Advances in Assistive Electronic Device Solutions for Urology
Abstract
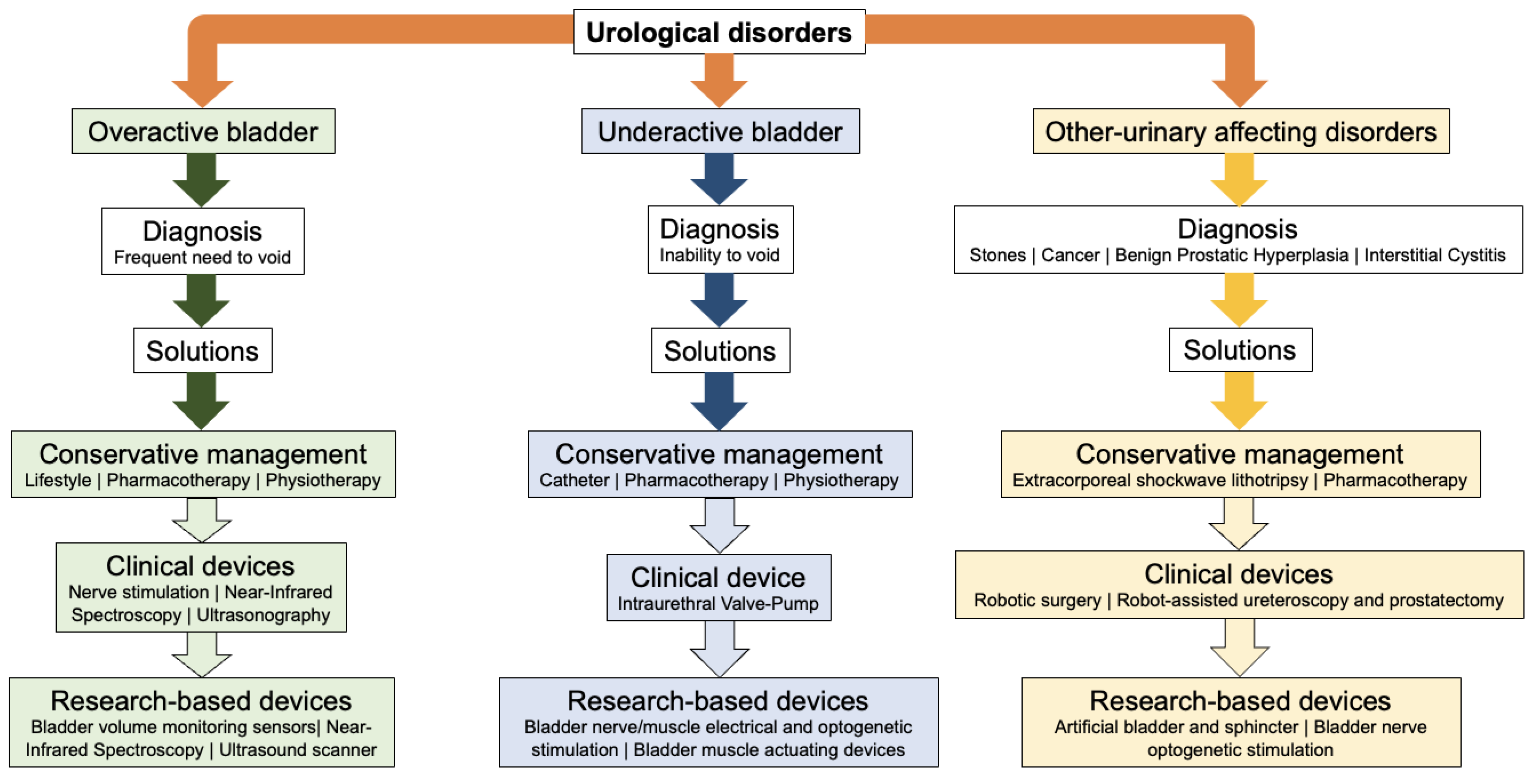
1. Introduction
2. Methodology
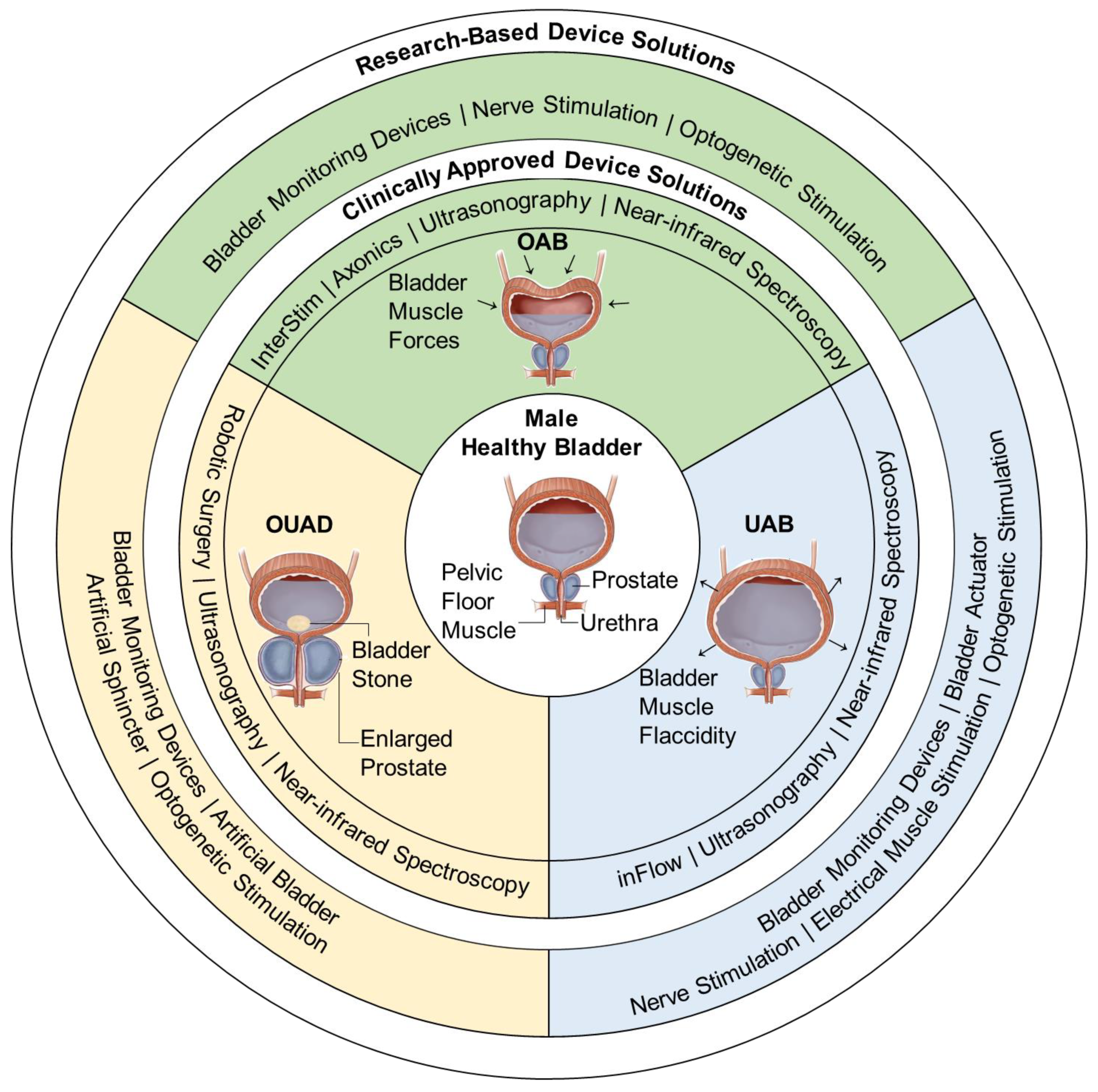
3. Overactive Bladder
3.1. Diagnosis
3.2. Clinically Approved Management Strategies
3.2.1. Conservative Management Strategies
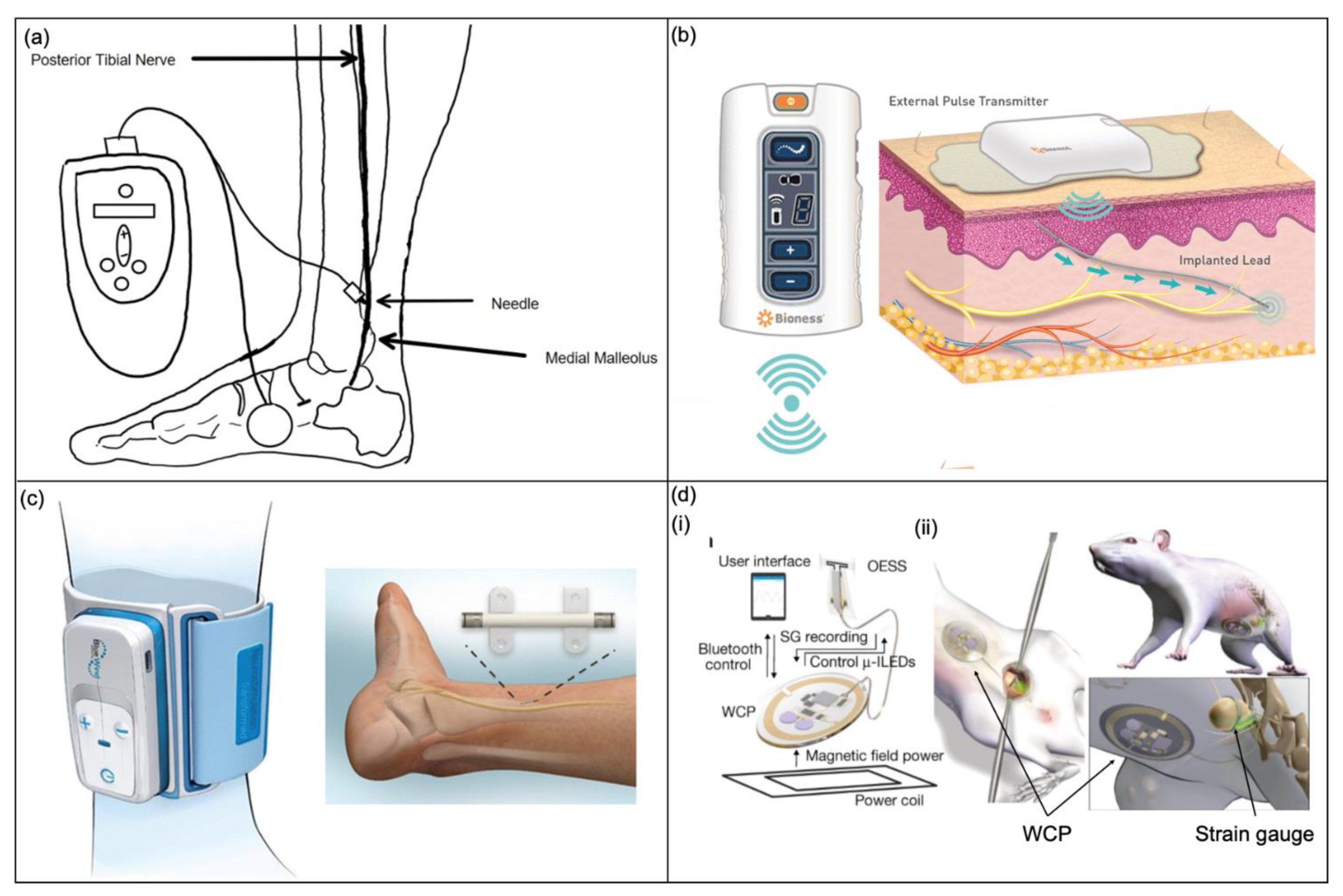
3.2.2. Nerve Stimulation Devices
InterStim
Axonics Modulation Technologies
3.2.3. Ultrasonography Systems
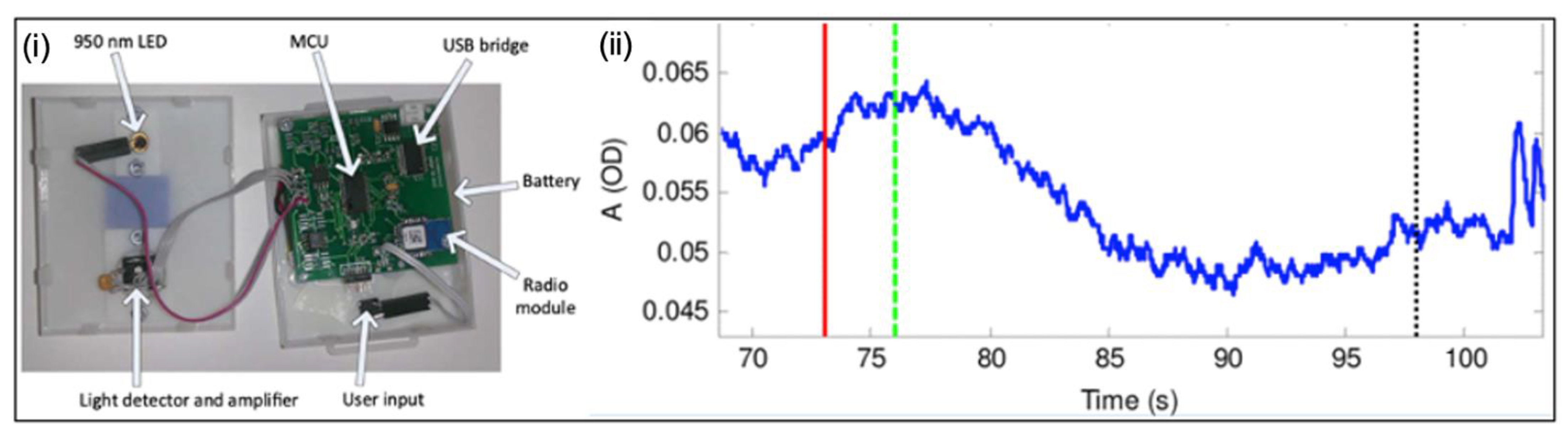
3.2.4. Near-Infrared Spectroscopy Systems
3.3. Research-Based Solutions
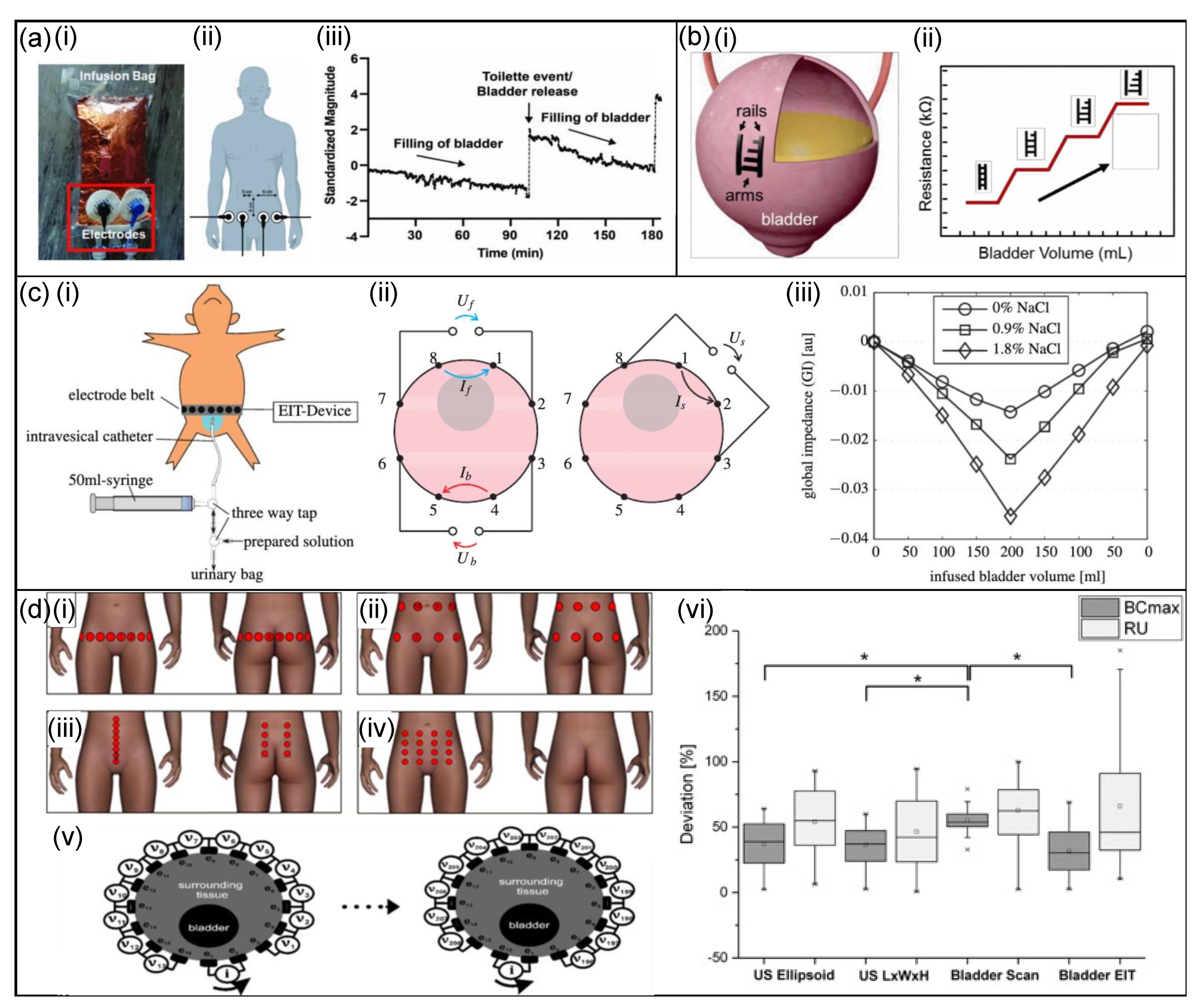
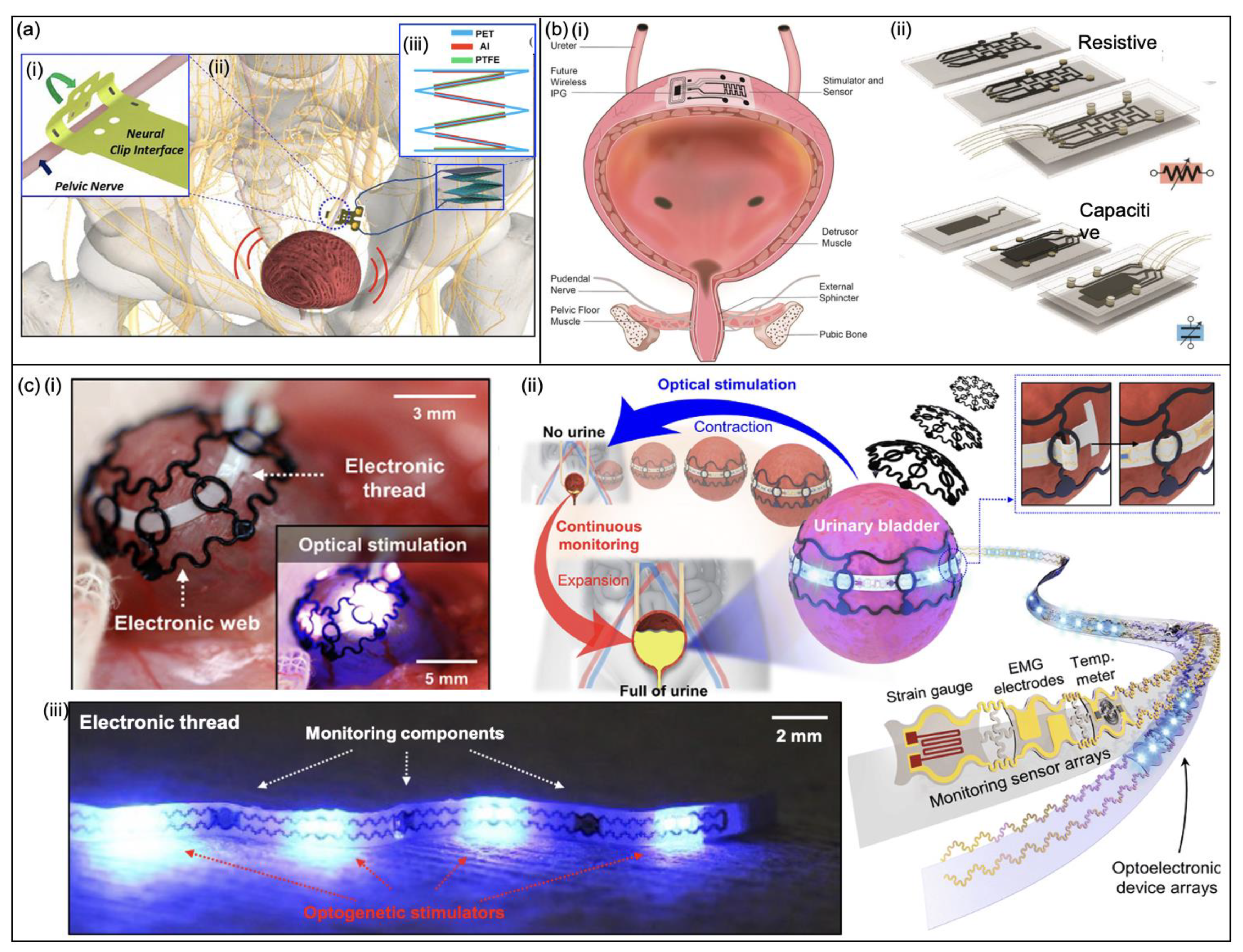
3.3.1. Bladder-Monitoring Devices
Near-Infrared Spectroscopy Systems
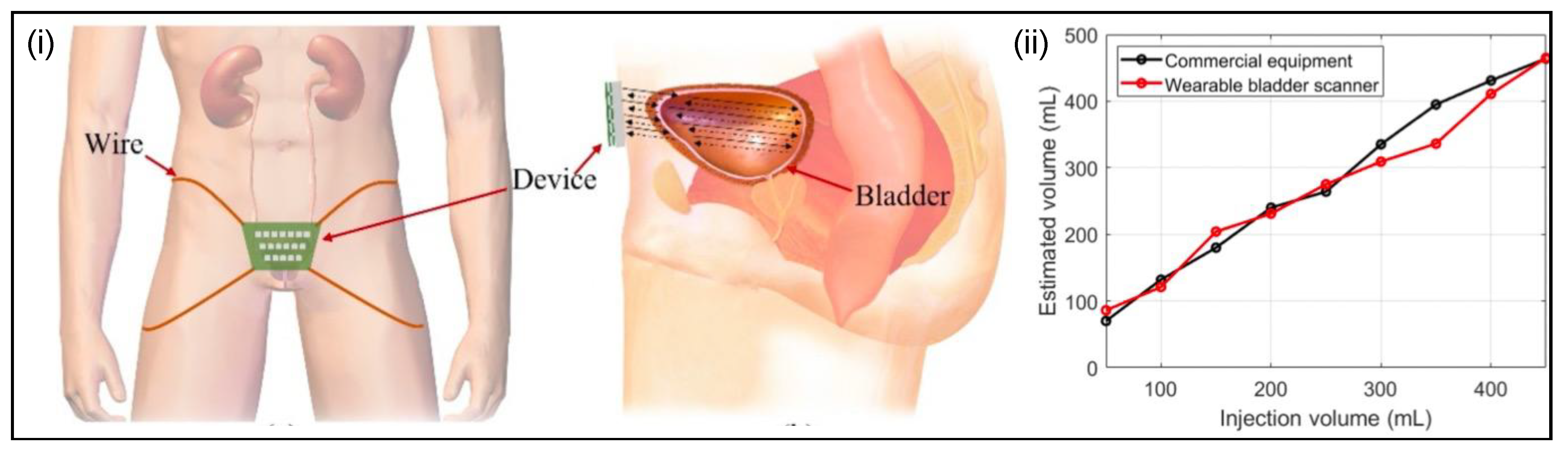
Ultrasound Scanner Systems
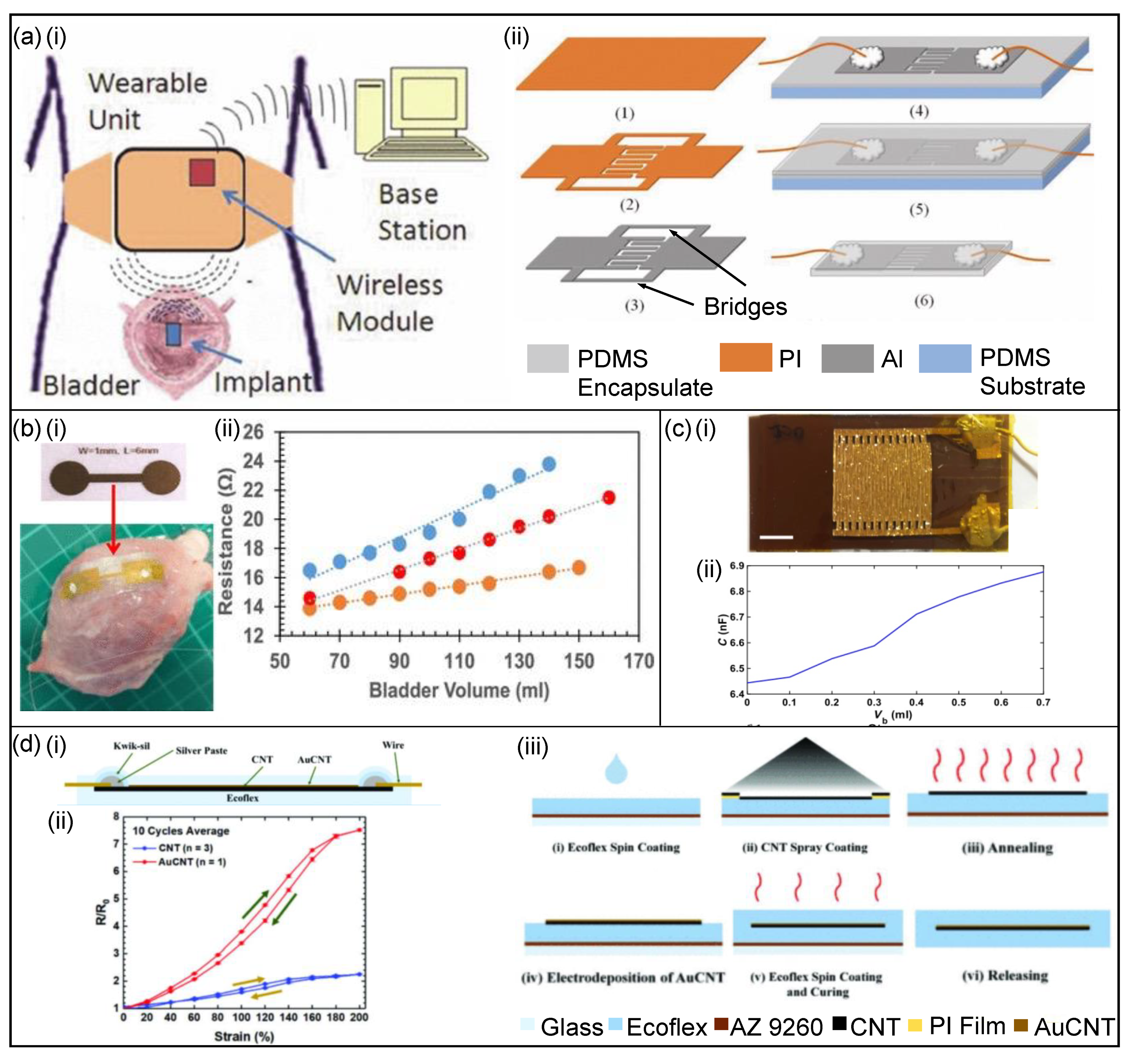
Bioimpedance Sensors
Pressure Sensors
Capacitance-Based Sensors
Strain Sensors
3.3.2. Stimulation Devices
Nerve Stimulation
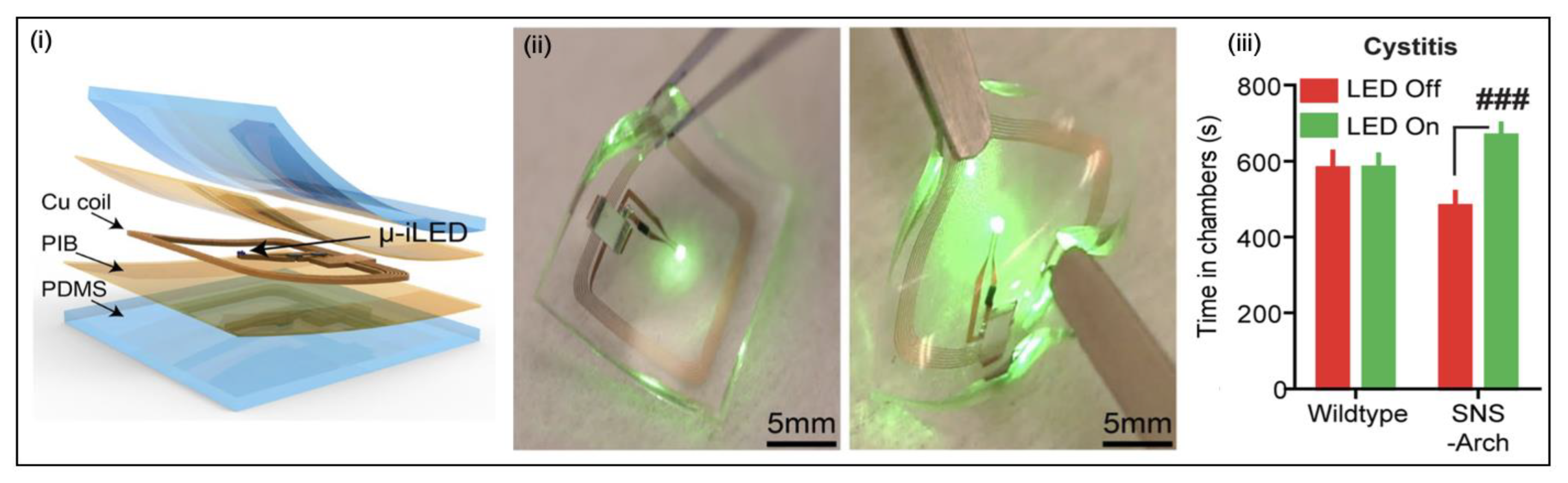
Optogenetic Stimulation
4. Underactive Bladder
4.1. Diagnosis
4.2. Clinically Approved Management Strategies
4.2.1. Conservative Management Strategies
4.2.2. Inflow™ Intraurethral Valve Pump
4.3. Research-Based Solutions
4.3.1. Stimulation Devices
Nerve Stimulation
Electrical Muscle Stimulation
Optogenetic Stimulation
4.3.2. Prosthetic Devices
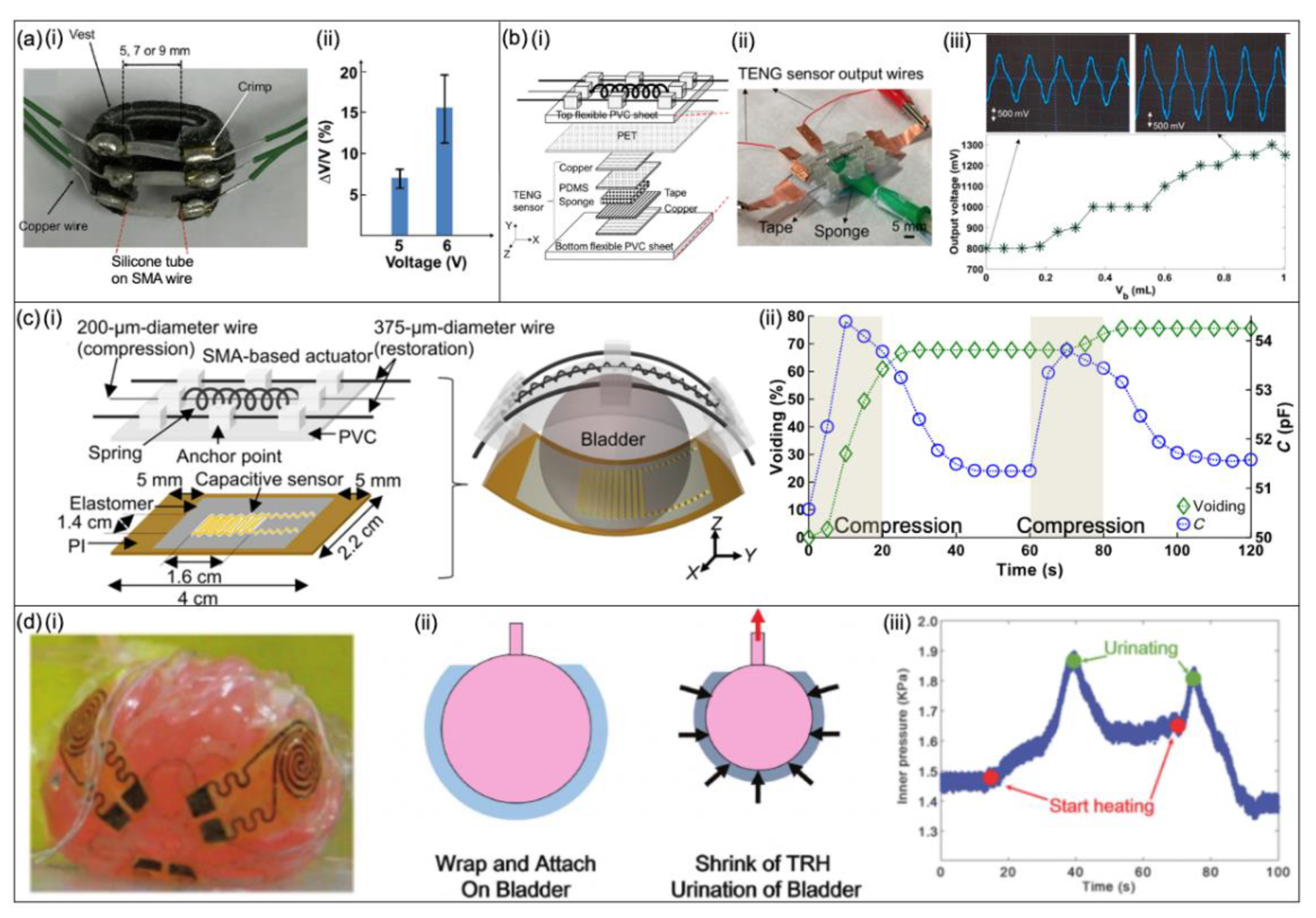
Shape Memory Alloy-Based Bladder Actuators
Hydrogel-Based Devices
5. Other-Urinary-Affecting-Disorders
5.1. Diagnosis
5.1.1. Kidney and Ureteral Stones
5.1.2. Cancer
5.1.3. Benign Prostatic Hyperplasia
5.1.4. Interstitial Cystitis
5.2. Clinically Approved Management Strategies
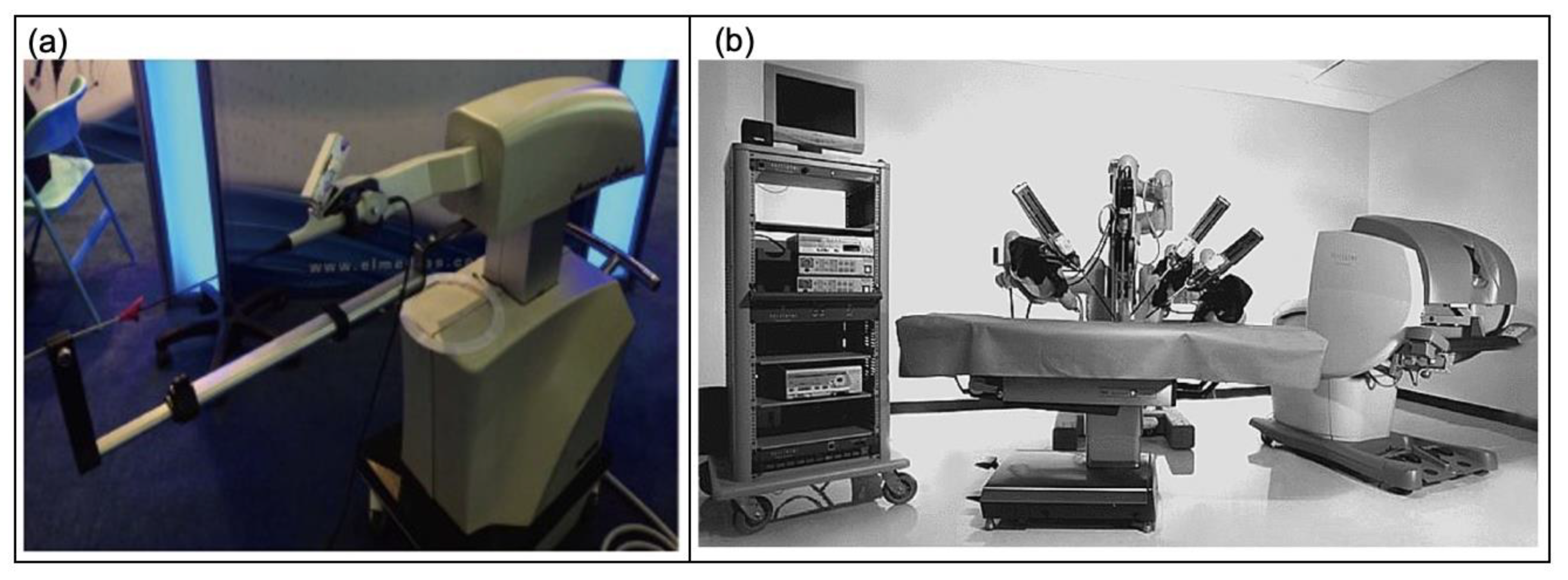
5.2.1. Robotic Surgery
5.2.2. Robot-Assisted Ureteroscopy
5.2.3. Robot-Assisted Prostatectomy
5.3. Research-Based Solutions
5.3.1. Prosthetic Devices
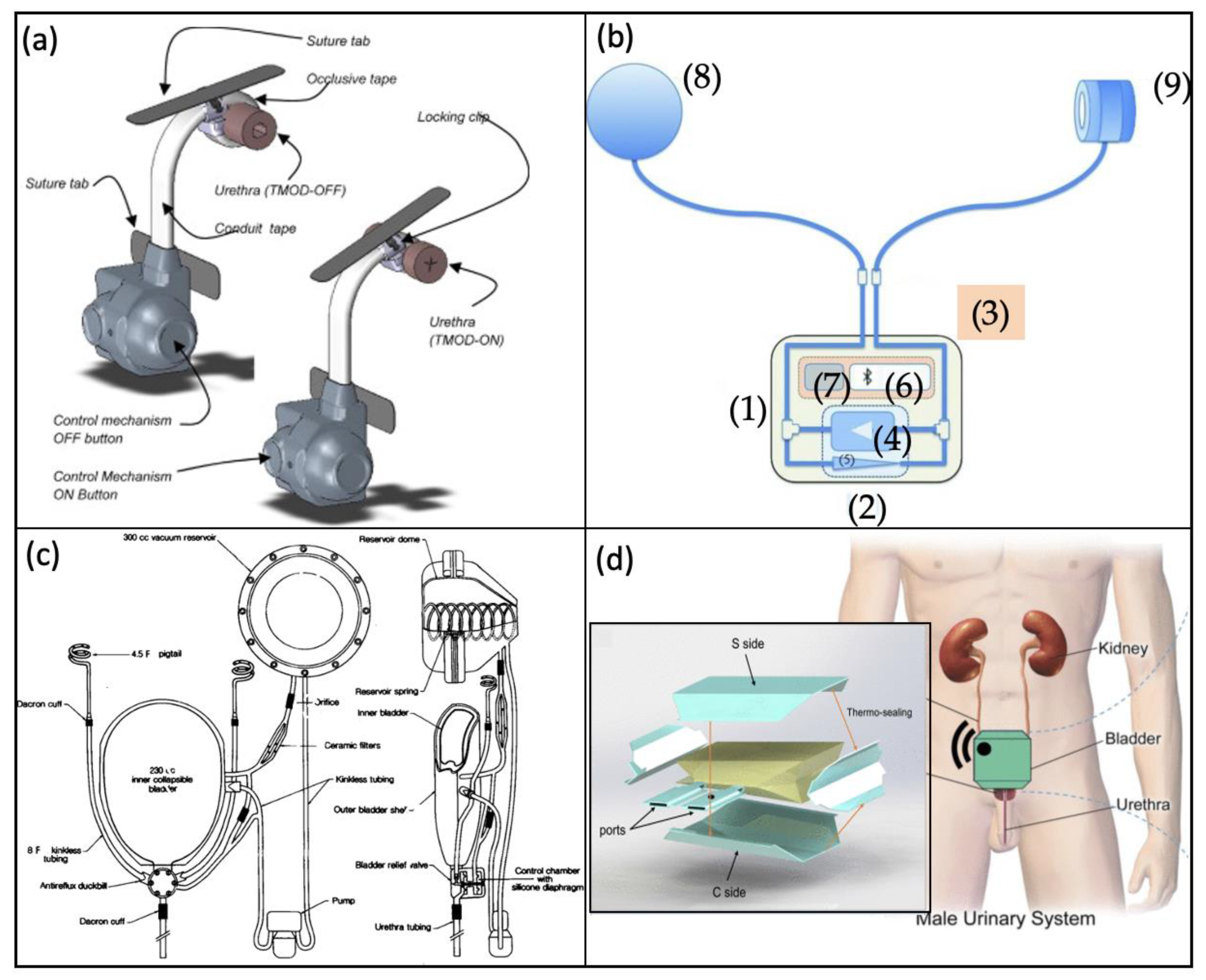
Artificial Urinary Sphincter
Artificial Bladder
5.3.2. Stimulation Devices
Optogenetic Stimulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harrison, S. Urology: GIRFT Programme National Speciality Report; GIRFT/NHS Improvement: London, UK, 2018; Available online: https://gettingitrightfirsttime.co.uk/wp-content/uploads/2018/07/Urology-June18-M.pdf (accessed on 1 February 2022).
- Aboumarzouk, O.M. Blandy’s Urology, 3rd ed.; John Wiley & Sons, Incorporated: Newark, UK, 2019. [Google Scholar]
- Parsons, J.K.; Eifler, J.B.; Han, M. Handbook of Urology, 1st ed.; John Wiley & Sons, Incorporated: Hoboken, UK, 2013. [Google Scholar]
- Bin Lim, K. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef]
- Leron, E.; Weintraub, A.Y.; Mastrolia, S.A.; Schwarzman, P. Overactive Bladder Syndrome: Evaluation and Management. Curr. Urol. 2018, 11, 117–125. [Google Scholar] [CrossRef]
- Van Dijk, M.M.; Wijkstra, H.; Debruyne, F.M.; De La Rosette, J.J.; Michel, M.C. The role of nocturia in the quality of life of men with lower urinary tract symptoms. Br. J. Urol. 2010, 105, 1141–1146. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Whitehurst, L.A.; Cook, P.; Somani, B. Kidney stone disease: An update on its management in primary care. Br. J. Gen. Pract. 2020, 70, 205–206. [Google Scholar] [CrossRef]
- Giarenis, I.; Cardozo, L. Managing urinary incontinence: What works? Climacteric 2014, 17, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Nguyen, L.; Sirls, L.; Peters, K. Current best practice management of interstitial cystitis/bladder pain syndrome. Ther. Adv. Urol. 2018, 10, 197–211. [Google Scholar] [CrossRef]
- Chen, F.-Z.; Zhao, X.-K. Prostate Cancer: Current Treatment and Prevention Strategies. Iran. Red Crescent Med. J. 2013, 15, 279–284. [Google Scholar] [CrossRef]
- Stovsky, M.D.; Rhee, K.; Hartke, D. Medical therapy versus surgery and minimally invasive surgical therapies for lower urinary tract symptoms and benign prostatic hyperplasia: What makes better economic sense? Curr. Urol. Rep. 2007, 8, 289–297. [Google Scholar] [CrossRef]
- NHS. Bladder Cancer-Overview. Bladder Cancer. 2021. Available online: https://www.nhs.uk/conditions/bladder-cancer/ (accessed on 5 October 2021).
- Irwin, D.E.; Milsom, I.; Kopp, Z.; Abrams, P.; Cardozo, L. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. Br. J. Urol. 2005, 97, 96–100. [Google Scholar] [CrossRef]
- Chung, K.J.; Han, A.N.Y.; Kim, K.H. Recommendations to the primary care practitioners and the patients for managing pelvic pain, especially in painful bladder syndrome for early and better prognosis. J. Exerc. Rehabil. 2015, 11, 251–254. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 175628721983217. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.W.; English, K.; Agalliu, I.; Leegant, A.; Abraham, N. Racial and Ethnic Differences in Urodynamic Parameters in Women With Overactive Bladder Symptoms. Female Pelvic Med. Reconstr. Surg. 2020, 26, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Eeden, S.K.V.D.; Shan, J.; Jacobsen, S.; Aaronsen, D.; Haque, R.; Quinn, V.P.; Quesenberry, C.P.; Project, U.D.I.A. Evaluating Racial/Ethnic Disparities in Lower Urinary Tract Symptoms in Men. J. Urol. 2012, 187, 185–189. [Google Scholar] [CrossRef][Green Version]
- Akbar, A.; Liu, K.; Michos, E.D.; Brubaker, L.; Markossian, T.; Bancks, M.P.; Kramer, H. Racial Differences in Urinary Incontinence Prevalence, Overactive Bladder and Associated Bother among Men: The Multi-Ethnic Study of Atherosclerosis. J. Urol. 2021, 205, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, O.; Dmochowski, R.R. Underactive bladder: A review of the current treatment concepts. Türk Urol. Derg. Turk. J. Urol. 2019, 45, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Santos-Pereira, M.; Charrua, A. Understanding underactive bladder: A review of the contemporary literature. Porto Biomed. J. 2020, 5, e070. [Google Scholar] [CrossRef]
- Geller, E.; Dieter, A.; Willis-Gray, M. Evaluation and management of overactive bladder: Strategies for optimizing care. Res. Rep. Urol. 2016, 8, 113–122. [Google Scholar] [CrossRef]
- Burgio, K.L. Update on Behavioral and Physical Therapies for Incontinence and Overactive Bladder: The Role of Pelvic Floor Muscle Training. Curr. Urol. Rep. 2013, 14, 457–464. [Google Scholar] [CrossRef] [PubMed]
- van Koeveringe, G.; Vahabi, B.; Andersson, K.; Kirschner-Herrmans, R.; Oelke, M. Detrusor underactivity: A plea for new approaches to a common bladder dysfunction. Neurourol. Urodyn. 2011, 30, 723–728. [Google Scholar] [CrossRef]
- Al Taweel, W.; Seyam, R. Neurogenic bladder in spinal cord injury patients. Res. Rep. Urol. 2015, 7, 85–99. [Google Scholar] [CrossRef]
- Price, N.; Dawood, F.R.; Jackson, S.R. Pelvic floor exercise for urinary incontinence: A systematic literature review. Maturitas 2010, 67, 309–315. [Google Scholar] [CrossRef]
- Radzimińska, A.; Strączyńska, A.; Weber-Rajek, M.; Styczyńska, H.; Strojek, K.; Piekorz, Z. The impact of pelvic floor muscle training on the quality of life of women with urinary incontinence: A systematic literature review. Clin. Interv. Aging 2018, 13, 957–965. [Google Scholar] [CrossRef]
- Brawer, M.K. Hormonal therapy for prostate cancer. Rev. Urol. 2006, 8 (Suppl. 2), S35–S47. [Google Scholar] [PubMed]
- Gay, H.A.; Michalski, J.M. Radiation therapy for prostate cancer. Mo. Med. 2018, 115, 146–150. [Google Scholar] [PubMed]
- Srikrishna, S.; Robinson, D.; Cardozo, L.; Vella, M. Management of overactive bladder syndrome. Postgrad. Med. J. 2007, 83, 481–486. [Google Scholar] [CrossRef][Green Version]
- Miyazato, M.; Yoshimura, N.; Chancellor, M.B. The other bladder syndrome: Underactive bladder. Rev. Urol. 2013, 15, 11–22. [Google Scholar]
- Wyndaele, J.; Madersbacher, H.; Kovindha, A. Conservative treatment of the neuropathic bladder in spinal cord injured patients. Spinal Cord 2001, 39, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Türk, C.; Petrik, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. EAU Guidelines on Diagnosis and Conservative Management of Urolithiasis. Eur. Urol. 2016, 69, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.R.; Davis, N.F.; Dalton, D.M.; McDermott, T.; Flynn, R.J.; Thomas, A.Z.; Manecksha, R.P. Quantitative Analysis of Technological Innovation in Urology. Urology 2018, 111, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Rassweiler, J.; Fiedler, M.; Charalampogiannis, N.; Kabakci, A.S.; Saglam, R.; Klein, J.-T. Robot-assisted flexible ureteroscopy: An update. Urolithiasis 2017, 46, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Pokorny, M.; Novara, G.; Geurts, N.; Dovey, Z.; De Groote, R.; Ploumidis, A.; Schatteman, P.; de Naeyer, G.; Mottrie, A. Robot-assisted Simple Prostatectomy for Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Enlargement: Surgical Technique and Outcomes in a High-volume Robotic Centre. Eur. Urol. 2015, 68, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, R.; Bonzo, J.; Lee, D. Robot-Assisted Simple Prostatectomy. J. Endourol. 2018, 32, S33–S38. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muhsin, H.M.; McAdams, S.B.; Nuñez, R.N.; Katariya, N.N.; Castle, E.P. Robot-assisted Transplanted Ureteral Stricture Management. Urology 2017, 105, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Buffi, N.M.; Lughezzani, G.; Hurle, R.; Lazzeri, M.; Taverna, G.; Bozzini, G.; Bertolo, R.; Checcucci, E.; Porpiglia, F.; Fossati, N.; et al. Robot-assisted Surgery for Benign Ureteral Strictures: Experience and Outcomes from Four Tertiary Care Institutions. Eur. Urol. 2017, 71, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, S.M.; Dmochowski, R.R. The inFlow intraurethral valve-pump for women with detrusor underactivity: A summary of peer-reviewed literature. J. Spinal Cord Med. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; White, M.D.; Longhurst, P.A. Sacral nerve stimulation for the management of voiding dysfunction. Rev. Urol. 2000, 2, 43–60. [Google Scholar] [PubMed]
- Nasrabadi, M.Z.; Tabibi, H.; Salmani, M.; Torkashvand, M.; Zarepour, E. A comprehensive survey on non-invasive wearable bladder volume monitoring systems. Med. Biol. Eng. Comput. 2021, 59, 1373–1402. [Google Scholar] [CrossRef] [PubMed]
- Padmapriya, B.; Kesavamurthy, T.; Selvi, M.T.; Gayathri, A. An Intensive Review on the Noninvasive Methods to Measure the Urinary Bladder Volume Using Ultrasound Images. Appl. Mech. Mater. 2014, 573, 803–807. [Google Scholar] [CrossRef]
- Mickle, A.; Won, S.M.; Noh, K.N.; Yoon, J.; Meacham, K.W.; Xue, Y.; McIlvried, L.A.; Copits, B.A.; Samineni, V.; Crawford, K.; et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 2019, 565, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.-M.; Lee, J.H.; Zhou, H.; Joo, J.; Lim, B.H.; Cheng, H.; Kim, S.H.; Kang, I.-S.; Lee, K.-S.; Park, E.; et al. Expandable and implantable bioelectronic complex for analyzing and regulating real-time activity of the urinary bladder. Sci. Adv. 2020, 6, eabc9675. [Google Scholar] [CrossRef] [PubMed]
- Samineni, V.K.; Mickle, A.D.; Yoon, J.; Grajales-Reyes, J.G.; Pullen, M.Y.; Crawford, K.E.; Noh, K.N.; Gereau, G.B.; Vogt, S.K.; Lai, H.H.; et al. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Sci. Rep. 2017, 7, 15865. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, J.; De Riese, W.; De Riese, C. New Implantable Tibial Nerve Stimulation Devices: Review of Published Clinical Results in Comparison to Established Neuromodulation Devices. Res. Rep. Urol. 2019, 11, 351–357. [Google Scholar] [CrossRef]
- Hassani, F.A.; Peh, W.Y.X.; Gammad, G.G.L.; Mogan, R.P.; Ng, T.K.; Kuo, T.L.C.; Ng, L.G.; Luu, P.; Yen, S.-C.; Lee, C. A 3D Printed Implantable Device for Voiding the Bladder Using Shape Memory Alloy (SMA) Actuators. Adv. Sci. 2017, 4, 1700143. [Google Scholar] [CrossRef] [PubMed]
- Hassani, F.A.; Jin, H.; Yokota, T.; Someya, T.; Thakor, N.V. Soft sensors for a sensing-actuation system with high bladder voiding efficiency. Sci. Adv. 2020, 6, eaba0412. [Google Scholar] [CrossRef] [PubMed]
- Hassani, F.A.; Gammad, G.G.L.; Mogan, R.P.; Ng, T.K.; Kuo, T.L.C.; Ng, L.G.; Luu, P.; Thakor, N.V.; Yen, S.-C.; Lee, C. Design and Anchorage Dependence of Shape Memory Alloy Actuators on Enhanced Voiding of a Bladder. Adv. Mater. Technol. 2017, 3, 1700184. [Google Scholar] [CrossRef]
- Barrett, D.M.; O’Sullivan, D.C.; Parulkar, B.G.; Donovan, M.G. Artificial Bladder Replacement: A New Design Concept. Mayo Clin. Proc. 1992, 67, 215–220. [Google Scholar] [CrossRef]
- Pane, S.; Mazzocchi, T.; Iacovacci, V.; Ricotti, L.; Menciassi, A. Smart Implantable Artificial Bladder: An Integrated Design for Organ Replacement. IEEE Trans. Biomed. Eng. 2021, 68, 2088–2097. [Google Scholar] [CrossRef]
- Yang, X.; An, C.; Liu, S.; Cheng, T.; Bunpetch, V.; Liu, Y.; Dong, S.; Li, S.; Zou, X.; Li, T.; et al. Soft Artificial Bladder Detrusor. Adv. Healthc. Mater. 2018, 7, e1701014. [Google Scholar] [CrossRef]
- Guzman-Negron, J.M.; Goldman, H.B. New Devices and Technologies for the Management of Overactive Bladder. Curr. Urol. Rep. 2017, 18, 94. [Google Scholar] [CrossRef]
- Wright, A.E.; Rukin, N.J.; Somani, B.K. Ureteroscopy and stones: Current status and future expectations. World J. Nephrol. 2014, 3, 243–248. [Google Scholar] [CrossRef]
- Murphy, D.; Challacombe, B.; Khan, M.S.; Dasgupta, P. Robotic technology in urology. Postgrad. Med. J. 2006, 82, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Schnider, P.; Birner, P.; Gendo, A.; Ratheiser, K.; Auff, E. Bladder volume determination: Portable 3-D versus stationary 2-D ultrasound device. Arch. Phys. Med. Rehabil. 2000, 81, 18–21. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Bih, L.-I.; Chen, S.-L.; Tsai, S.-J.; Teng, C.-H. The accuracy of ultrasonic estimation of bladder volume: A comparison of portable and stationary equipment. Arch. Phys. Med. Rehabil. 2004, 85, 138–141. [Google Scholar] [CrossRef]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, Techniques, and Limitations of Near Infrared Spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, M.; Schurle, S.; Magno, M. A Non-Invasive Wearable Bioimpedance System to Wirelessly Monitor Bladder Filling. In Proceedings of the 2020 Design, Automation & Test in Europe Conference & Exhibition (DATE), Grenoble, France, 9–13 March 2020. [Google Scholar] [CrossRef]
- Cao, H.; Tata, U.; Landge, V.; Li, A.-L.; Peng, Y.-B.; Chiao, J.-C. A Wireless Bladder Volume Monitoring System using a Flexible Capacitance-Based Sensor. In Proceedings of the 2013 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems, Austin, TX, USA, 20–23 January 2013. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, H.; Jung, Y.S.; Adem, K.M.A.; Bawazir, S.S.; Stefanini, C.; Lee, H.J. Implantable Bladder Volume Sensor Based on Resistor Ladder Network Composed of Conductive Hydrogel Composite. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017; pp. 1732–1735. [Google Scholar] [CrossRef]
- Weaver, J.N.; Alspaugh, J.C.; Behkam, B. Toward a Minimally Invasive Bladder Pressure monitoring System: Model Bladder for In Vitro Testing. In Proceedings of the 2010 3rd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Tokyo, Japan, 26–29 September 2010. [Google Scholar] [CrossRef]
- Hannah, S.; Brige, P.; Ravichandran, A.; Ramuz, M. Conformable, Stretchable Sensor to Record Bladder Wall Stretch. ACS Omega 2019, 4, 1907–1915. [Google Scholar] [CrossRef]
- Jo, Y.; Kang, M.; Shin, H.; Lee, S. Highly Stretchable Strain Sensor and Detecting System for Monitoring of Bladder Volume. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 555–558. [Google Scholar] [CrossRef]
- Lee, S.; Wang, H.; Peh, W.Y.X.; He, T.; Yen, S.-C.; Thakor, N.V.; Lee, C. Mechano-neuromodulation of autonomic pelvic nerve for underactive bladder: A triboelectric neurostimulator integrated with flexible neural clip interface. Nano Energy 2019, 60, 449–456. [Google Scholar] [CrossRef]
- Yan, D.; Bruns, T.M.; Wu, Y.; Zimmerman, L.L.; Stephan, C.; Cameron, A.P.; Yoon, E.; Seymour, J.P. Ultracompliant Carbon Nanotube Direct Bladder Device. Adv. Healthc. Mater. 2019, 8, e1900477. [Google Scholar] [CrossRef]
- Malaeb, B.S.; Elliott, S.P.; Lee, J.; Anderson, D.W.; Timm, G.W. Novel Artificial Urinary Sphincter in the Canine Model: The Tape Mechanical Occlusive Device. Urology 2011, 77, 211–216. [Google Scholar] [CrossRef]
- Biardeau, X.; Hached, S.; Loutochin, O.; Campeau, L.; Sawan, M.; Corcos, J. Montreal electronic artificial urinary sphincters: Our futuristic alternatives to the AMS800TM. Can. Urol. Assoc. J. 2017, 11, E396–E404. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef]
- Stewart, W.; Van Rooyen, J.; Cundiff, G.; Abrams, P.; Herzog, A.; Corey, R.; Hunt, T.; Wein, A. Prevalence and burden of overactive bladder in the United States. World J. Urol. 2003, 20, 327–336. [Google Scholar] [CrossRef]
- Sexton, C.C.; Coyne, K.S.; Thompson, C.; Bavendam, T.; Chen, C.-I.; Markland, A. Prevalence and Effect on Health-Related Quality of Life of Overactive Bladder in Older Americans: Results from the Epidemiology of Lower Urinary Tract Symptoms Study. J. Am. Geriatr. Soc. 2011, 59, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Milsom, I.; Abrams, P.; Cardozo, L.; Roberts, R.; Thüroff, J.; Wein, A. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. Br. J. Urol. 2001, 87, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Overactive Bladder: Information for Patients. NHS Foundation Trust. 2019. Available online: https://mft.nhs.uk/app/uploads/sites/4/2018/04/Overactive-bladder-August-2017.pdf (accessed on 29 December 2021).
- Tyagi, P. Pathophysiology of the urothelium and detrusor. Can. Urol. Assoc. J. 2011, 5, S128–S130. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C. A neurologic basis for the overactive bladder. Urology 1997, 50, 36–52. [Google Scholar] [CrossRef]
- Brading, A.F. Spontaneous activity of lower urinary tract smooth muscles: Correlation between ion channels and tissue function. J. Physiol. 2006, 570, 13–22. [Google Scholar] [CrossRef]
- Drake, M.; Mills, I.; Gillespie, J. Model of peripheral autonomous modules and a myovesical plexus in normal and overactive bladder function. Lancet 2001, 358, 401–403. [Google Scholar] [CrossRef]
- Coyne, K.S.; Sexton, C.C.; Irwin, D.E.; Kopp, Z.S.; Kelleher, C.J.; Milsom, I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: Results from the EPIC study. Br. J. Urol. 2008, 101, 1388–1395. [Google Scholar] [CrossRef]
- Vij, M.; Robinson, D.; Cardozo, L. Overactive Bladder: Diagnosis and Treatment. Women Health 2010, 6, 297–310. [Google Scholar] [CrossRef]
- Shafik, A.; Shafik, I.A. Overactive bladder inhibition in response to pelvic floor muscle exercises. World J. Urol. 2003, 20, 374–377. [Google Scholar] [CrossRef]
- Biastre, K.; Burnakis, T. Trospium Chloride Treatment of Overactive Bladder. Ann. Pharmacother. 2009, 43, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Madhuvrata, P.; Cody, J.D.; Ellis, G.; Herbison, G.P.; Hay-Smith, E.J.C. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst. Rev. 2012, 1, CD005429. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.M.; Carrico, D.J.; MacDiarmid, S.A.; Wooldridge, L.S.; Khan, A.U.; McCoy, C.E.; Franco, N.; Bennett, J.B. Sustained therapeutic effects of percutaneous tibial nerve stimulation: 24-month results of the STEP study. Neurourol. Urodyn. 2013, 32, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Blok, B.; Van Kerrebroeck, P.; de Wachter, S.; Ruffion, A.; Van der Aa, F.; Jairam, R.; Perrouin-Verbe, M.; Elneil, S. Three month clinical results with a rechargeable sacral neuromodulation system for the treatment of overactive bladder. Neurourol. Urodyn. 2018, 37, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Macnab, A.; Friedman, B.; Shadgan, B.; Stothers, L. Bladder anatomy physiology and pathophysiology: Elements that suit near infrared spectroscopic evaluation of voiding dysfunction. Biomed. Spectrosc. Imaging 2012, 1, 223–235. [Google Scholar] [CrossRef]
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 625–663. [Google Scholar] [CrossRef]
- Molavi, B.; Shadgan, B.; Macnab, A.J.; Dumont, G.A. Noninvasive Optical Monitoring of Bladder Filling to Capacity Using a Wireless Near Infrared Spectroscopy Device. IEEE Trans. Biomed. Circuits Syst. 2013, 8, 325–333. [Google Scholar] [CrossRef]
- Stothers, L.; Shadgan, B.; Macnab, A. Urological applications of near infrared spectroscopy. Can. J. Urol. 2008, 15, 4399–4409. [Google Scholar]
- Jo, H.G.; Park, B.H.; Joung, D.Y.; Jo, J.K.; Hoh, J.-K.; Choi, W.Y.; Park, K.K. Forward-Looking Ultrasound Wearable Scanner System for Estimation of Urinary Bladder Volume. Sensors 2021, 21, 5445. [Google Scholar] [CrossRef]
- Caffrey, C.M.; Vaajoki, A.; Halme, J.; Sillanpaa, T.; Revuelta, A.; Puukko, P. Design and Verification of a Wireless Readout System for Integrated Motor Axle Condition Monitoring. In Proceedings of the 2018 IEEE Sensors, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Schlebusch, T.; Orschulik, J.; Malmivuo, J.; Leonhardt, S.; Leonhäuser, D.; Grosse, J.; Kowollik, M.; Kirschner-Hermanns, R.; Walter, M. Impedance Ratio Method for Urine Conductivity-Invariant Estimation of Bladder Volume. J. Electr. Bioimpedance 2014, 5, 48–54. [Google Scholar] [CrossRef]
- Leonhäuser, D.; Castelar, C.; Schlebusch, T.; Rohm, M.; Rupp, R.; Leonhardt, S.; Walter, M.; Grosse, J.O. Evaluation of electrical impedance tomography for determination of urinary bladder volume: Comparison with standard ultrasound methods in healthy volunteers. Biomed. Eng. Online 2018, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Majerus, S.; Basu, A.S.; Makovey, I.; Wang, P.; Zhui, H.; Zorman, C.; Ko, W.; Damaser, M.S. Wireless Bladder Pressure Monitor for Closed-Loop Bladder Neuromodulation. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Soebadi, M.A.; Bakula, M.; Hakim, L.; Puers, R.; De Ridder, D. Wireless intravesical device for real-time bladder pressure measurement: Study of consecutive voiding in awake minipigs. PLoS ONE 2019, 14, e0225821. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.S.; Majerus, S.; Ferry, E.; Makovey, I.; Zhu, H.; Damaser, M.S. Is submucosal bladder pressure monitoring feasible? Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2019, 233, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Sukhu, T.; Kennelly, M.J.; Kurpad, R. Sacral neuromodulation in overactive bladder: A review and current perspectives. Res. Rep. Urol. 2016, 8, 193–199. [Google Scholar] [CrossRef]
- Deer, T.; Pope, J.; Benyamin, R.; Vallejo, R.; Friedman, A.; Caraway, D.; Staats, P.; Grigsby, E.; McRoberts, W.P.; McJunkin, T.; et al. Prospective, Multicenter, Randomized, Double-Blinded, Partial Crossover Study to Assess the Safety and Efficacy of the Novel Neuromodulation System in the Treatment of Patients With Chronic Pain of Peripheral Nerve Origin. Neuromodul. Technol. Neural Interface 2016, 19, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Elneil, S.; Digesu, A.; van Breda, H.; Heesakkers, J.; van Kerrebroeck, P. Safety and performance of a wireless implantable tibial nerve stimulator device for the treatment of patients with overactive bladder (OAB). Neurourol. Urodyn. 2016, 35, S45–S46. [Google Scholar]
- Birmingham, K.; Gradinaru, V.; Anikeeva, P.; Grill, W.M.; Pikov, V.; McLaughlin, B.; Pasricha, P.; Weber, D.; Ludwig, K.; Famm, K. Bioelectronic medicines: A research roadmap. Nat. Rev. Drug Discov. 2014, 13, 399–400. [Google Scholar] [CrossRef]
- Kavvadias, T.; Huebner, M.; Brucker, S.Y.; Reisenauer, C. Management of device-related complications after sacral neuromodulation for lower urinary tract disorders in women: A single center experience. Arch. Gynecol. Obstet. 2017, 30, 766–957. [Google Scholar] [CrossRef] [PubMed]
- del Valle, J.; Navarro, X. Interfaces with the Peripheral Nerve for the Control of Neuroprostheses. Int. Rev. Neurobiol. 2013, 109, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Yenli, E.; Aboah, K.; Gyasi-Sarpong, C.; Azorliade, R.; Arhin, A. Acute and chronic urine retention among adults at the urology section of the Accident and Emergency Unit of Komfo Anokye Teaching Hospital, Kumasi, Ghana. Afr. J. Urol. 2015, 21, 129–136. [Google Scholar] [CrossRef][Green Version]
- Deane, A.M.; Worth, P.H.L. Female Chronic Urinary Retention. Br. J. Urol. 1985, 57, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Kuchel, G.A. Detrusor Underactivity: Clinical Features and Pathogenesis of an Underdiagnosed Geriatric Condition. J. Am. Geriatr. Soc. 2006, 54, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.I.; Chapple, C.R.; Abrams, P.; Dmochowski, R.; Haab, F.; Nitti, V.; Koelbl, H.; van Kerrebroeck, P.; Wein, A.J. Detrusor Underactivity and the Underactive Bladder: A New Clinical Entity? A Review of Current Terminology, Definitions, Epidemiology, Aetiology, and Diagnosis. Eur. Urol. 2013, 65, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.L.; Muir, G.H. Chronic urinary retention in men: How we define it, and how does it affect treatment outcome. Br. J. Urol. 2012, 110, 1590–1594. [Google Scholar] [CrossRef]
- Abrams, P.H.; Dunn, M.; George, N. Urodynamic findings in chronic retention of urine and their relevance to results of surgery. BMJ 1978, 2, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.A.; Wein, A.J.; Staskin, D.R.; Roehrborn, C.G.; Steers, W.D. Urinary Retention and Post-Void Residual Urine in Men: Separating Truth from Tradition. J. Urol. 2008, 180, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Azuma, Y. Periodical measurement of urine volume in the bladder during sleep: Temporary volume reduction suggestive of absorption. Int. J. Urol. 2015, 23, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.G.; Michael, B. Practical Neuro-Urology: Genitourinary Complications in Neurologic Disease; Butterworth-Heinemann: Boston, MA, USA, 1995; Volume IX. [Google Scholar]
- Przydacz, M.; Denys, P.; Corcos, J. What do we know about neurogenic bladder prevalence and management in developing countries and emerging regions of the world? Ann. Phys. Rehabil. Med. 2017, 60, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.R.; Davis, N.F.; Quinlan, M.R.; Flynn, R.J.; McDermott, T.; Manecksha, R.; Thornhill, J.A. A prospective audit on the effect of training and educational workshops on the incidence of urethral catheterization injuries. Can. Urol. Assoc. J. 2017, 11, E302-6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, A.; Hu, D.; Xue, D.; Li, C.; Zhang, K.; Ma, H.; Yan, S.; Pan, Z. Indwelling versus Intermittent Urinary Catheterization following Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0130636. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Intermittent Catheters for Chronic Urinary Retention: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2019, 19, 1–153. [Google Scholar]
- Oh, S.-J.; Ku, J.H.; Jeon, H.G.; Shin, H.-I.; Paik, N.-J.; Yoo, T. Health-related quality of life of patients using clean intermittent catheterization for neurogenic bladder secondary to spinal cord injury. Urology 2005, 65, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Hassani, F.A.; Mogan, R.P.; Gammad, G.G.L.; Wang, H.; Yen, S.-C.; Thakor, N.V.; Lee, C. Toward Self-Control Systems for Neurogenic Underactive Bladder: A Triboelectric Nanogenerator Sensor Integrated with a Bistable Micro-Actuator. ACS Nano 2018, 12, 3487–3501. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion–contraction of photoresponsive artificial muscle regulated by host–guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef] [PubMed]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef] [PubMed]
- Sigurjonsdottir, V.K.; Runolfsdottir, H.L.; Indridason, O.S.; Palsson, R.; Edvardsson, V.O. Impact of nephrolithiasis on kidney function. BMC Nephrol. 2015, 16, 149. [Google Scholar] [CrossRef]
- Mikawlrawng, K.; Kumar, S.; Vandana, R. Current scenario of urolithiasis and the use of medicinal plants as antiurolithiatic agents in Manipur (North East India): A review. Int. J. Herb. Med. 2014, 2, 1–12. [Google Scholar]
- Rule, A.D.; Roger, V.L.; Melton, L.J.; Bergstralh, E.J.; Li, X.; Peyser, P.A.; Krambeck, A.E.; Lieske, J.C. Kidney Stones Associate with Increased Risk for Myocardial Infarction. J. Am. Soc. Nephrol. 2010, 21, 1641–1644. [Google Scholar] [CrossRef]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Obesity, Weight Gain, and the Risk of Kidney Stones. JAMA 2005, 293, 455–462. [Google Scholar] [CrossRef]
- Chauhan, C.K.; Joshi, M.J.; Vaidya, A.D.B. Growth inhibition of Struvite crystals in the presence of herbal extract Commiphora wightii. J. Mater. Sci. Mater. Electron. 2009, 20, 85–92. [Google Scholar] [CrossRef]
- Edvardsson, V.; Indridason, O.S.; Haraldsson, G.; Kjartansson, O.; Palsson, R. Temporal trends in the incidence of kidney stone disease. Kidney Int. 2013, 83, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney stones. Nat. Rev. Dis. Prim. 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.Y.; Taghipour-Gorgikolai, M.; Sharifian, R. Treatment of Kidney Stones Using Extracorporeal Shock Wave Lithotripsy (ESWL) and Double-J Stent in Infants. Adv. Urol. 2012, 2012, 589038. [Google Scholar] [CrossRef]
- NICE. Prostate Cancer: Diagnosis and Management NICE Guideline. 2019. Available online: https://www.nice.org.uk/guidance/ng131 (accessed on 1 February 2022).
- Lepor, H. Managing and preventing acute urinary retention. Rev. Urol. 2005, 7, S26–S33. [Google Scholar] [PubMed]
- Thomas, K.; Chow, K.; Kirby, R.S. Acute Urinary Retention: A review of the aetiology and management. Prostate Cancer Prost. Dis. 2004, 7, 32–37. [Google Scholar] [CrossRef][Green Version]
- Davis, N.F.; Brady, C.M.; Creagh, T. Interstitial cystitis/painful bladder syndrome: Epidemiology, pathophysiology and evidence-based treatment options. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 30–37. [Google Scholar] [CrossRef]
- Rosenberg, M.T.; Page, S.; Hazzard, M.A. Prevalence of Interstitial Cystitis in a Primary Care Setting. Urology 2007, 69, S48–S52. [Google Scholar] [CrossRef]
- Jones, C.A.; Nyberg, L. Epidemiology of interstitial cystitis. Urology 1997, 49, 2–9. [Google Scholar] [CrossRef]
- Payne, C.K.; Joyce, G.F.; Wise, M.; Clemens, J.Q.; Project, U.D.I.A. Interstitial Cystitis and Painful Bladder Syndrome. J. Urol. 2007, 177, 2042–2049. [Google Scholar] [CrossRef]
- Bitcon, C.; Anderson, K.; Cox, A. Treatment of interstitial cystitis/bladder pain syndrome: A contemporary review. EMJ 2020, 5, 91–100. [Google Scholar] [CrossRef]
- Rassweiler, J.J.; Teber, D. Advances in laparoscopic surgery in urology. Nat. Rev. Urol. 2016, 13, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Rassweiler, J.J.; Autorino, R.; Klein, J.; Mottrie, A.; Goezen, A.S.; Stolzenburg, J.-U.; Rha, K.H.; Schurr, M.; Kaouk, J.; Patel, V.; et al. Future of robotic surgery in urology. Br. J. Urol. 2017, 120, 822–841. [Google Scholar] [CrossRef] [PubMed]
- Saglam, R.; Muslumanoglu, A.Y.; Tokatlı, Z.; Çaşkurlu, T.; Sarica, K.; Taşçi, A.I.; Erkurt, B.; Süer, E.; Kabakci, A.S.; Preminger, G.; et al. A New Robot for Flexible Ureteroscopy: Development and Early Clinical Results (IDEAL Stage 1–2b). Eur. Urol. 2014, 66, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-W.; van Meer, F.; Bailly, Y.; Yeung, C.K. Design and Development of a Da Vinci Surgical System Simulator. In Proceedings of the 2007 International Conference on Mechatronics and Automation, Harbin, China, 5–9 August 2007; pp. 1050–1055. [Google Scholar] [CrossRef]
- Menon, M.; Tewari, A.; Peabody, J.O.; Shrivastava, A.; Kaul, S.; Bhandari, A.; Hemal, A.K. Vattikuti Institute prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate: Experience of over 1100 cases. Urol. Clin. N. Am. 2004, 31, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Yafi, F.A.; Powers, M.K.; Zurawin, J.; Hellstrom, W.J. Contemporary Review of Artificial Urinary Sphincters for Male Stress Urinary Incontinence. Sex. Med. Rev. 2016, 4, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kil Nam, J.; Kim, T.N.; Park, S.W.; Lee, S.D.; Chung, M.K. The Studer Orthotopic Neobladder: Long-Term (More Than 10 Years) Functional Outcomes, Urodynamic Features, and Complications. Yonsei Med. J. 2013, 54, 690–695. [Google Scholar] [CrossRef]
- Chow, B.Y.; Han, X.; Dobry, A.S.; Qian, X.; Chuong, A.S.; Li, M.; Henninger, M.A.; Belfort, G.M.; Lin, Y.; Monahan, P.E.; et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 2010, 463, 98–102. [Google Scholar] [CrossRef]


| Digital Library | Number of Articles |
|---|---|
| PubMed | 26 |
| IEEE | 9 |
| NCBI | 9 |
| Nature | 3 |
| Other | 15 |
| Total | 62 |
| Causes | Definitions |
|---|---|
| Neurogenic dysfunction [75] | A reduction in inhibitory neural impulses and an increase in the afferent impulses from the bladder trigger voiding reflex. |
| Myogenic dysfunction [76] | Altered structure or disordered function of the detrusor smooth muscle by an increase in sensitivity to cholinergic stimulation can lead to increased random activity. |
| Autonomous bladder theory [77] | An alteration of phasic activity is generated by muscarinic stimulation. |
| Other [78,79] | UTI, weak sphincter, bladder abnormalities, diabetes mellitus, excessive caffeine or alcohol and hypercalcemia. |
| Lifestyle [5,21] | Pharmacotherapy [5,29] | Physiotherapy [5,22] |
|---|---|---|
| Reduce intake of caffeine | Antimuscinaries | Pelvic floor muscle training |
| Alter daily fluid intake | Oxybutynin | Bladder training |
| Weight loss | Propiverine | Double void |
| Absorbent pads | Tolterodine | Vaginal weight training |
| Smoking cessation | Trospium | |
| Scheduled toileting | Solifenacin | |
| Bowel regime |
| Causes | Definitions |
|---|---|
| Nerve damage | Damage to the peripheral nerves may lessen or eliminate one’s ability to feel the filling of the bladder. |
| Diabetes | Increased high blood sugar can cause damage to peripheral nerves, resulting in incomplete emptying of the bladder. |
| Pelvic surgery | Surgery may lead to damaged nerves, causing decreased bladder contractions. |
| Aging | The volume and elasticity of the bladder tissue can decrease by aging. |
| Obstruction | An enlarged prostate or prostate cancer in men and vaginal prolapse in women can block urine flow. |
| Urinary tract infection (UTI) | An infection present in the bladder or urethra can lead to urinary retention. |
| Medications | Drugs with antimuscarinic properties block a chemical that relaxes the muscle, e.g., antidepressants, antihistamines, and muscle relaxants. |
| Spinal cord injury (SCI) | Injuries below lumbar vertebrae (L1) may have a flaccid bladder, which will not contract. |
| Other | Multiple sclerosis, Parkinson’s disease, herniated disc, lesion of the pudendal nerve, acquired immunodeficiency syndrome (AIDS) and neurosyphilis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes-Martin, K.; Zhu, M.; Xiao, S.; Arab Hassani, F. Advances in Assistive Electronic Device Solutions for Urology. Micromachines 2022, 13, 551. https://doi.org/10.3390/mi13040551
Holmes-Martin K, Zhu M, Xiao S, Arab Hassani F. Advances in Assistive Electronic Device Solutions for Urology. Micromachines. 2022; 13(4):551. https://doi.org/10.3390/mi13040551
Chicago/Turabian StyleHolmes-Martin, Kieran, Minghui Zhu, Shujun Xiao, and Faezeh Arab Hassani. 2022. "Advances in Assistive Electronic Device Solutions for Urology" Micromachines 13, no. 4: 551. https://doi.org/10.3390/mi13040551
APA StyleHolmes-Martin, K., Zhu, M., Xiao, S., & Arab Hassani, F. (2022). Advances in Assistive Electronic Device Solutions for Urology. Micromachines, 13(4), 551. https://doi.org/10.3390/mi13040551







