Three-Dimensional Kidney-on-a-Chip Assessment of Contrast-Induced Kidney Injury: Osmolality and Viscosity
Abstract
:1. Introduction
2. Materials and Methods
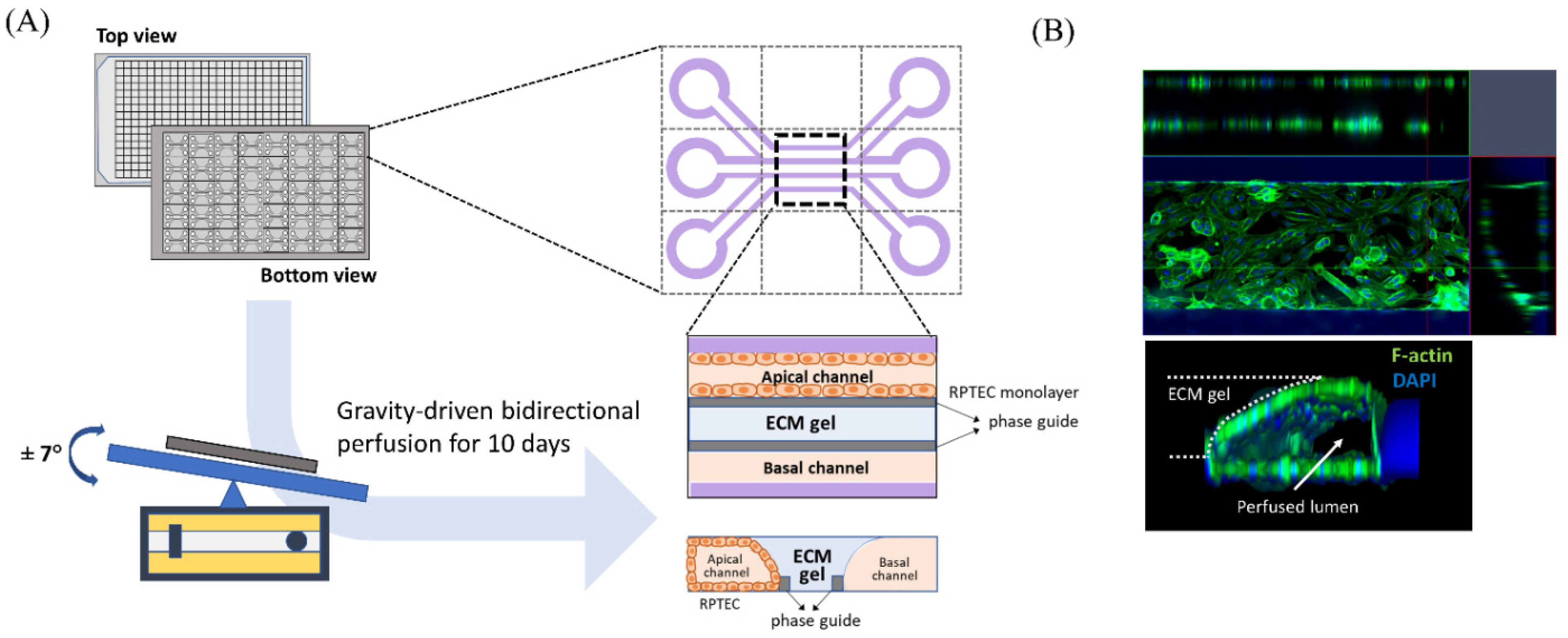
2.1. Cell Culture and Microfluid Device
2.2. Chemicals and Reagents
2.3. Experimental Model
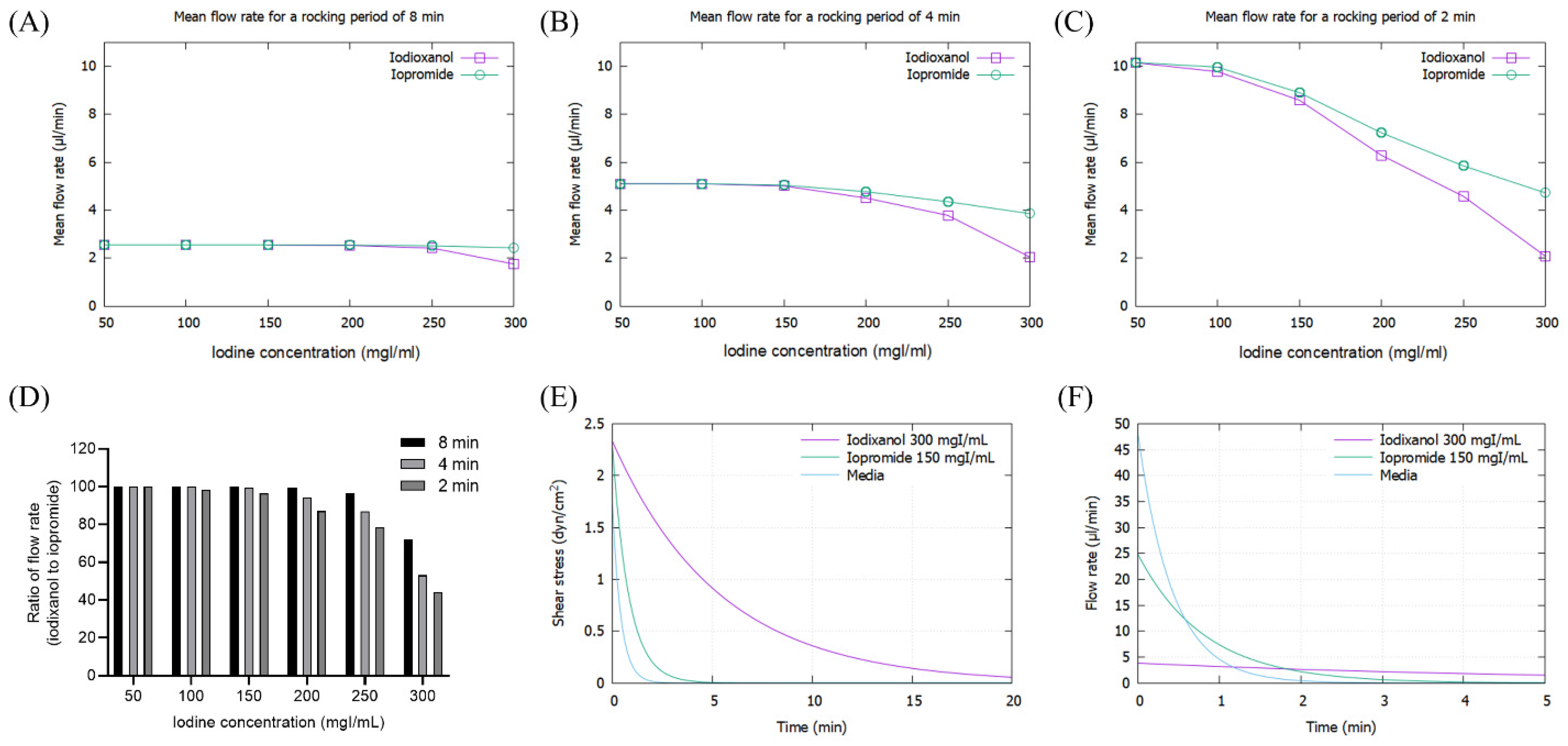
2.4. Mathematical Simulation
2.5. Image and Statistical Analysis
3. Results
3.1. Mathematical simulation
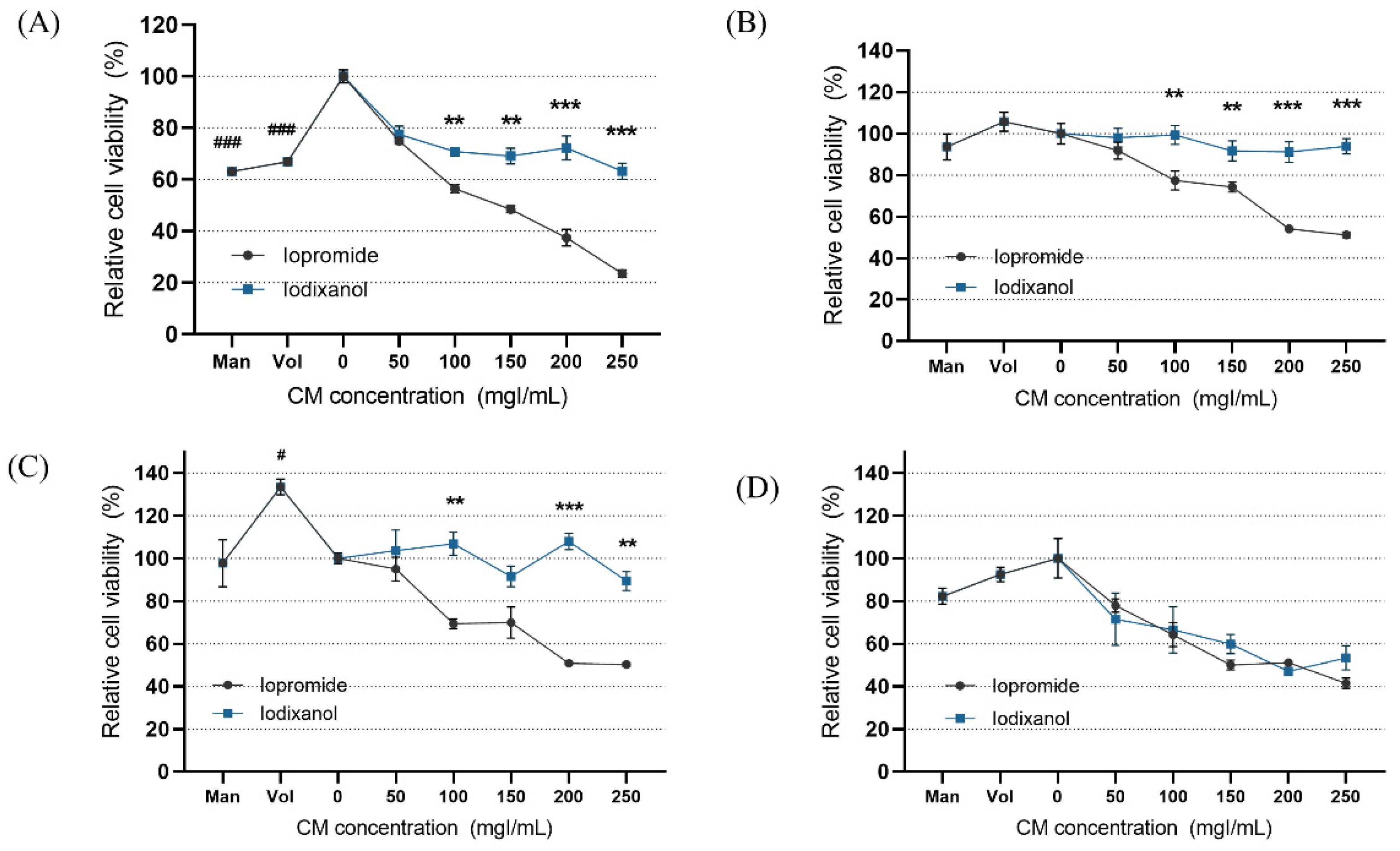
3.2. Modeling of Contrast-Induced Nephropathy in Kidney-on-a-Chip
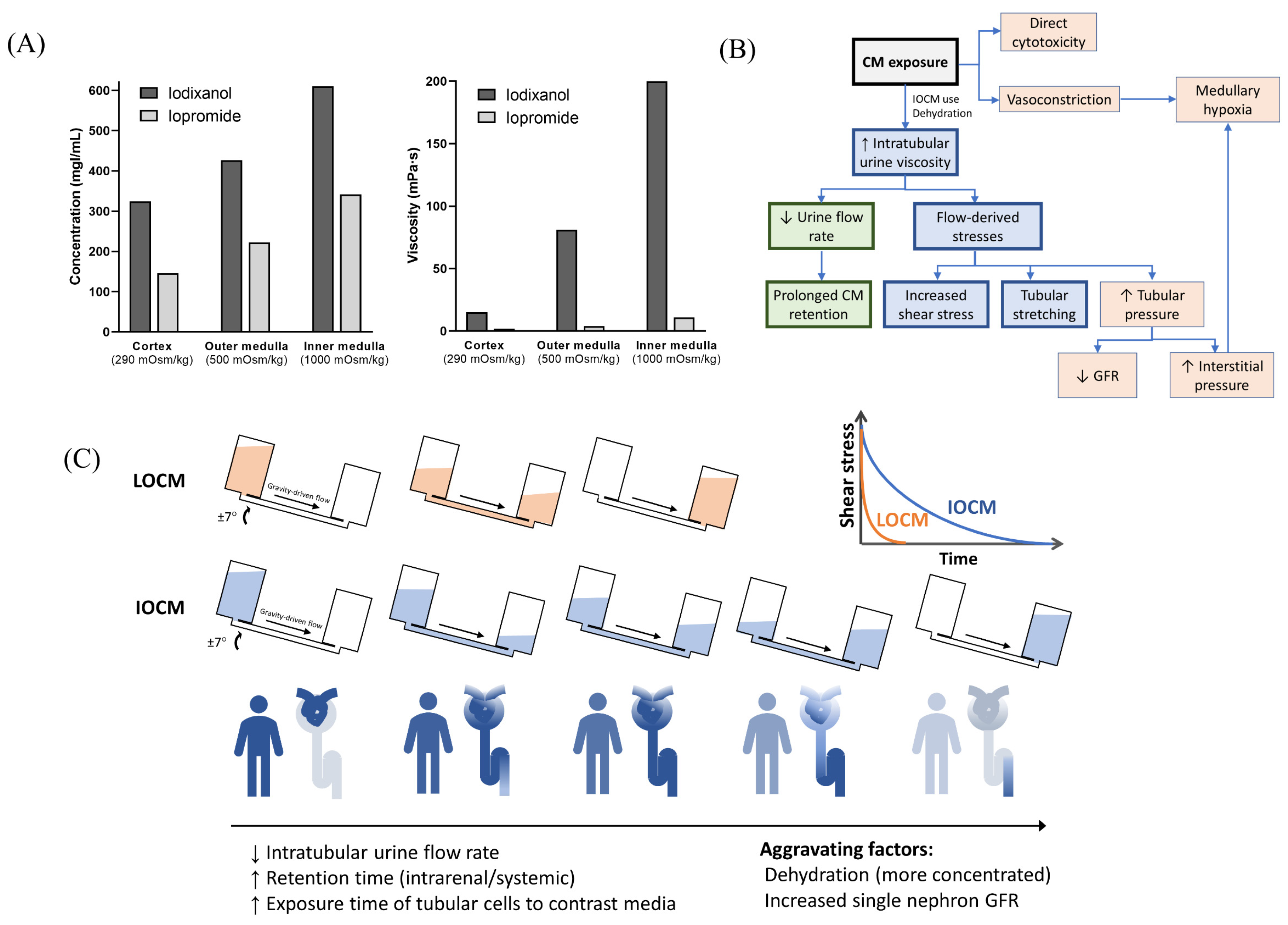
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nash, K.; Hafeez, A.; Hou, S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002, 39, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Kusirisin, P.; Chattipakorn, S.C.; Chattipakorn, N. Contrast-induced nephropathy and oxidative stress: Mechanistic insights for better interventional approaches. J. Transl. Med. 2020, 18, 400. [Google Scholar] [CrossRef]
- Mohammed, N.M.; Mahfouz, A.; Achkar, K.; Rafie, I.M.; Hajar, R. Contrast-induced Nephropathy. Heart Views 2013, 14, 106–116. [Google Scholar] [CrossRef]
- Barrett, B.J.; Carlisle, E.J. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology 1993, 188, 171–178. [Google Scholar] [CrossRef]
- Aspelin, P.; Aubry, P.; Fransson, S.G.; Strasser, R.; Willenbrock, R.; Berg, K.J.; NEPHRIC Study Investigators. Nephrotoxic effects in high-risk patients undergoing angiography. N. Engl. J. Med. 2003, 348, 491–499. [Google Scholar] [CrossRef]
- Jo, S.H.; Youn, T.J.; Koo, B.K.; Park, J.S.; Kang, H.J.; Cho, Y.S.; Chung, W.Y.; Joo, G.W.; Chae, I.H.; Choi, D.J.; et al. Renal toxicity evaluation and comparison between visipaque (iodixanol) and hexabrix (ioxaglate) in patients with renal insufficiency undergoing coronary angiography: The RECOVER study: A randomized controlled trial. J. Am. Coll. Cardiol. 2006, 48, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Eng, J.; Wilson, R.F.; Subramaniam, R.M.; Zhang, A.; Suarez-Cuervo, C.; Turban, S.; Choi, M.J.; Sherrod, C.; Hutfless, S.; Iyoha, E.E.; et al. Comparative Effect of Contrast Media Type on the Incidence of Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 417–424. [Google Scholar] [CrossRef]
- From, A.M.; Al Badarin, F.J.; McDonald, F.S.; Bartholmai, B.J.; Cha, S.S.; Rihal, C.S. Iodixanol versus low-osmolar contrast media for prevention of contrast induced nephropathy: Meta-analysis of randomized, controlled trials. Circ. Cardiovasc. Interv. 2010, 3, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Fahling, M.; Seeliger, E.; Patzak, A.; Persson, P.B. Understanding and preventing contrast-induced acute kidney injury. Nat. Rev. Nephrol. 2017, 13, 169–180. [Google Scholar] [CrossRef]
- Netti, G.S.; Prattichizzo, C.; Montemurno, E.; Simone, S.; Cafiero, C.; Rascio, F.; Stallone, G.; Ranieri, E.; Grandaliano, G.; Gesualdo, L. Exposure to low- vs iso-osmolar contrast agents reduces NADPH-dependent reactive oxygen species generation in a cellular model of renal injury. Free Radic. Biol. Med. 2014, 68, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lakhal, K.; Ehrmann, S.; Robert-Edan, V. Iodinated contrast medium: Is there a re(n)al problem? A clinical vignette-based review. Crit. Care 2020, 24, 641. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Kim, S. Kidney on chips. Methods Cell Biol. 2018, 146, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Suh, K.Y. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 2010, 10, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gotoh, N.; Yan, Q.; Du, Z.; Weinstein, A.M.; Wang, T.; Weinbaum, S. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 11418–11423. [Google Scholar] [CrossRef] [Green Version]
- Miravete, M.; Klein, J.; Besse-Patin, A.; Gonzalez, J.; Pecher, C.; Bascands, J.L.; Mercier-Bonin, M.; Schanstra, J.P.; Buffin-Meyer, B. Renal tubular fluid shear stress promotes endothelial cell activation. Biochem. Biophys. Res. Commun. 2011, 407, 813–817. [Google Scholar] [CrossRef]
- Maggiorani, D.; Dissard, R.; Belloy, M.; Saulnier-Blache, J.S.; Casemayou, A.; Ducasse, L.; Gres, S.; Belliere, J.; Caubet, C.; Bascands, J.L.; et al. Shear Stress-Induced Alteration of Epithelial Organization in Human Renal Tubular Cells. PLoS ONE 2015, 10, e0131416. [Google Scholar] [CrossRef]
- Vormann, M.K.; Gijzen, L.; Hutter, S.; Boot, L.; Nicolas, A.; van den Heuvel, A.; Vriend, J.; Ng, C.P.; Nieskens, T.T.G.; van Duinen, V.; et al. Nephrotoxicity and Kidney Transport Assessment on 3D Perfused Proximal Tubules. AAPS J. 2018, 20, 90. [Google Scholar] [CrossRef]
- Denic, A.; Mathew, J.; Lerman, L.O.; Lieske, J.C.; Larson, J.J.; Alexander, M.P.; Poggio, E.; Glassock, R.J.; Rule, A.D. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N. Engl. J. Med. 2017, 376, 2349–2357. [Google Scholar] [CrossRef]
- Liebich, H.-G. Funktionelle Histologie der Haussäugetiere und Vögel: Lehrbuch und Farbatlas fur Studium und Praxis; Schattauer: Stuttgart, Germany, 2010. [Google Scholar]
- Ganong, W.F. Review of Medical Physiology; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Lote, C.J. Principles of Renal Physiology; Springer: New York, NY, USA, 2012. [Google Scholar]
- Fattah, H.; Layton, A.; Vallon, V. How Do Kidneys Adapt to a Deficit or Loss in Nephron Number? Physiology 2019, 34, 189–197. [Google Scholar] [CrossRef]
- Kaufman, J.M.; DiMeola, H.J.; Siegel, N.J.; Lytton, B.; Kashgarian, M.; Hayslett, J.P. Compensatory adaptation of structure and function following progressive renal ablation. Kidney Int. 1974, 6, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, A.; Faga, T.; Pisani, A.; Riccio, E.; Bramanti, P.; Sabbatini, M.; Navarra, M.; Andreucci, M. Molecular mechanisms of renal cellular nephrotoxicity due to radiocontrast media. BioMed. Res. Int. 2014, 2014, 249810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, G.; Lengsfeld, P.; Lenhard, D.C.; Pietsch, H.; Hutter, J.; Sieber, M.A. Viscosity of iodinated contrast agents during renal excretion. Eur. J. Radiol. 2011, 80, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.A. Nephroscreen: A diagnostic test for predicting acute renal failure? Expert Rev. Mol. Diagn. 2005, 5, 633–642. [Google Scholar] [CrossRef]
- Grabias, B.M.; Konstantopoulos, K. The physical basis of renal fibrosis: Effects of altered hydrodynamic forces on kidney homeostasis. Am. J. Physiol. Ren. Physiol. 2014, 306, F473–F485. [Google Scholar] [CrossRef]
- Cho, E.; Ko, G.J. The Pathophysiology and the Management of Radiocontrast-Induced Nephropathy. Diagnostics 2022, 12, 180. [Google Scholar] [CrossRef]
- Liang, R.; Zhao, Q.; Jian, G.; Cheng, D.; Wang, N.; Zhang, G.; Wang, F. Tanshinone IIA Attenuates Contrast-Induced Nephropathy via Nrf2 Activation in Rats. Cell. Physiol. Biochem. 2018, 46, 2616–2623. [Google Scholar] [CrossRef]
- Zhao, Z.; Liao, G.; Zhou, Q.; Lv, D.; Holthfer, H.; Zou, H. Sulforaphane Attenuates Contrast-Induced Nephropathy in Rats via Nrf2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2016, 2016, 9825623. [Google Scholar] [CrossRef]
- Sendeski, M.M. Pathophysiology of renal tissue damage by iodinated contrast media. Clin. Exp. Pharmacol. Physiol. 2011, 38, 292–299. [Google Scholar] [CrossRef]
- Washino, S.; Hosohata, K.; Miyagawa, T. Roles Played by Biomarkers of Kidney Injury in Patients with Upper Urinary Tract Obstruction. Int. J. Mol. Sci. 2020, 21, 5490. [Google Scholar] [CrossRef]
- Garvin, J.L.; Hong, N.J. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 2008, 51, 488–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardiek, K.; Katholi, R.E.; Ramkumar, V.; Deitrick, C. Proximal tubule cell response to radiographic contrast media. Am. J. Physiol. Ren. Physiol. 2001, 280, F61–F70. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.J. Nephrotoxicity related to contrast media. Scand. J. Urol. Nephrol. 2000, 34, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Nygren, A.; Sjöquist, M.; Jacobsson, E.; Ulfendahl, H.R.; Araki, Y. Iodine concentrations in the rat kidney measured by X-ray microanalysis. Acta Radiol. 1998, 39, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Nygren, A.; Hansell, P.; Erikson, U. Influence of contrast media on single nephron glomerular filtration rate in rat kidney. A comparison between diatrizoate, iohexol, ioxaglate, and iotrolan. Acta Radiol. 1992, 33, 596–599. [Google Scholar] [CrossRef]
- Seeliger, E.; Flemming, B.; Wronski, T.; Ladwig, M.; Arakelyan, K.; Godes, M.; Mockel, M.; Persson, P.B. Viscosity of contrast media perturbs renal hemodynamics. J. Am. Soc. Nephrol. 2007, 18, 2912–2920. [Google Scholar] [CrossRef] [Green Version]
- Love, L.; Johnson, M.S.; Bresler, M.E.; Nelson, J.E.; Olson, M.C.; Flisak, M.E. The persistent computed tomography nephrogram: Its significance in the diagnosis of contrast-associated nephrotoxicity. Br. J. Radiol. 1994, 67, 951–957. [Google Scholar] [CrossRef]
- Widmann, G.; Bale, R.; Ulmer, H.; Putzer, D.; Schullian, P.; Wiedermann, F.J.; Lederer, W. Systemic Hypotension Following Intravenous Administration of Nonionic Contrast Medium during Computed Tomography: Iopromide Versus Iodixanol. Anesth. Analg. 2018, 126, 769–775. [Google Scholar] [CrossRef]
- Alexander, L.D.; Alagarsamy, S.; Douglas, J.G. Cyclic stretch-induced cPLA2 mediates ERK 1/2 signaling in rabbit proximal tubule cells. Kidney Int. 2004, 65, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Andreucci, M.; Fuiano, G.; Presta, P.; Esposito, P.; Faga, T.; Bisesti, V.; Procino, A.; Altieri, V.; Tozzo, C.; Memoli, B.; et al. Radiocontrast media cause dephosphorylation of Akt and downstream signaling targets in human renal proximal tubular cells. Biochem. Pharmacol. 2006, 72, 1334–1342. [Google Scholar] [CrossRef]
- Du, M.; Jiang, L.; Tang, X.; Gao, Z.; Xu, B.; Yuan, J. Contrast Induced Nephropathy and 2-Year Outcomes of Iso-Osmolar Compared with Low-Osmolar Contrast Media after Elective Percutaneous Coronary Intervention. Korean Circ. J. 2021, 51, 174–181. [Google Scholar] [CrossRef] [PubMed]


| Iodixanol (Visipaque™) | Iopromide (Ultravist™) | 6%HES (VOLULYTE®) | 15% Mannitol | Negative Control (Culture Media) | |
|---|---|---|---|---|---|
| Iodine concentration (mgI/dL) | 320 | 290 | - | - | - |
| Osmolality (mOsm/kg) | 290 | 607 | 283 | 823 | 288 |
| Viscosity (cP, at 37 °C) | 11.8 | 4.9 | 2 | 1 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Jeong, B.; Lee, Y.-M.; Son, H.-E.; Ryu, J.-Y.; Park, S.; Jeong, J.C.; Chin, H.J.; Kim, S. Three-Dimensional Kidney-on-a-Chip Assessment of Contrast-Induced Kidney Injury: Osmolality and Viscosity. Micromachines 2022, 13, 688. https://doi.org/10.3390/mi13050688
Kim K, Jeong B, Lee Y-M, Son H-E, Ryu J-Y, Park S, Jeong JC, Chin HJ, Kim S. Three-Dimensional Kidney-on-a-Chip Assessment of Contrast-Induced Kidney Injury: Osmolality and Viscosity. Micromachines. 2022; 13(5):688. https://doi.org/10.3390/mi13050688
Chicago/Turabian StyleKim, Kipyo, Beomgyun Jeong, Yun-Mi Lee, Hyung-Eun Son, Ji-Young Ryu, Seokwoo Park, Jong Cheol Jeong, Ho Jun Chin, and Sejoong Kim. 2022. "Three-Dimensional Kidney-on-a-Chip Assessment of Contrast-Induced Kidney Injury: Osmolality and Viscosity" Micromachines 13, no. 5: 688. https://doi.org/10.3390/mi13050688
APA StyleKim, K., Jeong, B., Lee, Y.-M., Son, H.-E., Ryu, J.-Y., Park, S., Jeong, J. C., Chin, H. J., & Kim, S. (2022). Three-Dimensional Kidney-on-a-Chip Assessment of Contrast-Induced Kidney Injury: Osmolality and Viscosity. Micromachines, 13(5), 688. https://doi.org/10.3390/mi13050688







