Autonomous Exercise Generator for Upper Extremity Rehabilitation: A Fuzzy-Logic-Based Approach
Abstract
1. Introduction
2. Design Requirements and Background
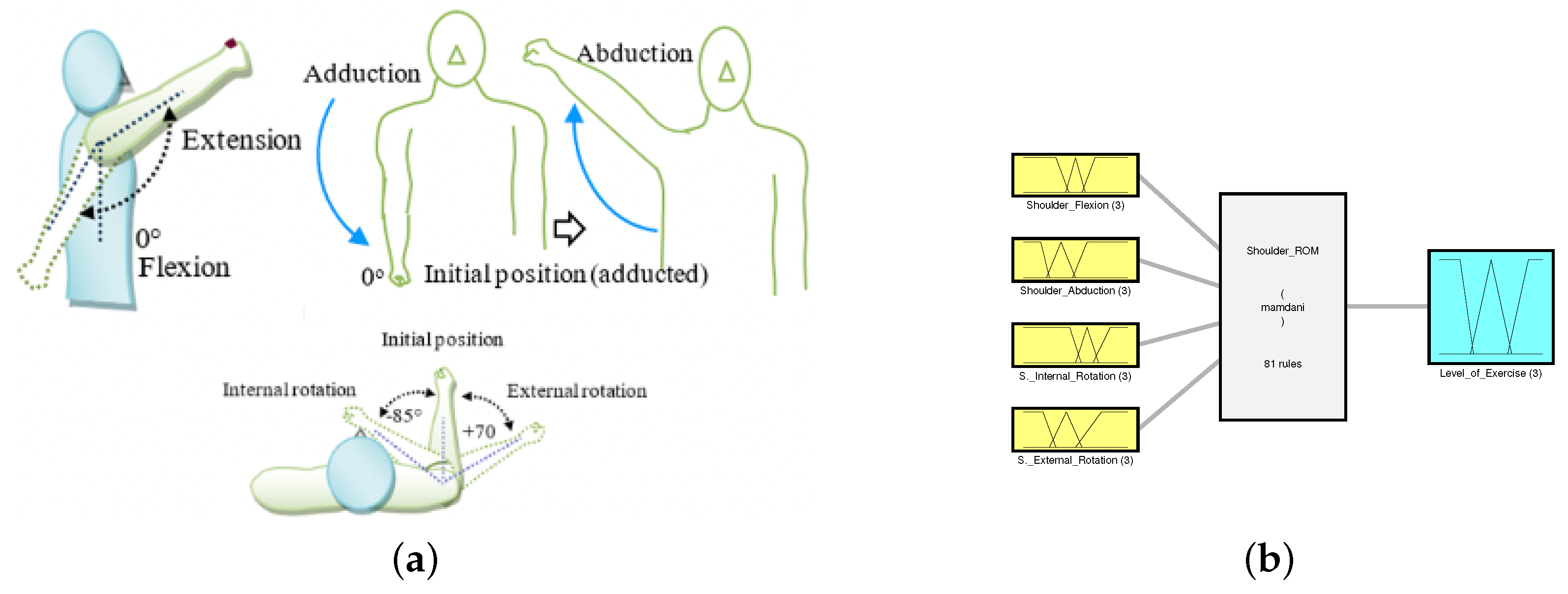
2.1. Range of Motion
2.2. Muscle Strength
2.3. Spasticity
3. Methods and Materials of the Fuzzy-Based Decision-Making Scheme
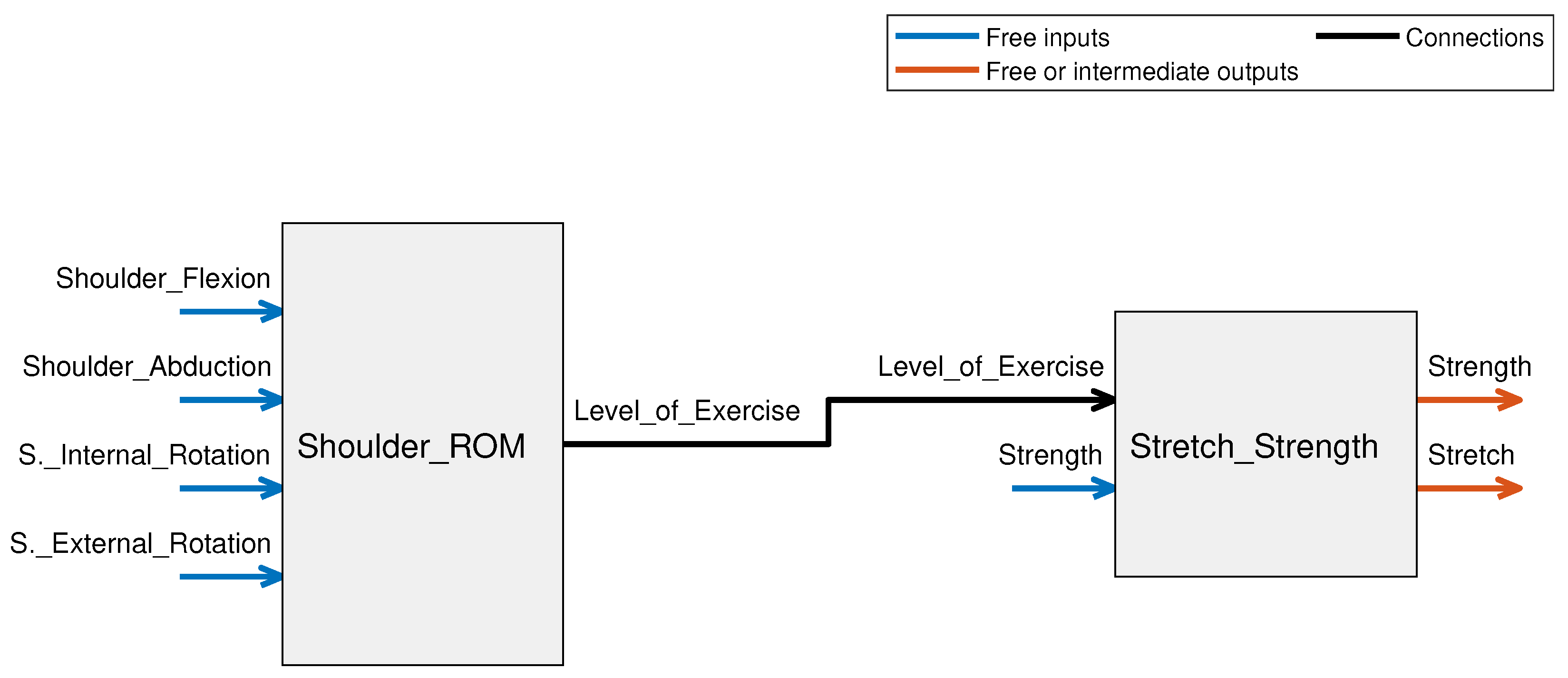
3.1. Shoulder ROM FIS
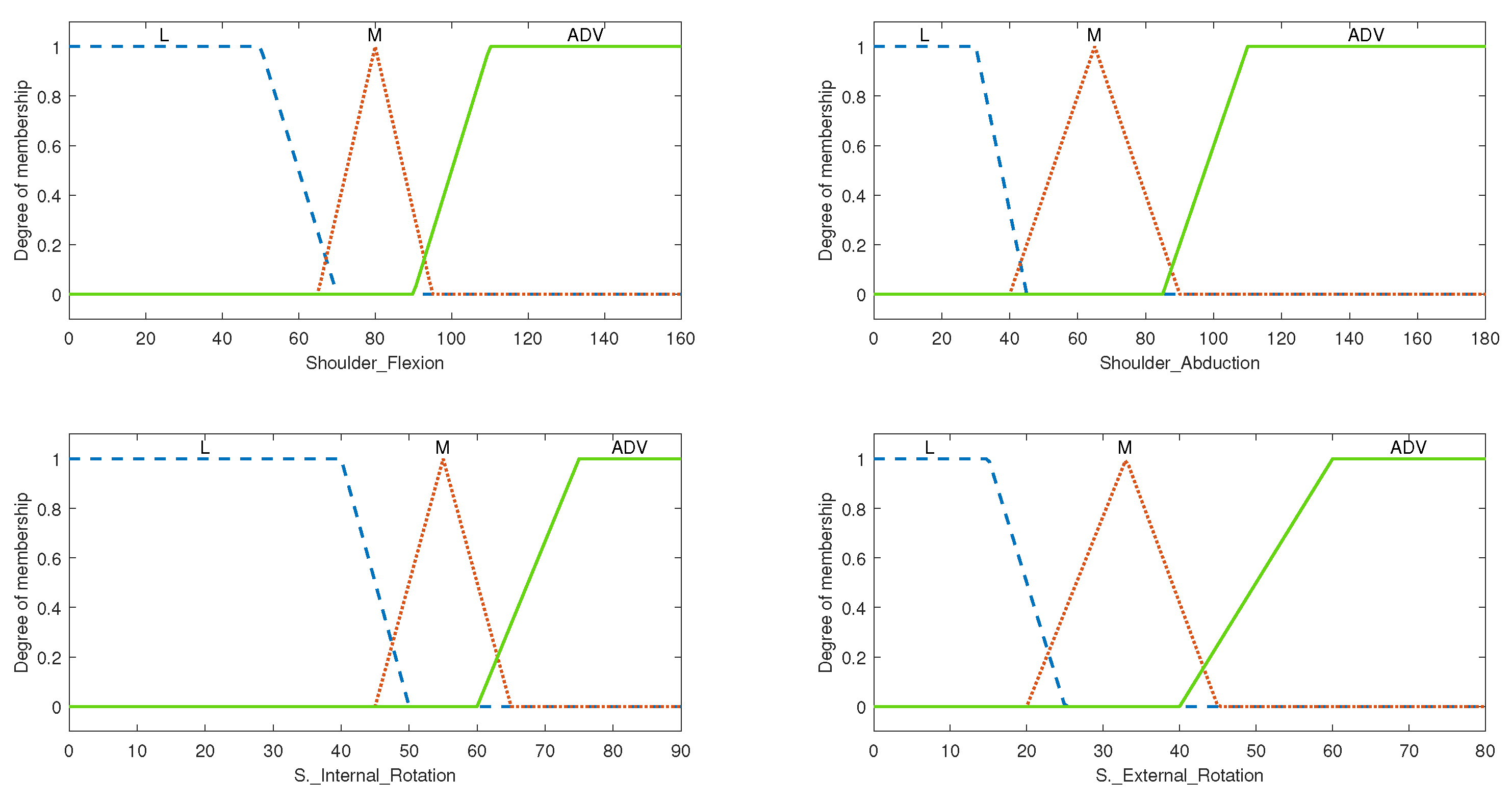
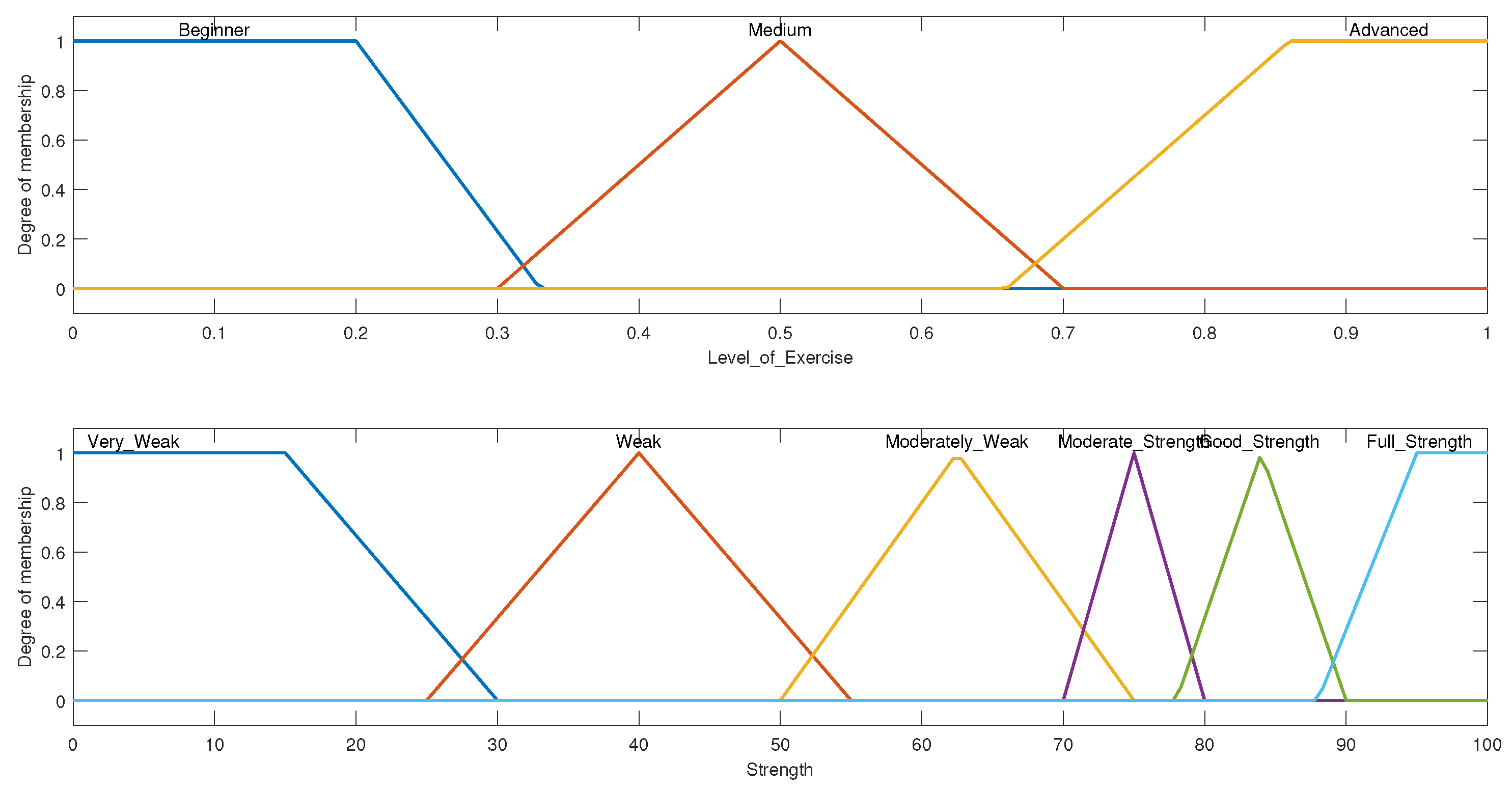
3.1.1. System Variables
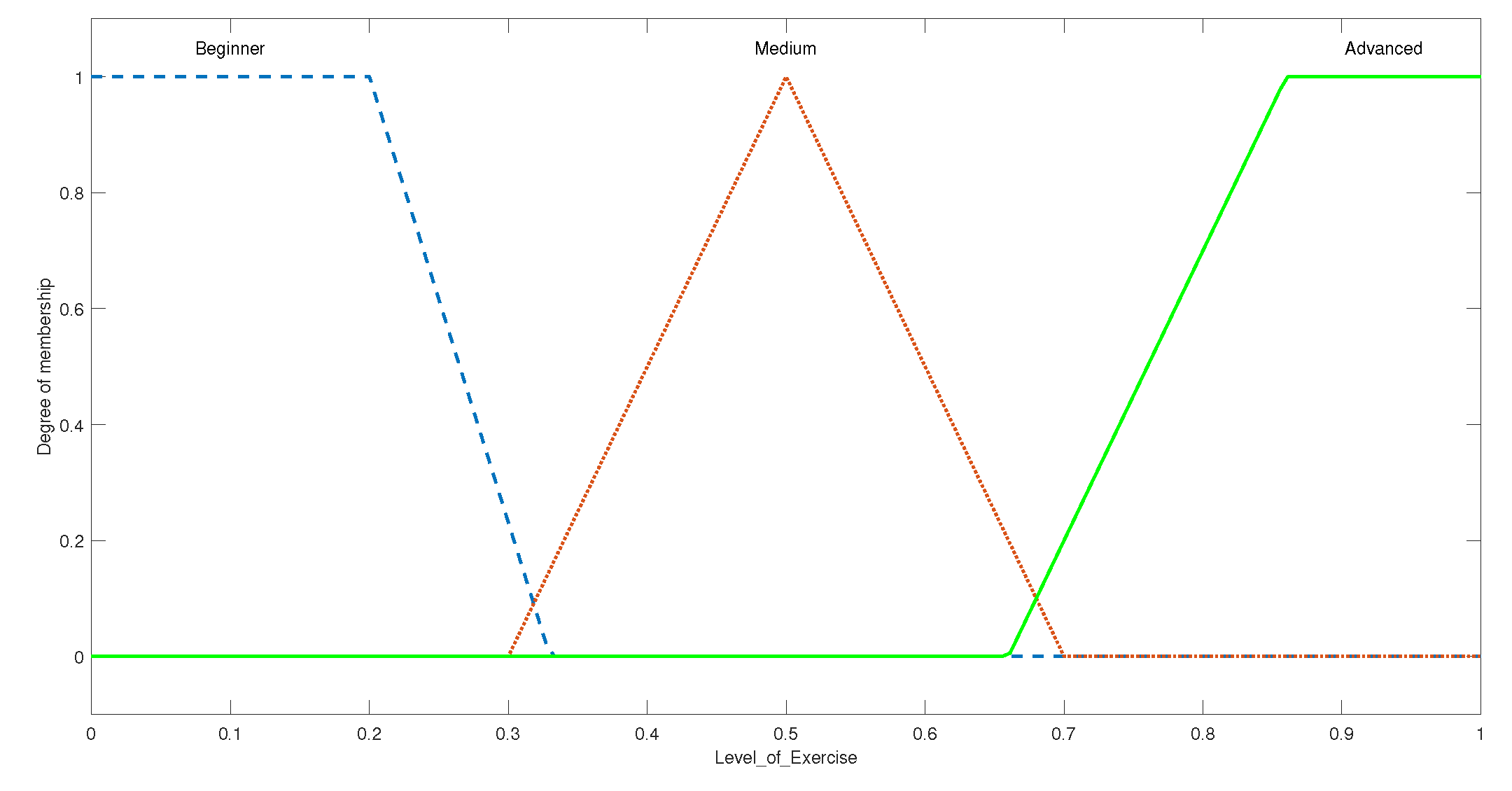
3.1.2. Rule Evaluation and Overall Setup for Shoulder ROM FIS
3.2. Stretch and Strength FIS
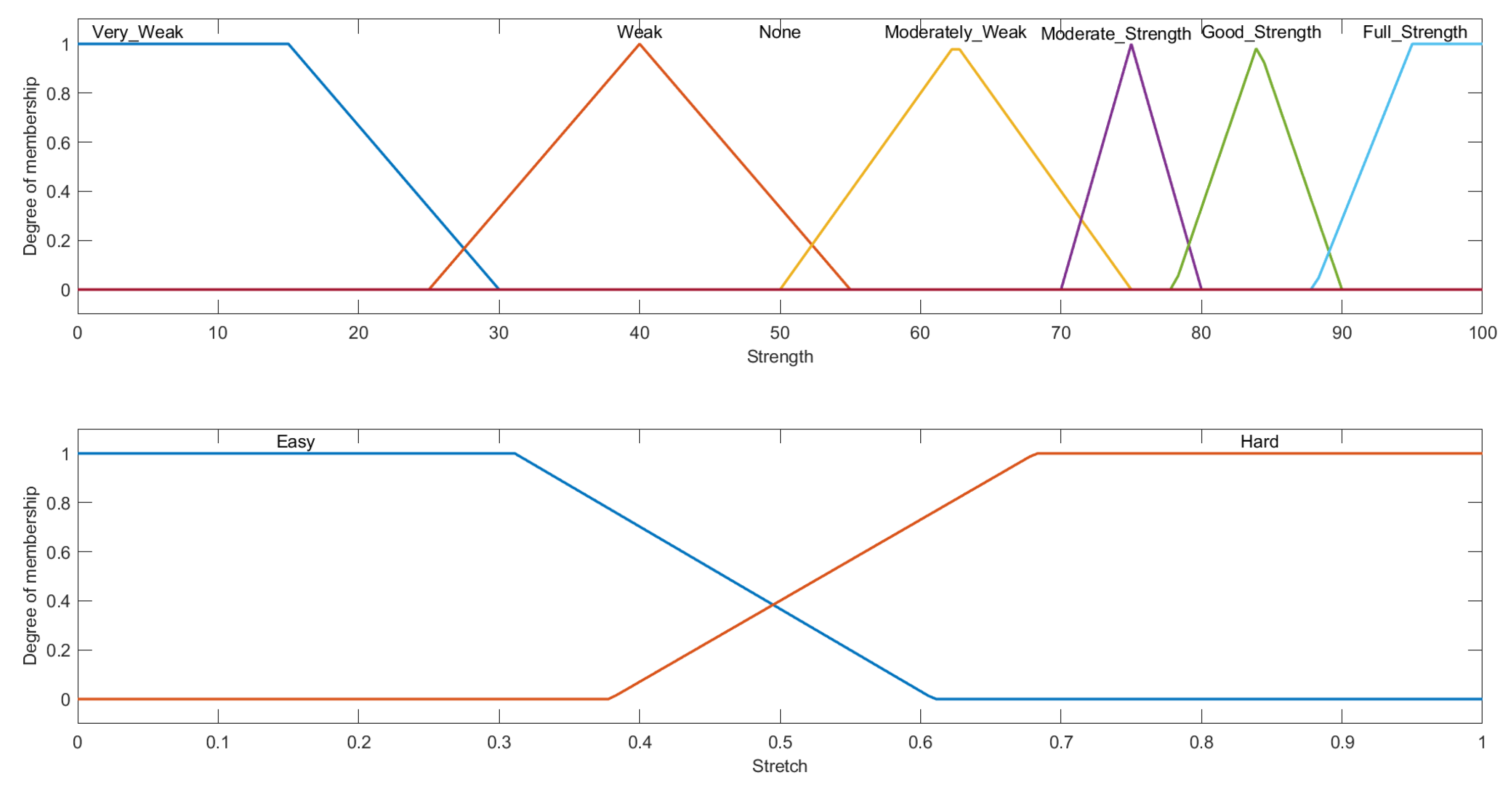
3.2.1. System Variables
3.2.2. Rule Evaluation and Overall Setup for Stretch and Strength FIS
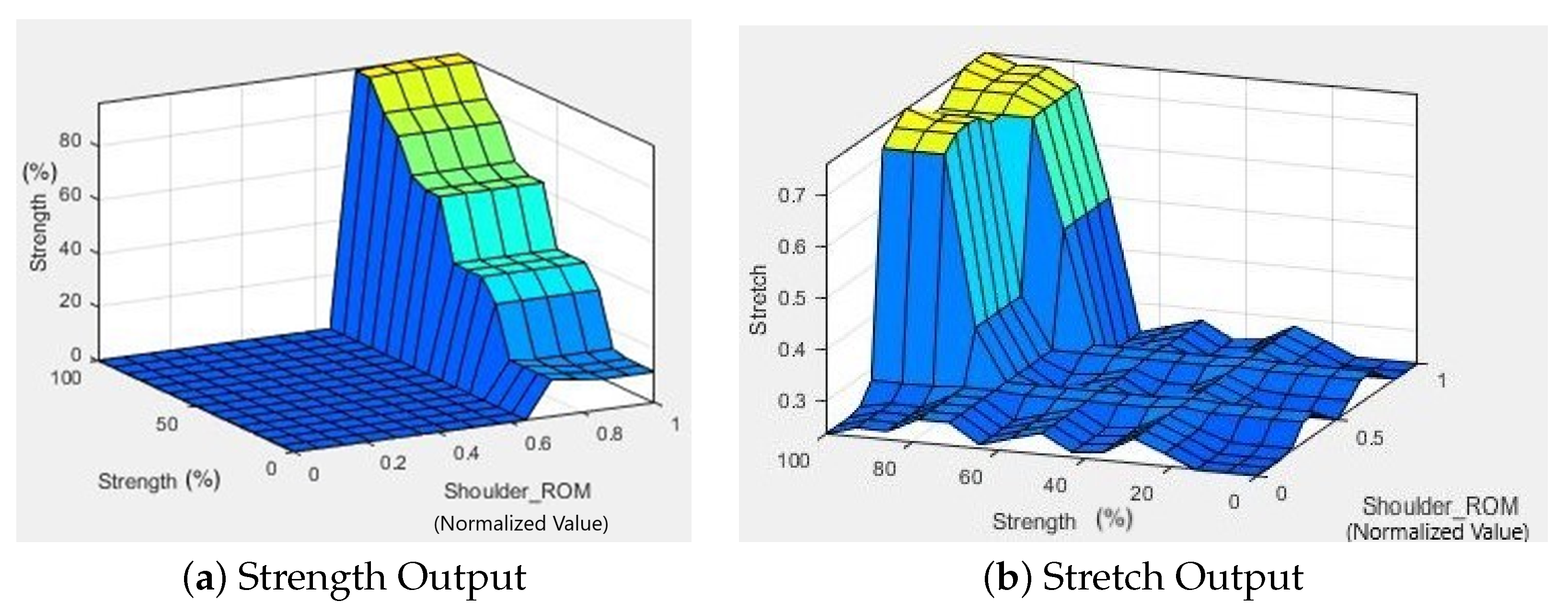
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ward, N.S. Restoring brain function after stroke—Bridging the gap between animals and humans. Nat. Rev. Neurol. 2017, 13, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Warlow, C. Stroke: Practical Management; Blackwell Pub: Malden, MA, USA, 2008. [Google Scholar]
- World Health Organization. The World Health Report 2003: Shaping The Future; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Langhorne, P.; Sandercock, P.; Prasad, K. Evidence-based practice for stroke. Lancet Neurol. 2009, 8, 308–309. [Google Scholar] [CrossRef]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. Neuroeng. Rehabil. 2014, 11, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Oña, E.D.; de la Cuerda, R.C.; Sánchez-Herrera, P.; Balaguer, C.; Jardón, A. A Review of Robotics in Neurorehabilitation: Towards an Automated Process for Upper Limb. J. Healthc. Eng. 2018, 2018, 9758939. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Disability; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Islam, M.R.; Spiewak, C.; Rahman, M.H.; Fareh, R. A brief review on robotic exoskeletons for upper extremity rehabilitation to find the gap between research porotype and commercial type. Adv. Robot. Autom. 2017, 6, 2. [Google Scholar] [CrossRef]
- Bertani, R.; Melegari, C.; Cola, M.C.D.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, G.; Cho, D.Y.; Kim, H.Y.; Lee, J.Y.; Kim, S.; Park, S.B.; Shin, J.H. Comparisons between end-effector and exoskeleton rehabilitation robots regarding upper extremity function among chronic stroke patients with moderate-to-severe upper limb impairment. Sci. Rep. 2020, 10, 1806. [Google Scholar] [CrossRef]
- Lum, P.S.; Burgar, C.G.; der Loos, M.V.; Shor, P.C.; Majmundar, M.; Yap, R. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: A follow-up study. J. Rehabil. Res. Dev. 2006, 43, 631. [Google Scholar] [CrossRef]
- Amirabdollahian, F.; Loureiro, R.; Gradwell, E.; Collin, C.; Harwin, W.; Johnson, G. Multivariate analysis of the Fugl-Meyer outcome measures assessing the effectiveness of GENTLE/S robot-mediated stroke therapy. J. Neuroeng. Rehabil. 2007, 4, 4. [Google Scholar] [CrossRef]
- Krebs, H.I.; Volpe, B.T.; Williams, D.; Celestino, J.; Charles, S.K.; Lynch, D.; Hogan, N. Robot-Aided Neurorehabilitation: A Robot for Wrist Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Reinkensmeyer, D.J.; Kahn, L.E.; Averbuch, M.; McKenna-Cole, A.; Schmit, B.D.; Rymer, W.Z. Understanding and treating arm movement impairment after chronic brain injury: Progress with the ARM guide. J. Rehabil. Res. Dev. 2000, 37, 653–662. [Google Scholar] [PubMed]
- Hesse, S.; Schulte-Tigges, G.; Konrad, M.; Bardeleben, A.; Werner, C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects11An organization with which 1 or more of the authors is associated has received or will receive financial benefits from a commercial party having a direct financial interest in the results of the research supporting this article. Arch. Phys. Med. Rehabil. 2003, 84, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, S.; Sun, Q. Development and Assist-As-Needed Control of an End-Effector Upper Limb Rehabilitation Robot. Appl. Sci. 2020, 10, 6684. [Google Scholar] [CrossRef]
- Eslami, M.; Mokhtarian, A.; Pirmoradian, M.; Seifzadeh, A.; Rafiaei, M. Design and fabrication of a passive upper limb rehabilitation robot with adjustable automatic balance based on variable mass of end-effector. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 629. [Google Scholar] [CrossRef]
- Meng, Q.; Jiao, Z.; Yu, H.; Zhang, W. Design and evaluation of a novel upper limb rehabilitation robot with space training based on an end effector. Mech. Sci. 2021, 12, 639–648. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Niu, B.; Zhang, Y.; Du, J.; Niu, J.; Sun, L. Mechanical Design and Analysis of the End-Effector Finger Rehabilitation Robot (EFRR) for Stroke Patients. Machines 2021, 9, 110. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Ji, L.; Bi, S.; Zhang, X.; Huo, J.; Ji, R. Development and Implementation of an End-Effector Upper Limb Rehabilitation Robot for Hemiplegic Patients with Line and Circle Tracking Training. J. Healthc. Eng. 2017, 2017, 4931217. [Google Scholar] [CrossRef]
- Sanchez, R.; Reinkensmeyer, D.; Shah, P.; Liu, J.; Rao, S.; Smith, R.; Cramer, S.; Rahman, T.; Bobrow, J. Monitoring functional arm movement for home-based therapy after stroke. In Proceedings of the the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar] [CrossRef]
- Pignolo, L.; Dolce, G.; Basta, G.; Lucca, L.; Serra, S.; Sannita, W. Upper limb rehabilitation after stroke: ARAMIS a “robo-mechatronic” innovative approach and prototype. In Proceedings of the 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob), Rome, Italy, 24–27 June 2012; pp. 1410–1414. [Google Scholar]
- Nef, T.; Mihelj, M.; Kiefer, G.; Perndl, C.; Muller, R.; Riener, R. ARMin-Exoskeleton for Arm Therapy in Stroke Patients. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007; pp. 68–74. [Google Scholar] [CrossRef]
- Sugar, T.; He, J.; Koeneman, E.; Koeneman, J.; Herman, R.; Huang, H.; Schultz, R.; Herring, D.; Wanberg, J.; Balasubramanian, S.; et al. Design and Control of RUPERT: A Device for Robotic Upper Extremity Repetitive Therapy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 336–346. [Google Scholar] [CrossRef]
- Kiguchi, K.; Rahman, M.H.; Sasaki, M.; Teramoto, K. Development of a 3DOF mobile exoskeleton robot for human upper-limb motion assist. Robot. Auton. Syst. 2008, 56, 678–691. [Google Scholar] [CrossRef]
- Rahman, M.H.; Rahman, M.J.; Cristobal, O.L.; Saad, M.; Kenné, J.P.; Archambault, P.S. Development of a whole arm wearable robotic exoskeleton for rehabilitation and to assist upper limb movements. Robotica 2014, 33, 19–39. [Google Scholar] [CrossRef]
- Islam, M.R.; Assad-Uz-Zaman, M.; Brahmi, B.; Bouteraa, Y.; Wang, I.; Rahman, M.H. Design and Development of an Upper Limb Rehabilitative Robot with Dual Functionality. Micromachines 2021, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, B.; Brahmi, A.; Saad, M.; Gauthier, G.; Rahman, M.H. Robust Adaptive Tracking Control of Uncertain Rehabilitation Exoskeleton Robot. J. Dyn. Syst. Meas. Control 2019, 141, 121007. [Google Scholar] [CrossRef]
- Brahmi, B.; Driscoll, M.; Bojairami, I.K.E.; Saad, M.; Brahmi, A. Novel adaptive impedance control for exoskeleton robot for rehabilitation using a nonlinear time-delay disturbance observer. ISA Trans. 2021, 108, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Casas, R.; Lum, P. An Elbow Exoskeleton for Upper Limb Rehabilitation With Series Elastic Actuator and Cable-Driven Differential. IEEE Trans. Robot. 2019, 35, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Lien, W.Y.; Chen, C.T.; Wu, Y.C. Implementation of an Upper-Limb Exoskeleton Robot Driven by Pneumatic Muscle Actuators for Rehabilitation. Actuators 2020, 9, 106. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, D.; Qian, W.; Xiao, X.; Guo, Z. Modeling and Control of a Cable-Driven Rotary Series Elastic Actuator for an Upper Limb Rehabilitation Robot. Front. Neurorobot. 2020, 14, 13. [Google Scholar] [CrossRef]
- Islam, M.R.; Assad-Uz-Zaman, M.; Rahman, M.H. Design and control of an ergonomic robotic shoulder for wearable exoskeleton robot for rehabilitation. Int. J. Dyn. Control 2019, 8, 312–325. [Google Scholar] [CrossRef]
- Haghshenas-Jaryani, M.; Patterson, R.M.; Bugnariu, N.; Wijesundara, M.B. A pilot study on the design and validation of a hybrid exoskeleton robotic device for hand rehabilitation. J. Hand Ther. 2020, 33, 198–208. [Google Scholar] [CrossRef]
- Otten, P.; Kim, J.; Son, S. A Framework to Automate Assessment of Upper-Limb Motor Function Impairment: A Feasibility Study. Sensors 2015, 15, 20097–20114. [Google Scholar] [CrossRef]
- Hobart, J.C.; Lamping, D.L.; Freeman, J.A.; Langdon, D.W.; McLellan, D.L.; Greenwood, R.J.; Thompson, A.J. Evidence-based measurement: Which disability scale for neurologic rehabilitation? Neurology 2001, 57, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.W.; Manuel, S.; Lloyd, A.; Gaasch, D. Development of an Exercise Expert System for Older Adults. Med. Sci. Sports Exerc. 1999, 31, S49. [Google Scholar] [CrossRef][Green Version]
- González, J.C.; Pulido, J.C.; Fernández, F. A three-layer planning architecture for the autonomous control of rehabilitation therapies based on social robots. Cogn. Syst. Res. 2017, 43, 232–249. [Google Scholar] [CrossRef]
- Schipor, O.; Geman, O.; Chiuchisan, I.; Covasa, M. From Fuzzy Expert System to Artificial Neural Network: Application to Assisted Speech Therapy. In Artificial Neural Networks-Models and Applications; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Ivanova, M.; Ilieva, R.; Lu, Z. Construction of Fuzzy-Classification Expert System in Cerebral Palsy for Learning Performance Facilitation. In Methodologies and Intelligent Systems for Technology Enhanced Learning, 10th International Conference. Workshops; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 5–14. [Google Scholar] [CrossRef]
- Kiguchi, K.; Rahman, M.; Yamaguchi, T. Adaptation Strategy for the 3DOF Exoskeleton for Upper-Limb Motion Assist. In Proceedings of the 2005 IEEE International Conference on Robotics and Automation, Barcelona, Spain, 18–22 April 2005. [Google Scholar] [CrossRef]
- Kiguchi, K.; Rahman, M.; Sasaki, M. Neuro-fuzzy based motion control of a robotic exoskeleton: Considering end-effector force vectors. In Proceedings of the Proceedings 2006 IEEE International Conference on Robotics and Automation, 2006. ICRA 2006, Orlando, FL, USA, 15–19 May 2006. [Google Scholar] [CrossRef]
- Negnevitsky, M. Artificial Intelligence: A guide to Intelligent Systems; Addison-Wesley: Harlow, UK; New York, NY, USA, 2005. [Google Scholar]
- Mamdani. Application of Fuzzy Logic to Approximate Reasoning Using Linguistic Synthesis. IEEE Trans. Comput. 1977, C-26, 1182–1191. [Google Scholar] [CrossRef]
- Doğan, M.; Koçak, M.; Kılınç, Ö.O.; Ayvat, F.; Sütçü, G.; Ayvat, E.; Kılınç, M.; Özgür, Ü.; Yıldırım, S.A. Functional range of motion in the upper extremity and trunk joints: Nine functional everyday tasks with inertial sensors. Gait Osture 2019, 70, 141–147. [Google Scholar] [CrossRef]
- Visser, J.; Mans, E.; de Visser, M.; van den Berg-Vos, R.; Franssen, H.; de Jong, J.; van den Berg, L.; Wokke, J.; de Haan, R. Comparison of maximal voluntary isometric contraction and hand-held dynamometry in measuring muscle strength of patients with progressive lower motor neuron syndrome. Neuromuscul. Disord. 2003, 13, 744–750. [Google Scholar] [CrossRef]
- Patten, C.; Lexell, J.; Brown, H.E. Weakness and strength training in persons with poststroke hemiplegia: Rationale, method, and efficacy. J. Rehabil. Res. Dev. 2004, 41, 293. [Google Scholar] [CrossRef]
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenbeg Lecture. Neurology 1980, 30, 1303. [Google Scholar] [CrossRef]
- Nam, K.E.; Lim, S.H.; Kim, J.S.; Hong, B.Y.; Jung, H.Y.; Lee, J.K.; Yoo, S.D.; Pyun, S.B.; Lee, K.M.; Lee, K.J.; et al. When does spasticity in the upper limb develop after a first stroke? A nationwide observational study on 861 stroke patients. J. Clin. Neurosci. 2019, 66, 144–148. [Google Scholar] [CrossRef]
- Siddique, T.; Marzooqi, R.A.; Alleem, H.R.; Fareh, R.; Baziyad, M.S.; Elsabe, A.Y.H. Evaluation of UE Exercises using NAO Robot for Poststroke Disabilities. In Proceedings of the 2022 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 21–24 February 2022. [Google Scholar] [CrossRef]

| Low Range | Medium Range | Advanced Range | |
|---|---|---|---|
| (Degrees) | (Degrees) | (Degrees) | |
| S. Extension | ROM < 30 | 30 < ROM < 38 | 38 < ROM < 50 |
| S. Flexion | ROM < 70 | 70 < ROM < 92 | 92 < ROM < 110 |
| S. Abduction | ROM < 45 | 45 < ROM < 90 | 90 < ROM < 110 |
| S. Medial Rotation | ROM < 45 | 45 < ROM < 64 | 64 < ROM < 70 |
| S. Lateral Rotation | ROM < 20 | 20 < ROM < 40 | 40 < ROM < 60 |
| Low Range | Medium Range | Advanced Range | |
|---|---|---|---|
| (Degrees) | (Degrees) | (Degrees) | |
| E. Flexion | ROM < 110 | 110 < ROM < 124 | 124 < ROM < 135 |
| E. Extension | ROM > 10 | 10 > ROM > 5 | 5 > ROM > 0 |
| Supination | ROM < 18 | 18 < ROM < 30 | 30 < ROM < 50 |
| Pronation | ROM < 13 | 13 < ROM < 30 | 30 < ROM < 50 |
| Low Range | Medium Range | Advanced Range | |
|---|---|---|---|
| (Degrees) | (Degrees) | (Degrees) | |
| W. Flexion | ROM < 20 | 20 < ROM < 30 | 30 < ROM < 50 |
| W. Extension | ROM < 30 | 30 < ROM < 40 | 40 < ROM < 60 |
| U. deviation | ROM < 15 | 15 < ROM < 19 | 19 < ROM < 25 |
| R. deviation | ROM < 10 | 10 < ROM < 18 | 18 < ROM < 20 |
| Linguistic Range | Strength Range in Percentage |
|---|---|
| Very Weak | 0–25% |
| Weak | 25–50% |
| Moderately Weak | 50–70% |
| Moderate Strength | 70–80% |
| Good Strength | 80–90% |
| Full Strength | 90–100% |
| Shoulder Flexion ROM | Shoulder Abduction ROM | Shoulder Internal Rotation ROM | Shoulder External Rotation ROM | Level of Exercise (Output) | |
|---|---|---|---|---|---|
| Case 1 | 38 | 29 | 35 | 10 | 0.1326 |
| Case 2 | 55 | 40 | 47 | 22 | 0.1529 |
| Case 3 | 71 | 34 | 50 | 25 | 0.1503 |
| Case 4 | 55 | 47 | 51 | 23 | 0.3255 |
| Case 5 | 88 | 59 | 62 | 37 | 0.5000 |
| Case 6 | 73 | 46 | 40 | 15 | 0.5000 |
| Case 7 | 93 | 78 | 70 | 41 | 0.5000 |
| Case 8 | 95 | 88 | 81 | 58 | 0.6920 |
| Case 9 | 97 | 91 | 85 | 61 | 0.8443 |
| Case 10 | 108 | 108 | 90 | 75 | 0.8722 |
| Case 11 | 130 | 180 | 100 | 80 | 0.5000 |
| Case 12 | 100 | 95 | 40 | 15 | 0.1498 |
| Case 13 | 60 | 100 | 65 | 50 | 0.1518 |
| Case 14 | 105 | 40 | 68 | 53 | 0.1518 |
| Case 15 | 85 | 105 | 70 | 55 | 0.5000 |
| Shoulder Flexion ROM | Shoulder Abduction ROM | Shoulder Internal Rotation ROM | Shoulder External Rotation ROM | Strength (Percentage) | Output Strength (Percentage) | Output Stretch | |
|---|---|---|---|---|---|---|---|
| Case 1 | 38 | 29 | 35 | 10 | 30 | 0 | 0.2781 |
| Case 2 | 55 | 40 | 47 | 22 | 85 | 0 | 0.2454 |
| Case 3 | 71 | 34 | 50 | 25 | 60 | 0 | 0.2474 |
| Case 4 | 55 | 47 | 51 | 23 | 70 | 0 | 0.2928 |
| Case 5 | 88 | 59 | 62 | 37 | 50 | 0 | 0.2781 |
| Case 6 | 73 | 46 | 40 | 15 | 25 | 0 | 0.2781 |
| Case 7 | 93 | 78 | 70 | 41 | 70 | 0 | 0.2735 |
| Case 8 | 95 | 88 | 81 | 58 | 42 | 40 | 0.2905 |
| Case 9 | 97 | 91 | 85 | 61 | 88 | 84 | 0.7163 |
| Case 10 | 108 | 108 | 90 | 75 | 92 | 95.1905 | 0.7328 |
| Case 11 | 130 | 180 | 100 | 80 | 100 | 0 | 0.7595 |
| Case 12 | 100 | 95 | 40 | 15 | 60 | 0 | 0.2474 |
| Case 13 | 60 | 100 | 65 | 50 | 75 | 0 | 0.2356 |
| Case 14 | 105 | 40 | 68 | 53 | 90 | 0 | 0.2814 |
| Case 15 | 85 | 105 | 70 | 55 | 30 | 0 | 0.2781 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, T.; Fareh, R.; Abdallah, M.; Ahmed, Z.; Rahman, M.H. Autonomous Exercise Generator for Upper Extremity Rehabilitation: A Fuzzy-Logic-Based Approach. Micromachines 2022, 13, 842. https://doi.org/10.3390/mi13060842
Siddique T, Fareh R, Abdallah M, Ahmed Z, Rahman MH. Autonomous Exercise Generator for Upper Extremity Rehabilitation: A Fuzzy-Logic-Based Approach. Micromachines. 2022; 13(6):842. https://doi.org/10.3390/mi13060842
Chicago/Turabian StyleSiddique, Tanjulee, Raouf Fareh, Mahmoud Abdallah, Zaina Ahmed, and Mohammad Habibur Rahman. 2022. "Autonomous Exercise Generator for Upper Extremity Rehabilitation: A Fuzzy-Logic-Based Approach" Micromachines 13, no. 6: 842. https://doi.org/10.3390/mi13060842
APA StyleSiddique, T., Fareh, R., Abdallah, M., Ahmed, Z., & Rahman, M. H. (2022). Autonomous Exercise Generator for Upper Extremity Rehabilitation: A Fuzzy-Logic-Based Approach. Micromachines, 13(6), 842. https://doi.org/10.3390/mi13060842








