Electrical Impedance Tomography Technical Contributions for Detection and 3D Geometric Localization of Breast Tumors: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Methodological Quality
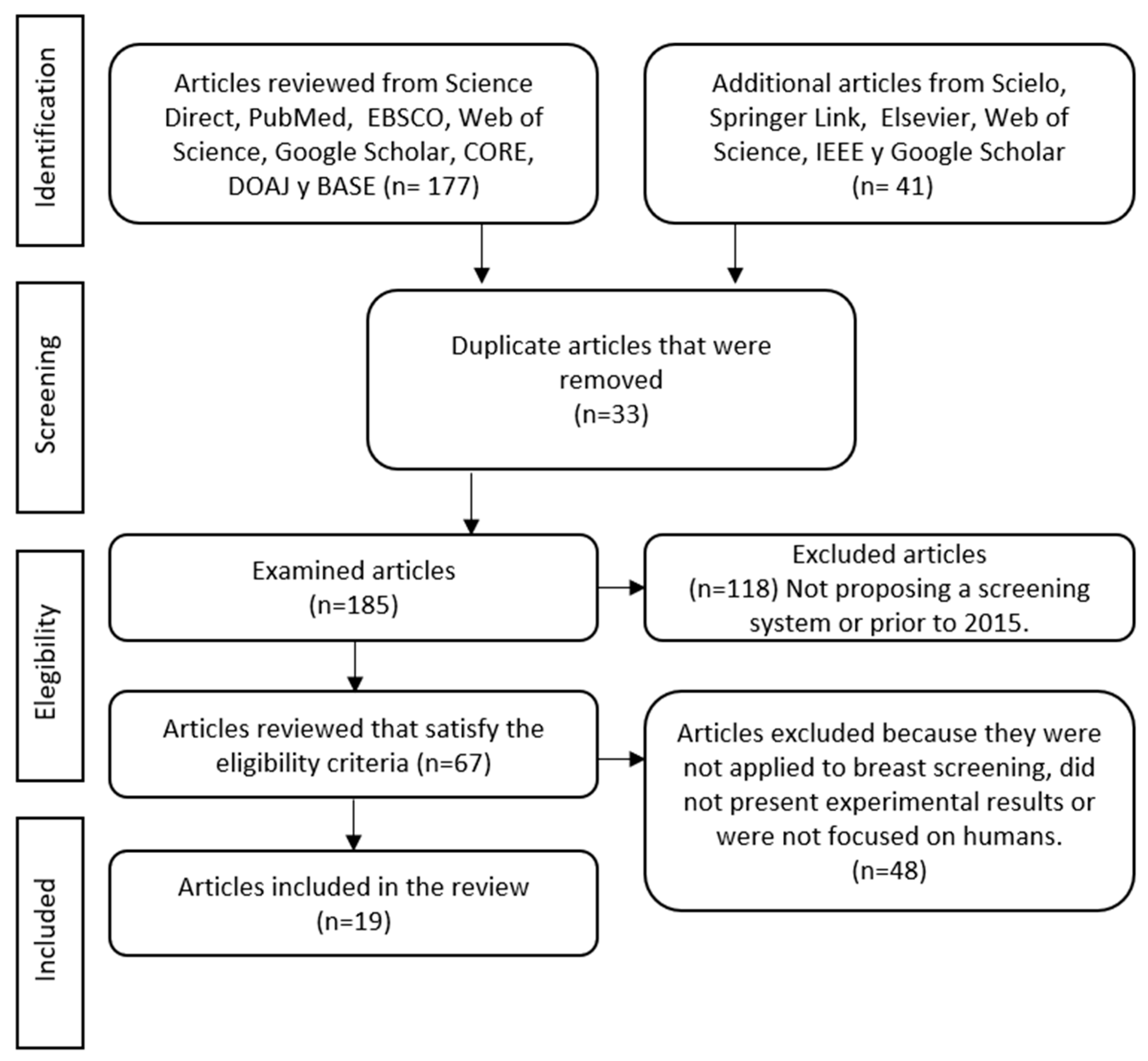
2.4. PRISMA Flowchart
3. Results
| Author (Year) | Electrode Arrangement | Working Frequency | Electric Current Injection | Medical Standard Validation |
|---|---|---|---|---|
| Choridah et al. (2021) [47] | 16 and 32 electrodes | - | - | - |
| Gomes et al. (2020) [42] | 16 electrodes in a ring a array | - | - | - |
| Hu Jing et al. (2020) [48] | 16 electrodes in a ring array | 50 Hz to 250 kHz | 1–7 mA | SwissTom Pioneer commercial system |
| Lee Jaehyuk et al. (2020) [49] | 16 electrodes distributed in 2 levels | 10 kHz to 10 MHz | 0.1 to 3 mA pp | - |
| Mansouri et al. (2020) [50] | 4 electrodes in a ring array | 1 kHz | 0.9 mA | Study approved by Research Ethics Committee in Health and Science Disciplines |
| Murillo-Ortiz et al. (2020) [51] | 2 electrodes one on each arm | 50 kHz | 0.5 mA | MEIK v.5.6 commercial system |
| Chen et al. (2020) [52] | 16 electrodes in a ring array | - | - | - |
| Gutierrez et al. (2019) [53] | 8 electrodes in a ring array | 500 Hz, 1 kHz, 5 kHz And 10 kHz | 60 μA pp | IEC/TS 60479-1 |
| Rao et al. (2019) [54] | 16 electrodes in a ring array | 100 Hz to 10 MHz | 1.2 mA pp | - |
| Mothi et al. (2018) [55] | 16 electrodes in a ring array | 260 kHz | 7 mA | SwissTom commercial system |
| Wu et al. (2018) [56] | 16 microelectrodes in A ring array | 10 kHz | - | - |
| Zarafshani et al. (2018) [57] | 85 electrodes in a planar array | 10 kHz to 3 MHz | 10 mA | IEC 60601-1 |
| Singh et al. (2017) [43] | 16 electrodes in a ring array | 1kHz to 1 MHz | 0.5 mA pp | - |
| Yunjie Yang et al. (2016) [58] | 16 microelectrodes in a ring array | 10 kHz | 1.5 mA pp | Class II, type BF |
| Murphy et al. (2016) [59] | 16 electrodes in a ring arrangement distributed in 2 rings (rotary system) | 10 kHz, 100 kHz, 1 MHz and 10 MHz | - | - |
| Hong Sunjoo et al. (2015) [60] | 90 electrodes distributed in multiple levels using ring arrangement | 100 Hz to 100 kHz | 10 to 400 μA pp | IEC 60601-1 |
| Khan Shadab et al. (2015) [61] | 16 electrodes in a ring array | 1 kHz to 100 kHz | 100 μA rms | IEC 60601 |
| Zhang et al. (2015) [62] | 85 electrodes in a planar array | 500 kHz | - | - |
| Halther et al. (2015) [63] | 16 electrodes in a ring array | 127 kHz | 1 V pp | Institutional Review Board-approved study at Dartmouth-Hitchcock Medical Center (Lebanon, NH, USA). |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO, World Health Organization. Cancer. Available online: https://www.who.int/health-topics/cancer (accessed on 18 February 2022).
- International Agency for Research on Cancer. World Health Organization. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2018 (accessed on 18 February 2022).
- Smith, R.A.; Manassaram-Baptiste, D.; Brooks, D.; Cokkinides, V.; Doroshenk, M.; Saslow, D.; Wender, R.C.; Brawley, O.W. Cancer screening in the United States, 2014: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2014, 64, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Horner, M.J.; Ries, L.; Krapcho, M.; Neyman, N.; Aminou, R.; Howlader, N.; Altekruse, S.F.; Feuer, E.J.; Huang, L.; Mariotto, A.; et al. SEER Cancer Statistics Review (1975–2006); National Cancer Institute: Bethesda, MD, USA, 2009.
- Coleman, M.; Quaresma, M.; Berrino, F.; Lutz, J.-M.; De Angelis, R.; Capocaccia, R.; Baili, P.; Rachet, B.; Gatta, G.; Hakulinen, T.; et al. Cancer survival in five continents: A worldwide population-based study (CONCORD). Lancet Oncol. 2008, 9, 730–756. [Google Scholar] [CrossRef]
- IMSS—Nstituto Mexicano del Seguro Social. Cáncer de Mama. Available online: http://www.imss.gob.mx/salud-en-linea/cancer-mama (accessed on 18 February 2022).
- Holder, D.S. Electrical Impedance Tomography: Methods, History and Applications; IOP Publishing: Bristol, UK, 2005. [Google Scholar]
- Liu, S.; Jia, J.; Zhang, Y.D.; Yang, Y. Image Reconstruction in Electrical Impedance Tomography Based on Structure-Aware Sparse Bayesian Learning. IEEE Trans. Med. Imaging 2018, 37, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.H.; Wauters, J. The Role for Bio-electrical Impedance Analysis in Critically Ill Patients. ICU Manag. Pract. 2014, 14. [Google Scholar] [CrossRef]
- Gong, B.; Schullcke, B.; Krueger-Ziolek, S.; Mueller-Lisse, U.; Moeller, K. Sparse regularization for EIT reconstruction incorporating structural information derived from medical imaging. Physiol. Meas. 2016, 37, 843–862. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, O.G. Bioelectricity and Bioimpedance Basics, 2nd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Huang, C.N.; Yu, F.M.; Chung, H.Y. The scanning data collection strategy for enhancing the quality of electrical impedance tomography. IEEE Trans. Instrum. Meas. 2008, 57, 1193–1198. [Google Scholar] [CrossRef]
- Pethig, R. Dielectric properties of body tissues. Clin. Phys. Physiol. Meas. 1987, 8, 5–12. [Google Scholar] [CrossRef]
- Cherepenin, V.; Karpov, A.; Korjenevsky, A.; Kornienko, V.; Mazaletskaya, A.; Mazourov, D.; Meister, D. A 3D electrical impedance tomography (EIT) system for breast cancer detection. Physiol. Meas. 2001, 22, 9–18. [Google Scholar] [CrossRef]
- Jossinet, J. Variability of impedivity in normal and pathological breast tissue. Med. Biol. Eng. Comput. 1996, 34, 346–350. [Google Scholar] [CrossRef]
- Bayford, R. Bioimpedance tomography (electrical impedance tomography). Annu. Rev. Biomed. Eng. 2006, 8, 63–91. [Google Scholar] [CrossRef]
- Arshad, S.H.; Kunzika, J.S.; Murphy, E.K.; Odame, K.; Halter, R.J. Towards a smart phone-based cardiac monitoring device using electrical impedance tomography. In Proceedings of the 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS), Atlanta, GA, USA, 22–24 October 2015. [Google Scholar] [CrossRef]
- Gabriel, C.; Gabriel, S.; Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 1996, 41, 2231–2249. [Google Scholar] [CrossRef]
- Lazebnik, M.; Popovic, D.; McCartney, L.; Watkins, C.B.; Lindstrom, M.J.; Harter, J.; Sewall, S.; Ogilvie, T.; Magliocco, A.; Breslin, T.M.; et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys. Med. Biol. 2007, 52, 6093. [Google Scholar] [CrossRef] [PubMed]
- Stelter, J.; Wtorek, J.; Nowakowski, A.; Kopacz, A.; Jastrzembski, T. Complex permittivity of breast tumor tissue. In Proceedings of the Tenth International Conference on Electrical Bio-Impedance, Barcelona, Spain, 5–9 April 1998; pp. 59–62. [Google Scholar]
- Jossinet, J.; Schmitt, M. A review of parameters for the bioelectrical characterization of breast tissue. Ann. N. Y. Acad. Sci. 1999, 873, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, A.; Stuchly, S.; Barr, J.; Swarup, A. Dielectric properties of breast carcinoma and the surrounding tissues. IEEE Trans. Biomed. Eng. 1988, 35, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, N.; Hamzaoui, L.; Rochaix, P.; Rigaud, B.; Voigt, J.J.; Morucci, J.P. Ex vivo discrimination between normal and patological tissues in human breast surgical biopsies using bioimpedance spectroscopy. Ann. N. Y. Acad. Sci. 1999, 873, 42–50. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, Z. A review of electrical impedance techniques for breast cancer detection. Med. Eng. Phys. 2003, 25, 79–90. [Google Scholar] [CrossRef]
- Melloul, M.; Paz, A.; Ohana, G.; Laver, O.; Michalevich, D.; Koren, R.; Wolloch, Y.; Gal, R. Double phase 99mTc-sestamibiscintimammography and trans-scan in diagnosing breast cancer. J. Nucl. Med. 1999, 40, 376–380. [Google Scholar]
- Wolf, I.; Sadetzki, S.; Catane, R.; Karasik, A.; Kaufman, B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005, 6, 103–111. [Google Scholar] [CrossRef]
- Ye, G.; Lim, K.H.; George, R.T., Jr.; Ybarra, G.A.; Joines, W.T.; Liu, Q.H. 3D EIT for breast cancer imaging: System, measurements, and reconstruction. Microw. Opt. Technol. Lett. 2008, 50, 3261–3271. [Google Scholar] [CrossRef]
- Halter, R.J.; Zhou, T.; Meaney, P.M.; Hartov, A.; Barth, R.J., Jr.; Rosenkranz, K.M.; Wells, W.A.; Kogel, C.A.; Borsic, A.; Rizzo, E.J.; et al. The correlation of in vivo and ex vivo tissue dielectric properties to validate electromagnetic breast imaging: Initial clinical experience. Physiol. Meas. 2009, 30, S121. [Google Scholar] [CrossRef]
- Wang, K.; Dong, X.; Fu, F.; Liao, Q.; Liu, R.; Ji, Z.; Wang, T. A Primary Research of the Relationship between Breast Tissues Impedance Spectroscopy and Electrical Impedance Scanning. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 1575–1579. [Google Scholar]
- Martellosio, A.; Bellomi, M.; Pasian, M.; Bozzi, M.; Perregrini, L.; Mazzanti, A.; Svelto, F.; Summers, P.E.; Renne, G.; Preda, L. Dielectric Properties Characterization from 0.5 to 50 GHz of Breast Cancer Tissues. IEEE Trans. Microw. Theory Tech. 2017, 65, 998–1011. [Google Scholar] [CrossRef]
- Wang, K.; Wang, T.; Fu, F.; Ji, Z.-Y.; Liu, R.-G.; Liao, Q.-M.; Dong, X.-Z. Electrical impedance scanning in breast tumor imaging: Correlation with the growth pattern of lesion. Chin. Med. J. 2009, 122, 1501–1506. [Google Scholar] [PubMed]
- Yorkey, T.J.; Webster, J.G.; Tompkins, W.J. Comparing Reconstruction Algorithms for Electrical Impedance Tomography. IEEE Trans. Biomed. Eng. 1987, 34, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Lionheart, W.; Polydorides, N.; Borsic, A. “The Reconstruction Problem” in Electrical Impedance Tomography: Methods, History and Applications, 1st ed.; Holder, D.S., Ed.; IOP: London, UK, 2005; Volume 1, pp. 1–64. [Google Scholar]
- Kantartzis, P.; Abdi, M.; Liatsis, P. Stimulation and measurement patterns versus prior information for fast 3D EIT: A breast screening case study. Signal Process. 2012, 93, 2838–2850. [Google Scholar] [CrossRef]
- Markar, S.R.; Wiggins, T.; Antonowicz, S.; Chin, S.; Romano, A.; Nikolic, K.; Evans, B.; Cunningham, D.; Mughal, M.; Lagergren, J.; et al. Assessment of a Noninvasive Exhaled Breath Test for the Diagnosis of Oesophagogastric Cancer. JAMA Oncol. 2018, 4, 970–976. [Google Scholar] [CrossRef]
- Young, D.O.; Kumar, A.S. Local excision of rectal cancer. Surg. Clin. N. Am. 2017, 97, 573–585. [Google Scholar] [CrossRef]
- Oberlin, D.T.; Flum, A.S.; Lai, J.D.; Meeks, J.J. The effect of minimally invasive prostatectomy on practice patterns of American urologists. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 255.e1–255.e5. [Google Scholar] [CrossRef]
- St John, E.A.; Ashrafian, H.; Athanasiou, T.; Takats, Z.; Hadjiminas, D.J.; Darzi, A.; Leff, D.R. Diagnostic accuracy of intraoperative techniques for margin assessment in breast cancer surgery: A meta-analysis. Ann. Surg. 2016, 265, 300–310. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, B.; Wang, H.; Ye, J. New Flexible Tactile Sensor Based on Electrical Impedance Tomography. Micromachines 2022, 13, 185. [Google Scholar] [CrossRef]
- Zain, M.N.; Kanaga, K. A Review on breast electrical impedance tomography clinical accuracy. ARPN J. Eng. Appl. Sci. 2015, 10, 6230–6234. [Google Scholar]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clínica 2016, 147, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.C.; Barbosa, V.A.F.; Ribeiro, D.E.; De Souza, R.E.; Dos Santos, W.P. Electrical impedance tomography image reconstruction based on backprojection and extreme learning machines. Res. Biomed. Eng. 2020, 36, 399–410. [Google Scholar] [CrossRef]
- Singh, G.; Anand, S.; Lall, B.; Srivastava, A.; Singh, V. A Low-Cost Portable Wireless Multi-frequency Electrical Impedance Tomography System. Arab. J. Sci. Eng. 2019, 44, 2305–2320. [Google Scholar] [CrossRef]
- Kumar, S.P.; Sriraam, N.; Benakop, P.; Jinaga, B.C. Reconstruction of brain electrical impedance tomography images using particle swarm optimization. In Proceedings of the 2010 5th International Conference on Industrial and Information Systems, Mangalore, India, 29 July–1 August 2010; pp. 339–342. [Google Scholar]
- Tehrani, J.N.; Jin, C.; McEwan, A.; van Schaik, A. A comparison between compressed sensing algorithms in Electrical Impedance Tomography. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 3109–3112. [Google Scholar]
- Tang, M.; Wang, W.; Wheeler, J.; McCormick, M.; Dong, X. The number of electrodes and basis functions in EIT image reconstruction. Physiol. Meas. 2002, 23, 129–140. [Google Scholar] [CrossRef]
- Choridah, L.; Kurniadi, D.; Ain, K.; Ulum, M.; Mukhaiyar, U.; Garnadi, A.; Setyawan, N. Comparison of electrical impedance tomography and ultrasonography for determination of solid and cystic lesion resembling breast tumor embedded in chicken phantom. J. Electr. Bioimpedance 2021, 12, 63–68. [Google Scholar] [CrossRef]
- Hu, J.; Soleimani, M. Combining Multiple Boundary Shapes in Deformable EIT a Potential Use in Breast Imaging. IEEE Sens. Lett. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Lee, J.; Gweon, S.; Lee, K.; Um, S.; Lee, K.-R.; Kim, K.; Lee, J.; Yoo, H.-J. A 9.6 mW/Ch 10 MHz Wide-bandwidth Electrical Impedance Tomography IC with Accurate Phase Compensation for Breast Cancer Detection. In Proceedings of the 2020 IEEE Custom Integrated Circuits Conference (CICC), Boston, MA, USA, 22–25 March 2020. [Google Scholar] [CrossRef]
- Mansouri, S.; Alhadidi, T.; Ben Azouz, M. Breast Cancer Detection Using Low-Frequency Bioimpedance Device. Breast Cancer Targets Ther. 2020, 12, 109–116. [Google Scholar] [CrossRef]
- Murillo-Ortiz, B.; Hernández-Ramírez, A.; Rivera-Villanueva, T.; Suárez-García, D.; Murguía-Pérez, M.; Martínez-Garza, S.; Rodríguez-Penin, A.; Romero-Coripuna, R.; López-Partida, X.M. Monofrequency electrical impedance mammography (EIM) diagnostic system in breast cancer screening. BMC Cancer 2020, 20, 876. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Jia, J.; Bagnaninchi, P. Deep Learning Based Cell Imaging with Electrical Impedance Tomography. In Proceedings of the 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Dubrovnik, Croatia, 25–28 May 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Gutierrez-Lopez, M.; Prado-Olivarez, J.; Diaz-Carmona, J.; Herrera-Ramírez, C.A.; Gutierrez-Gnecchi, J.A.; Medina-Sánchez, C.G. Electrical Impedance-Based Methodology for Locating Carcinoma Emulators on Breast Models. J. Sens. 2019, 2019, 8587191. [Google Scholar] [CrossRef]
- Rao, A.J.; Murphy, E.K.; Shahghasemi, M.; Odame, K.M. Current-conveyor-based wide-band current driver for electrical impedance tomography. Physiol. Meas. 2019, 40, 034005. [Google Scholar] [CrossRef]
- Mothi, V.; Chiew, Y.S.; Tan, C.P. Development of Electrical Impedance Tomography for Breast Phantom Monitoring. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Y.; Bagnaninchi, P.O.; Jia, J. Electrical impedance tomography for real-time and label-free cellular viability assays of 3D tumour spheroids. Analyst 2018, 143, 4189–4198. [Google Scholar] [CrossRef] [PubMed]
- Zarafshani, A.; Bach, T.; Chatwin, C.R.; Tang, S.; Xiang, L.; Zheng, B. Conditioning Electrical Impedance Mammography System. Measurement 2018, 116, 38–48. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, J.; Smith, S.; Jamil, N.; Gamal, W.; Bagnaninchi, P.-O. A Miniature Electrical Impedance Tomography Sensor and 3-D Image Reconstruction for Cell Imaging. IEEE Sens. J. 2016, 17, 514–523. [Google Scholar] [CrossRef]
- Murphy, E.K.; Mahara, A.; Halter, R.J. Absolute Reconstructions Using Rotational Electrical Impedance Tomography for Breast Cancer Imaging. IEEE Trans. Med. Imaging 2017, 36, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, K.; Ha, U.; Kim, H.; Lee, Y.; Kim, Y.; Yoo, H.-J. A 4.9 mΩ-Sensitivity Mobile Electrical Impedance Tomography IC for Early Breast-Cancer Detection System. IEEE J. Solid-State Circuits 2015, 50, 245–257. [Google Scholar] [CrossRef]
- Khan, S.; Manwaring, P.; Borsic, A.; Halter, R. FPGA-Based Voltage and Current Dual Drive System for High Frame Rate Electrical Impedance Tomography. IEEE Trans. Med. Imaging 2015, 34, 888–901. [Google Scholar] [CrossRef]
- Zhang, X.; Chatwin, C.; Barber, D.C. A feasibility study of a rotary planar electrode array for electrical impedance mammography using a digital breast phantom. Physiol. Meas. 2015, 36, 1311–1335. [Google Scholar] [CrossRef][Green Version]
- Halter, R.J.; Hartov, A.; Poplack, S.P.; DiFlorio-Alexander, R.; Wells, W.A.; Rosenkranz, K.M.; Barth, R.J.; Kaufman, P.A.; Paulsen, K.D. Real-Time Electrical Impedance Variations in Women With and Without Breast Cancer. IEEE Trans. Med. Imaging 2015, 34, 38–48. [Google Scholar] [CrossRef]
- Takkar, N.; Kochhar, S.; Garg, P.; Pandey, A.K.; Dalal, U.R.; Handa, U. Screening methods (clinical breast examination and mammography) to detect breast cancer in women aged 40–49 years. J. Mid-Life Health 2017, 8, 2–10. [Google Scholar] [CrossRef]
- Cheng, H.D.; Shan, J.; Ju, W.; Guo, Y.; Zhang, L. Automated breast cancer detection and classification using ultrasound images: A survey. Pattern Recognit. 2010, 43, 299–317. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, Breast Ultrasound, and Magnetic Resonance Imaging for Surveillance of Women at High Familial Risk for Breast Cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Isaacs, C.; Schnall, M.D.; Pisano, E.D.; Ascher, S.M.; Weatherall, P.T.; Bluemke, D.A.; Bowen, D.J.; Marcom, P.K.; Armstrong, D.K.; et al. Cancer Yield of Mammography, MR, and US in High-Risk Women: Prospective Multi-Institution Breast Cancer Screening Study. Radiology 2007, 244, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Imaging Technique | Proposal Validation | Tumor Size |
|---|---|---|---|
| Choridah et al. (2021) [47] | Imaging using a single layer (2D) | E: Chicken phantom filled with an artificial solid tumor | - |
| Gomes et al. (2020) [42] | Imaging using a single layer (2D) | S: Images generated in MATLAB | - |
| Hu Jing et al. (2020) [48] | Imaging using a single layer (2D) | E: 3D printed samples and phantoms | From 5 mm. |
| Lee Jaehyuk et al. (2020) [49] | Imaging using a single layer (2D) | E: Agar phantom using carrots as tumors | From 5 mm. |
| Mansouri et al. (2020) [50] | Impedance measurements between left and right breast | CT: 40 women. | - |
| Murillo-Ortiz et al. (2020) [51] | Single layer imaging (2D), tumor classification | CT: 1200 women | - |
| Chen et al. (2020) [52] | Single layer imaging(2D) and image processing | E: Phantom in micro scale | - |
| Gutierrez et al. (2019) [53] | Normalized Impedance plots | E: Agar breast phantom model | From 10 mm. |
| Rao et al. (2019) [54] | Single layer imaging (2D) | E: Saline tank setup | From 13 mm. |
| Mothi et al. (2018) [55] | Single layer imaging on EIDORS Software (2D) | E: Gelatine breast phantom model | From 10 mm. |
| Wu et al. (2018) [56] | Single layer imaging (2D) | E: Miniature EIT sensor using solution | From 1.2 mm. |
| Zarafshani et al. (2018) [57] | Single layer imaging (2D) | E: E-phantom realistic model | - |
| Singh et al. (2017) [43] | Single layer imaging on EIDORS software (2D) | E: Plastic tank phantom and background solution | - |
| Yunjie Yang et al. (2016) [58] | Multiple layers imaging (3D) | E: Miniature phantom | From 0.55 mm. |
| Murphy et al. (2016) [59] | Imaging of electrical conductivity cross-section (2D) | E: Tank filled with saline solution | From 10 mm. |
| Hong Sunjoo et al. (2015) [60] | 3D reconstruction on a mobile device (3D) | E: Agar breast phantom model | From 5 mm. |
| Khan Shadab et al. (2015) [61] | Single layer imaging (2D) | E: Tank filled with saline solution | From 25 mm. |
| Zhang et al. (2015) [62] | Multiple layers imaging (3D) | S: Digital breast phantom | From 15 mm. |
| Halther et al. (2015) [63] | Single layer imaging (2D) | CT: 19 women | From 20 mm. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Cortés, J.C.; Díaz-Carmona, J.J.; Padilla-Medina, J.A.; Calderon, A.E.; Gutiérrez, A.I.B.; Gutiérrez-López, M.; Prado-Olivarez, J. Electrical Impedance Tomography Technical Contributions for Detection and 3D Geometric Localization of Breast Tumors: A Systematic Review. Micromachines 2022, 13, 496. https://doi.org/10.3390/mi13040496
Gómez-Cortés JC, Díaz-Carmona JJ, Padilla-Medina JA, Calderon AE, Gutiérrez AIB, Gutiérrez-López M, Prado-Olivarez J. Electrical Impedance Tomography Technical Contributions for Detection and 3D Geometric Localization of Breast Tumors: A Systematic Review. Micromachines. 2022; 13(4):496. https://doi.org/10.3390/mi13040496
Chicago/Turabian StyleGómez-Cortés, Juan Carlos, José Javier Díaz-Carmona, José Alfredo Padilla-Medina, Alejandro Espinosa Calderon, Alejandro Israel Barranco Gutiérrez, Marcos Gutiérrez-López, and Juan Prado-Olivarez. 2022. "Electrical Impedance Tomography Technical Contributions for Detection and 3D Geometric Localization of Breast Tumors: A Systematic Review" Micromachines 13, no. 4: 496. https://doi.org/10.3390/mi13040496
APA StyleGómez-Cortés, J. C., Díaz-Carmona, J. J., Padilla-Medina, J. A., Calderon, A. E., Gutiérrez, A. I. B., Gutiérrez-López, M., & Prado-Olivarez, J. (2022). Electrical Impedance Tomography Technical Contributions for Detection and 3D Geometric Localization of Breast Tumors: A Systematic Review. Micromachines, 13(4), 496. https://doi.org/10.3390/mi13040496









