. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Gruner, P.; Riechers, B.; Semin, B.; Lim, J.; Johnston, A.; Short, K.; Baret, J. Controlling molecular transport in minimal emulsions. Nat. Commun. 2016, 7, 10392. [Google Scholar] [CrossRef] [Green Version]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.H.; Gartner, Z.J.; Abate, A.R. Multicolor Fluorescence Detection for Droplet Microfluidics Using Optical Fibers. J. Vis. Exp. 2016, 111, 54010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, R.H.; de Lange, N.; Gartner, Z.J.; Abate, A.R. Compact and modular multicolour fluorescence detector for droplet microfluidics. Lab Chip 2015, 15, 2754–2758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Li, J.; Li, W.; Zhang, Y.; Yang, X.; Chen, N.; Sun, Y.; Zhao, Y.; Fan, C.; Huang, Q. The Biocompatibility of Nanodiamonds and Their Application in Drug Delivery Systems. Theranostics 2012, 2, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Caen, O.; Vrignon, J.; Konrad, M.; Taly, V.; Baret, J. Parallelized ultra-high throughput microfluidic emulsifier for multiplex kinetic assays. Biomicrofluidics 2015, 9, 034101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Eustace, D.; Merten, C.A. Efficient cell pairing in droplets using dual-color sorting. Lab Chip 2015, 15, 3989–3993. [Google Scholar] [CrossRef] [Green Version]
- Agresti, J.J.; Antipov, E.; Abate, A.R.; Ahn, K.; Rowat, A.C.; Baret, J.; Marquez, M.; Klibanov, A.M.; Griffiths, A.D.; Weitz, D.A. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 4004–4009. [Google Scholar] [CrossRef] [Green Version]
- Baret, J.; Miller, O.J.; Taly, V.; Ryckelynck, M.; El-Harrak, A.; Frenz, L.; Rick, C.; Samuels, M.L.; Hutchison, J.B.; Agresti, J.J.; et al. Fluorescence-activated droplet sorting (FADS): Efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 2009, 9, 1850. [Google Scholar] [CrossRef]
- Ahn, K.; Kerbage, C.; Hunt, T.P.; Westervelt, R.M.; Link, D.R.; Weitz, D.A. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 2006, 88, 024104. [Google Scholar] [CrossRef] [Green Version]
- Abalde-Cela, S.; Gould, A.; Liu, X.; Kazamia, E.; Smith, A.G.; Abell, C. High-throughput detection of ethanol-producing cyanobacteria in a microdroplet platform. J. R. Soc. Interface 2015, 12, 20150216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leman, M.; Abouakil, F.; Griffiths, A.D.; Tabeling, P. Droplet-based microfluidics at the femtolitre scale. Lab Chip 2015, 15, 753–765. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Dai, X.; Gao, Y.; Jiang, W.; Han, J.; Wang, Z.; Zhang, R. Correlation between electron delocalization and structural planarization in small water rings. Int. J. Quantum Chem. 2015, 115, 817–819. [Google Scholar] [CrossRef]
- Ottino, J.M.; Ottino, J. The Kinematics of Mixing: Stretching, Chaos, and Transport; Cambridge University Press: Cambridge, UK, 1989; Volume 3. [Google Scholar]
- Song, H.; Tice, J.D.; Ismagilov, R.F. A Microfluidic System for Controlling Reaction Networks in Time. Angew. Chem. Int. Ed. 2003, 42, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, F.; Prat, L.; Di Miceli, N.; Cristobal, G.; Link, D.; Weitz, D. Mixing characterization inside microdroplets engineered on a microcoalescer. Chem. Eng. Sci. 2007, 62, 1042–1048. [Google Scholar] [CrossRef]
- Gallé, N.; Steinberg, V. On-chip encapsulation via chaotic mixing. Microfluid. Nanofluid. 2016, 20, 156. [Google Scholar] [CrossRef]
- Yakimov, A.; Denisov, I.; Bukatin, A.; Lukyanenko, K.; Belousov, K.; Kukhtevich, I.; Esimbekova, E.; Evstrapov, A.; Belobrov, P. Droplet reactors with bioluminescent enzymes for real time water pollution monitoring. In Proceedings of the 1st International Electronic Conference on Biosensors, Online, 2–17 November 2020; p. 7046. [Google Scholar]
- Bukatin, A.S.; Mukhin, I.S.; Malyshev, E.I.; Kukhtevich, I.V.; Evstrapov, A.A.; Dubina, M.V. Fabrication of high-aspect-ratio microstructures in polymer microfluid chips for in vitro single-cell analysis. Tech. Phys. 2016, 61, 1566–1571. [Google Scholar] [CrossRef]
- Belousov, K.I.; Filatov, N.A.; Kukhtevich, I.V.; Kantsler, V.; Evstrapov, A.A.; Bukatin, A.S. An asymmetric flow-focusing droplet generator promotes rapid mixing of reagents. Sci. Rep. 2021, 11, 8797. [Google Scholar] [CrossRef]
- Olsson, E.; Kreiss, G. A conservative level set method for two phase flow. J. Comput. Phys. 2005, 210, 225–246. [Google Scholar] [CrossRef]
- Kockmann, N. Transport Phenomena in Micro Process Engineering; Springer Science & Business Media: New York, NY, USA, 2008; p. 365. [Google Scholar]
- Filatov, N.A.; Denisov, I.A.; Evstrapov, A.A.; Bukatin, A.S. Open-Source Pressure Controller Based on Compact Electro-Pneumatic Regulators for Droplet Microfluidics Applications. IEEE Trans. Instrum. Meas. 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Sakurai, R.; Yamamoto, K.; Motosuke, M. Concentration-adjustable micromixers using droplet injection into a microchannel. Analyst 2019, 144, 2780–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denisov, I.A. Luciferase-based bioassay for rapid pollutants detection and classification by means of multilayer artificial neural networks. Sens. Actuators B Chem. 2017, 242, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Guo, X.; Li, Z.; Wang, Y.; Wei, G. Multisensors Cooperative Detection Task Scheduling Algorithm Based on Hybrid Task Decomposition and MBPSO. Math. Probl. Eng. 2017, 2017, 3453589. [Google Scholar] [CrossRef]
- Oyewunmi, O.D.; Safiabadi-Tali, S.H.; Jahanshahi-Anbuhi, S. Dual-Modal Assay Kit for the Qualitative and Quantitative Determination of the Total Water Hardness Using a Permanent Marker Fabricated Microfluidic Paper-Based Analytical Device. Chemosensors 2020, 8, 97. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A New Paper-Based Microfluidic Device for Improved Detection of Nitrate in Water. Sensors 2020, 21, 102. [Google Scholar] [CrossRef]
- Pattanayak, P.; Singh, S.K.; Gulati, M.; Vishwas, S.; Kapoor, B.; Chellappan, D.K.; Anand, K.; Gupta, G.; Jha, N.K.; Gupta, P.K.; et al. Microfluidic chips: Recent advances, critical strategies in design, applications and future perspectives. Microfluid. Nanofluid. 2021, 25, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Belobrov, P.I.; Evstrapov, A.A.; Esimbekova, E.N.; Denisov, I.A.; Lukyanenko, K.A.; Osipova, E.D.; Yakimov, A.S. Towards biological quantity theory for nominal property metrology in polyenzymatic devices with living cells. J. Phys. Conf. Ser. 2019, 1379, 012036. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakimov, A.S.; Denisov, I.A.; Bukatin, A.S.; Lukyanenko, K.A.; Belousov, K.I.; Kukhtevich, I.V.; Esimbekova, E.N.; Evstrapov, A.A.; Belobrov, P.I. Droplet Microfluidic Device for Chemoenzymatic Sensing. Micromachines 2022, 13, 1146. https://doi.org/10.3390/mi13071146
Yakimov AS, Denisov IA, Bukatin AS, Lukyanenko KA, Belousov KI, Kukhtevich IV, Esimbekova EN, Evstrapov AA, Belobrov PI. Droplet Microfluidic Device for Chemoenzymatic Sensing. Micromachines. 2022; 13(7):1146. https://doi.org/10.3390/mi13071146
Chicago/Turabian StyleYakimov, Anton S., Ivan A. Denisov, Anton S. Bukatin, Kirill A. Lukyanenko, Kirill I. Belousov, Igor V. Kukhtevich, Elena N. Esimbekova, Anatoly A. Evstrapov, and Peter I. Belobrov. 2022. "Droplet Microfluidic Device for Chemoenzymatic Sensing" Micromachines 13, no. 7: 1146. https://doi.org/10.3390/mi13071146
APA StyleYakimov, A. S., Denisov, I. A., Bukatin, A. S., Lukyanenko, K. A., Belousov, K. I., Kukhtevich, I. V., Esimbekova, E. N., Evstrapov, A. A., & Belobrov, P. I. (2022). Droplet Microfluidic Device for Chemoenzymatic Sensing. Micromachines, 13(7), 1146. https://doi.org/10.3390/mi13071146









