Contactless Micro-Droplet Manipulation of Liquid Released from a Parallel Plate to an Open Region in Electrowetting-on-Dielectric Platform
Abstract
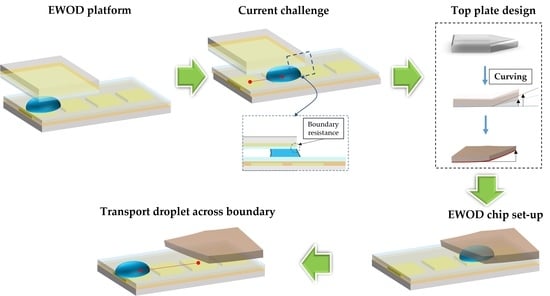
:1. Introduction
2. Experimental Materials and Methods
2.1. Edge-Effect Surface-Tension Resistance
2.2. Top-Plate Design
2.3. EWOD Chip Design and Fabrication
2.4. Experiment Design
3. Results
The Manipulation of Propylene Carbonate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ho, C.-M.; Tai, Y.-C. Micro-electro-mechanical-systems (MEMS) and fluid flows. Annu. Rev. Fluid Mech. 1998, 30, 579–612. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro electromechanical systems (MEMS) based microfluidic devices for biomedical applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, E.; De Rooij, N.F. Microfluidics meets MEMS. Proc. IEEE 2003, 91, 930–953. [Google Scholar] [CrossRef] [Green Version]
- Tng, D.J.H.; Hu, R.; Song, P.; Roy, I.; Yong, K.-T. Approaches and challenges of engineering implantable microelectromechanical systems (MEMS) drug delivery systems for in vitro and in vivo applications. Micromachines 2012, 3, 615–631. [Google Scholar] [CrossRef]
- Miao, P.; Mitcheson, P.; Holmes, A.; Yeatman, E.; Green, T.; Stark, B. MEMS inertial power generators for biomedical applications. Microsyst. Technol. 2006, 12, 1079–1083. [Google Scholar] [CrossRef] [Green Version]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar] [CrossRef] [Green Version]
- Jebrail, M.J.; Bartsch, M.S.; Patel, K.D. Digital microfluidics: A versatile tool for applications in chemistry, biology and medicine. Lab Chip 2012, 12, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Vergauwe, N.; Witters, D.; Ceyssens, F.; Vermeir, S.; Verbruggen, B.; Puers, R.; Lammertyn, J. A versatile electrowetting-based digital microfluidic platform for quantitative homogeneous and heterogeneous bio-assays. J. Micromech. Microeng. 2011, 21, 054026. [Google Scholar] [CrossRef]
- George, S.M.; Moon, H. Digital microfluidic three-dimensional cell culture and chemical screening platform using alginate hydrogels. Biomicrofluidics 2015, 9, 024116. [Google Scholar] [CrossRef] [Green Version]
- Coudron, L.; McDonnell, M.B.; Munro, I.; McCluskey, D.K.; Johnston, I.D.; Tan, C.K.; Tracey, M.C. Fully integrated digital microfluidics platform for automated immunoassay; A versatile tool for rapid, specific detection of a wide range of pathogens. Biosens. Bioelectron. 2019, 128, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-J.; Yao, D.-J.; Lin, H.-C.; Chang, W.-Y.; Chang, H.-Y. DNA ligation of ultramicro volume using an EWOD microfluidic system with coplanar electrodes. J. Micromech. Microeng. 2008, 18, 045017. [Google Scholar] [CrossRef]
- Wheeler, A.R.; Moon, H.; Kim, C.-J.C.; Loo, J.A.; Garrell, R.L. Electrowetting-based microfluidics for analysis of peptides and proteins by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 2004, 76, 4833–4838. [Google Scholar] [CrossRef]
- Shen, H.-H.; Fan, S.-K.; Kim, C.-J.; Yao, D.-J. EWOD microfluidic systems for biomedical applications. Microfluid. Nanofluidics 2014, 16, 965–987. [Google Scholar] [CrossRef]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef] [PubMed]
- Barbulovic-Nad, I.; Au, S.H.; Wheeler, A.R. A microfluidic platform for complete mammalian cell culture. Lab Chip 2010, 10, 1536–1542. [Google Scholar] [CrossRef]
- Cho, S.K.; Moon, H.; Kim, C.-J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Lee, J.; Moon, H.; Fowler, J.; Schoellhammer, T.; Kim, C.-J. Electrowetting and electrowetting-on-dielectric for microscale liquid handling. Sens. Actuators A Phys. 2002, 95, 259–268. [Google Scholar] [CrossRef]
- Yi, U.-C.; Kim, C.-J. Characterization of electrowetting actuation on addressable single-side coplanar electrodes. J. Micromech. Microeng. 2006, 16, 2053. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Moon, H.; Fowler, J.; Kim, C.-J.; Schoellhammer, T. Addressable micro liquid handling by electric control of surface tension. In Proceedings of the Technical Digest, MEMS 2001, 14th IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 01CH37090), Interlaken, Switzerland, 25–25 January 2001; pp. 499–502. [Google Scholar]
- Saeki, F.; Baum, J.; Moon, H.; Yoon, J.-Y.; Kim, C.; Garrell, R. Electrowetting on dielectrics (EWOD): Reducing voltage requirements for microfluidics. Polym. Mater. Sci. Eng 2001, 85, 12–13. [Google Scholar]
- Nelson, W.C.; Kim, C.-J.C. Droplet actuation by electrowetting-on-dielectric (EWOD): A review. J. Adhes. Sci. Technol. 2012, 26, 1747–1771. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.M.; Wheeler, A.R. Digital bioanalysis. Anal. Bioanal. Chem. 2009, 393, 419–426. [Google Scholar] [CrossRef]
- Alias, A.B.; Chiang, C.-E.; Huang, H.-Y.; Lin, K.-T.; Lu, P.-J.; Wang, Y.-W.; Wu, T.-H.; Jiang, P.-S.; Chen, C.-A.; Yao, D.-J. Extraction of cell-free Dna from An embryo-culture Medium Using Micro-scale Bio-reagents on ewod. Sci. Rep. 2020, 10, 9708. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, S.; Otts, D. Electrowetting Lenses Having Oleophobic Surfaces. Google Patents US11191636B2, 7 December 2021. [Google Scholar]
- Huang, H.-Y.; Shen, H.-H.; Tien, C.-H.; Li, C.-J.; Fan, S.-K.; Liu, C.-H.; Hsu, W.-S.; Yao, D.-J. Digital microfluidic dynamic culture of mammalian embryos on an electrowetting on dielectric (EWOD) chip. PLoS ONE 2015, 10, e0124196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciou, Y.-J.; Lee, H.-T.; Lin, Y.-W.; Yao, D.-J. Microfluidic patterning using a digital microfluidic system. AIP Adv. 2020, 10, 125115. [Google Scholar] [CrossRef]
- Chiang, C.-E.; Huang, H.-Y.; Lin, K.-T.; Alias, A.B.; Lu, P.-J.; Wang, Y.-W.; Wu, T.-H.; Jiang, P.-S.; Chen, C.-A.; Yao, D.-J. A medical innovation: A new and improved method of DNA extraction with electrowetting-on-dielectric of genetic testing in-vitro fertilization (IVF). Microfluid. Nanofluidics 2020, 24, 55. [Google Scholar] [CrossRef]
- Marshall, S.J.; Bayne, S.C.; Baier, R.; Tomsia, A.P.; Marshall, G.W. A review of adhesion science. Dent. Mater. 2010, 26, e11–e16. [Google Scholar] [CrossRef]
- Oliver, J.; Huh, C.; Mason, S. Resistance to spreading of liquids by sharp edges. J. Colloid Interface Sci. 1977, 59, 568–581. [Google Scholar] [CrossRef]
- Baratian, D.; Ruiz-Gutiérrez, É.; Mugele, F.; Ledesma-Aguilar, R. Slippery when wet: Mobility regimes of confined drops in electrowetting. Soft Matter 2019, 15, 7063–7070. [Google Scholar] [CrossRef] [Green Version]
- Baratian, D.; Cavalli, A.; Van Den Ende, D.; Mugele, F. On the shape of a droplet in a wedge: New insight from electrowetting. Soft Matter 2015, 11, 7717–7721. [Google Scholar] [CrossRef]
- Egunov, A.; Korvink, J.; Luchnikov, V. Polydimethylsiloxane bilayer films with an embedded spontaneous curvature. Soft Matter 2016, 12, 45–52. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-N.; Yao, D.-J. Contactless Micro-Droplet Manipulation of Liquid Released from a Parallel Plate to an Open Region in Electrowetting-on-Dielectric Platform. Micromachines 2022, 13, 898. https://doi.org/10.3390/mi13060898
Chang Y-N, Yao D-J. Contactless Micro-Droplet Manipulation of Liquid Released from a Parallel Plate to an Open Region in Electrowetting-on-Dielectric Platform. Micromachines. 2022; 13(6):898. https://doi.org/10.3390/mi13060898
Chicago/Turabian StyleChang, Yii-Nuoh, and Da-Jeng Yao. 2022. "Contactless Micro-Droplet Manipulation of Liquid Released from a Parallel Plate to an Open Region in Electrowetting-on-Dielectric Platform" Micromachines 13, no. 6: 898. https://doi.org/10.3390/mi13060898
APA StyleChang, Y.-N., & Yao, D.-J. (2022). Contactless Micro-Droplet Manipulation of Liquid Released from a Parallel Plate to an Open Region in Electrowetting-on-Dielectric Platform. Micromachines, 13(6), 898. https://doi.org/10.3390/mi13060898