Intravenous Calcium Alginate Microspheres as Drug Delivery Vehicles in Acute Kidney Injury Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Size Control of CAM
2.3. Drug Loading and Release
2.4. Swelling Property
2.5. Mice Surgical AKI Model
3. Results and Discussions
3.1. Emulsion Patterns in Different Flow Rate
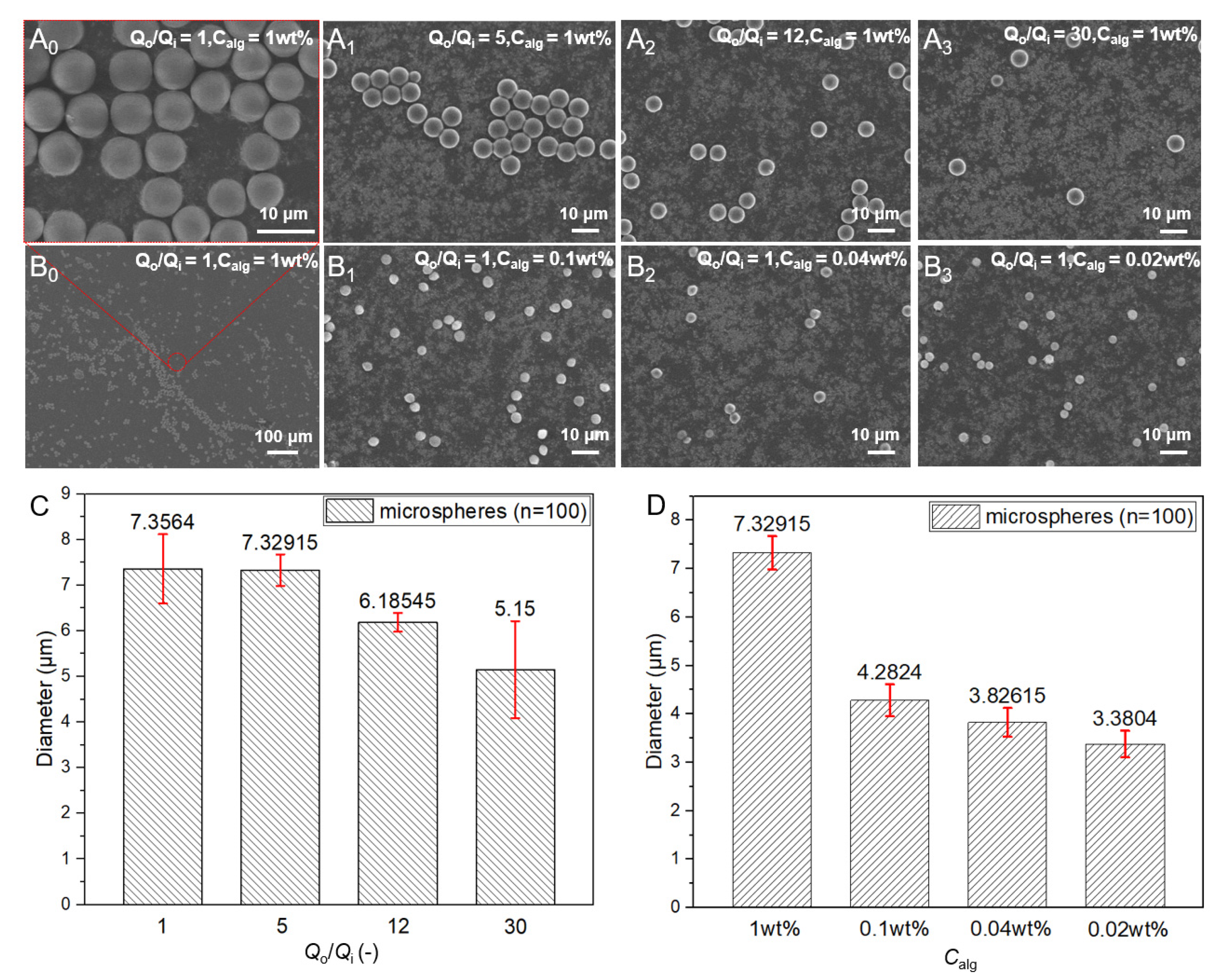
3.2. Control of the Size of Microspheres
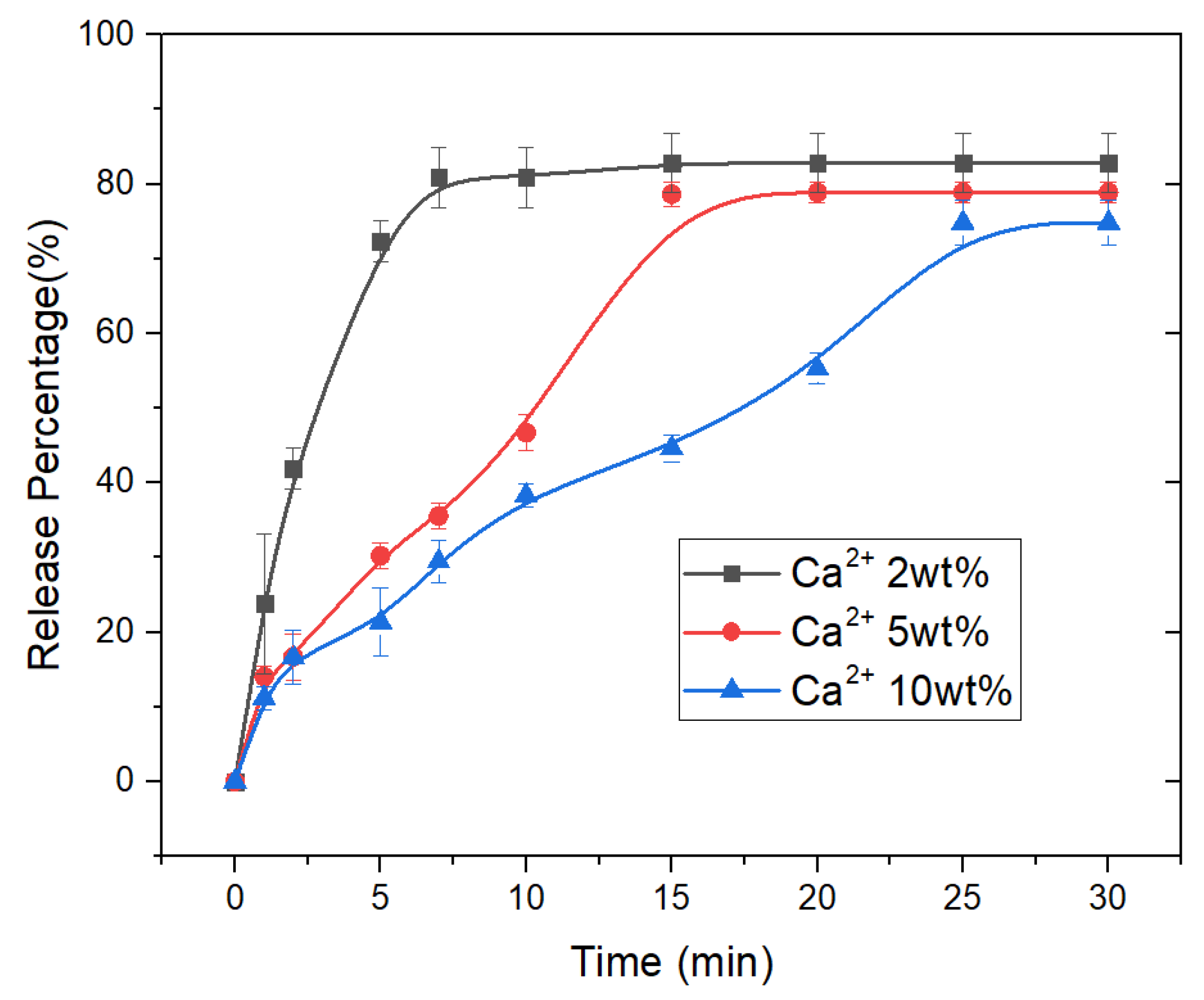
3.3. Drug Release Properties of CAM
3.4. Swelling Properties of CAM
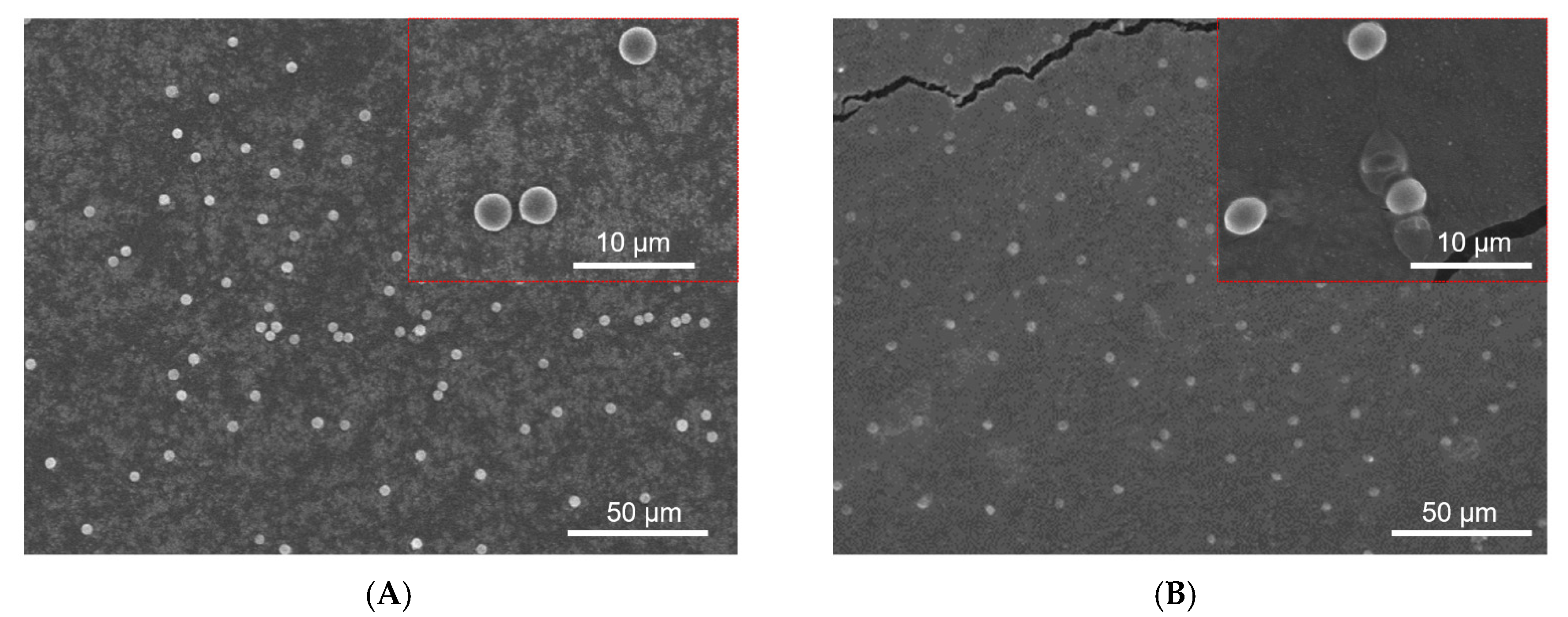
3.5. Drug Loaded CAM for AKI by Intravenous Injection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.J.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Seller-Pérez, G.; Más-Font, S.; Pérez-Calvo, C.; Villa-Díaz, P.; Celaya-López, M.; Herrera-Gutiérrez, M.E. Acute kidney injury: Renal disease in the ICU. Med. Intensiva 2016, 40, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Livingston, M.J.; Dong, G.; Tang, C.; Su, Y.; Wu, G.; Yin, X.-M.; Dong, Z. Histone deacetylase inhibitors protect against cisplatin-induced acute kidney injury by activating autophagy in proximal tubular cells. Cell Death Dis. 2018, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaraj, S.; Palanisamy, U.M.; Kadhar Mohamed, M.S.B.; Gangasalam, A.; Maria, G.A.; Kandasamy, R. Curcumin drug delivery by vanillin-chitosan coated with calcium ferrite hybrid nanoparticles as carrier. Eur. J. Pharm. Sci. 2018, 116, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Sangi, S.; SreeHarsha, N.; Bawadekji, A.; Ali, M.A. Chemotherapeutic drug targeting to lungs by way of microspheres after intravenous administration. Drug Des. Dev. Ther. 2018, 12, 3051–3060. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; Brugger, A.; Khare, A.; Chaubal, M.; Papadopoulos, P.; Rabinow, B.; Kipp, J.; Ning, J. Suspensions for intravenous (IV) injection: A review of development, preclinical and clinical aspects. Adv. Drug Del. Rev. 2008, 60, 939–954. [Google Scholar] [CrossRef]
- Yaowalak, S.; Prasong, S. Preparation and Characterization of Keratin/Alginate Blend Microparticles. Adv. Mater. Sci. Eng. 2018, 2018, 1–8. [Google Scholar]
- Raha, A.; Bhattacharjee, S.; Mukherjee, P.; Paul, M.; Bagchi, A. Design and Characterization of Ibuprofen Loaded Alginate Microspheres Prepared by Ionic Gelation Method. Int. J. Pharma. Res. Health Sci. 2018, 6, 2713–2729. [Google Scholar]
- Berkland, C.; Kim, K.; Pack, D.W. PLG microsphere size controls drug release rate through several competing factors. Pharm. Res. 2003, 20, 1055–1062. [Google Scholar] [CrossRef]
- Chopra, R.; Alderborn, G.; Podczeck, F.; Newton, J.M. The influence of pellet shape and surface properties on the drug release from uncoated and coated pellets. Int. J. Pharm. 2002, 239, 171–178. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, T.Y.; Choi, T.M.; Shim, T.S.; Kim, S.H.; Yang, S.M.J.L. Droplet Microfluidics for Producing Functional Microparticles. Langmuir 2014, 30, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Heida, T.; Neubauer, J.W.; Seuss, M.; Hauck, N.; Thiele, J.; Fery, A. Mechanically Defined Microgels by Droplet Microfluidics. Macromol. Chem. Phys. 2017, 218, 1600418. [Google Scholar] [CrossRef]
- Nunes, J.K.; Tsai, S.S.H.; Wan, J.; Stone, H.A. Dripping and jetting in microfluidic multiphase flows applied to particle and fibre synthesis. J. Phys. D Appl. Phys. 2013, 46, 114002. [Google Scholar] [CrossRef] [PubMed]
- Umbanhowar, P.B.; Prasad, V.; Weitz, D.A. Monodisperse Emulsion Generation via Drop Break Off in a Coflowing Stream. Langmuir 2000, 16, 347–351. [Google Scholar] [CrossRef]
- Utada, A.S.; Fernandez-Nieves, A.; Stone, H.A.; Weitz, D.A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 2007, 99, 094502. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, J.; Wang, X.; Li, J.; Cui, X.; Hua, Z.; Li, J.; Mao, Z.; Zhang, S. Intravenous Calcium Alginate Microspheres as Drug Delivery Vehicles in Acute Kidney Injury Treatment. Micromachines 2022, 13, 538. https://doi.org/10.3390/mi13040538
Man J, Wang X, Li J, Cui X, Hua Z, Li J, Mao Z, Zhang S. Intravenous Calcium Alginate Microspheres as Drug Delivery Vehicles in Acute Kidney Injury Treatment. Micromachines. 2022; 13(4):538. https://doi.org/10.3390/mi13040538
Chicago/Turabian StyleMan, Jia, Xiaojie Wang, Jianyong Li, Xiaoyang Cui, Zesheng Hua, Jianfeng Li, Zebing Mao, and Shanguo Zhang. 2022. "Intravenous Calcium Alginate Microspheres as Drug Delivery Vehicles in Acute Kidney Injury Treatment" Micromachines 13, no. 4: 538. https://doi.org/10.3390/mi13040538
APA StyleMan, J., Wang, X., Li, J., Cui, X., Hua, Z., Li, J., Mao, Z., & Zhang, S. (2022). Intravenous Calcium Alginate Microspheres as Drug Delivery Vehicles in Acute Kidney Injury Treatment. Micromachines, 13(4), 538. https://doi.org/10.3390/mi13040538





