Antibody-Conjugated Magnetic Beads for Sperm Sexing Using a Multi-Wall Carbon Nanotube Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
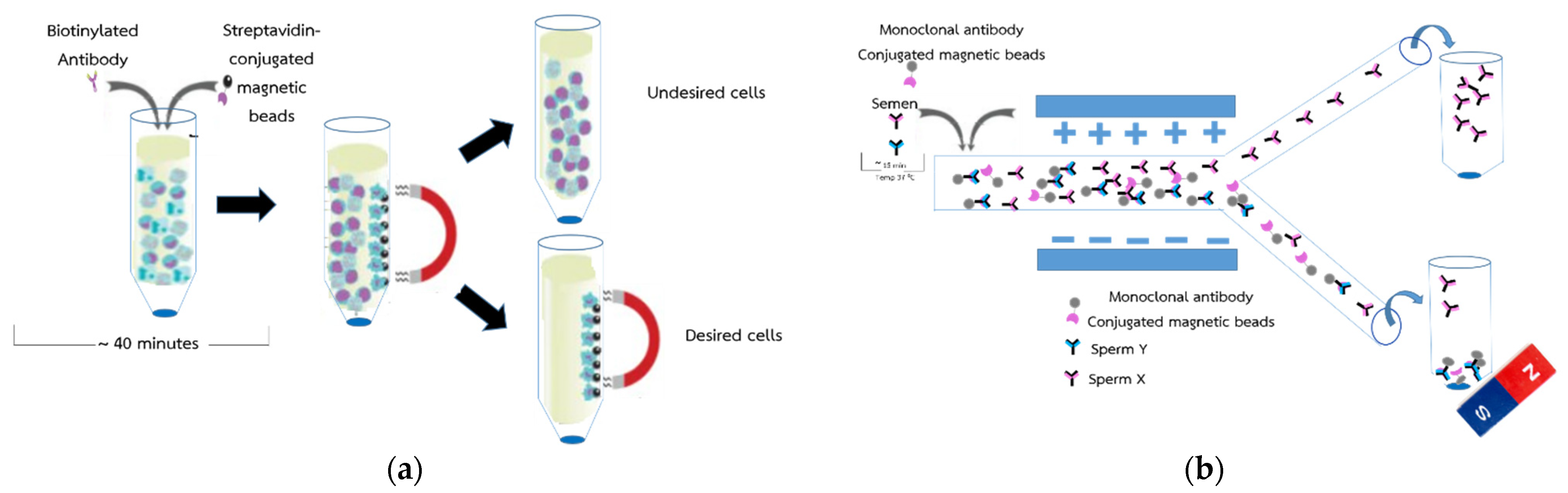
2.1. Conceptual Design for Novel Sperm Sorting Method
2.2. CNTs (MWCNTs)
2.3. Magnetic Beads
2.4. Preparation of Monoclonal Antibodies Conjugated with Sperm
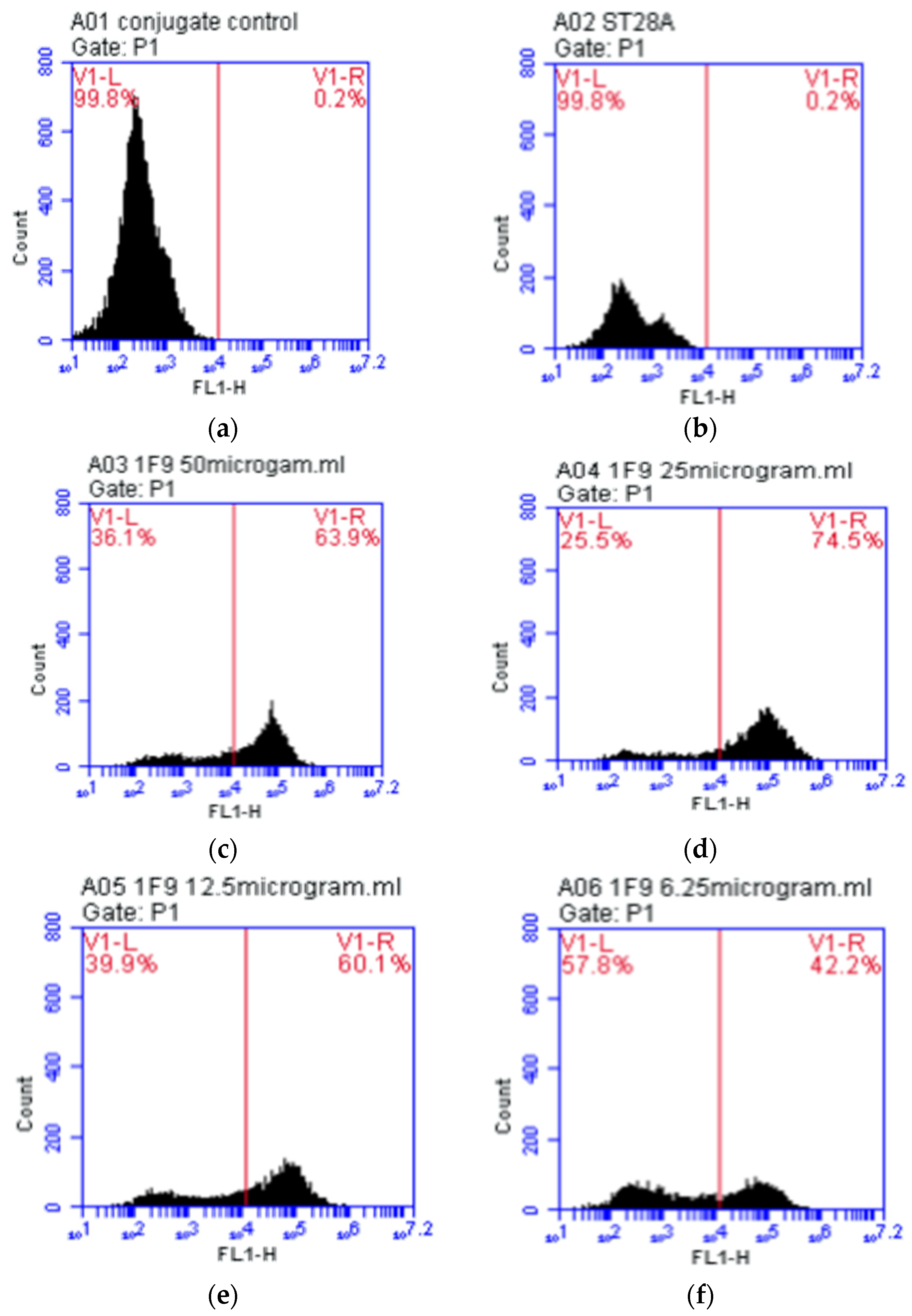
2.4.1. Control Sample
2.4.2. Monoclonal Antibody 1F9
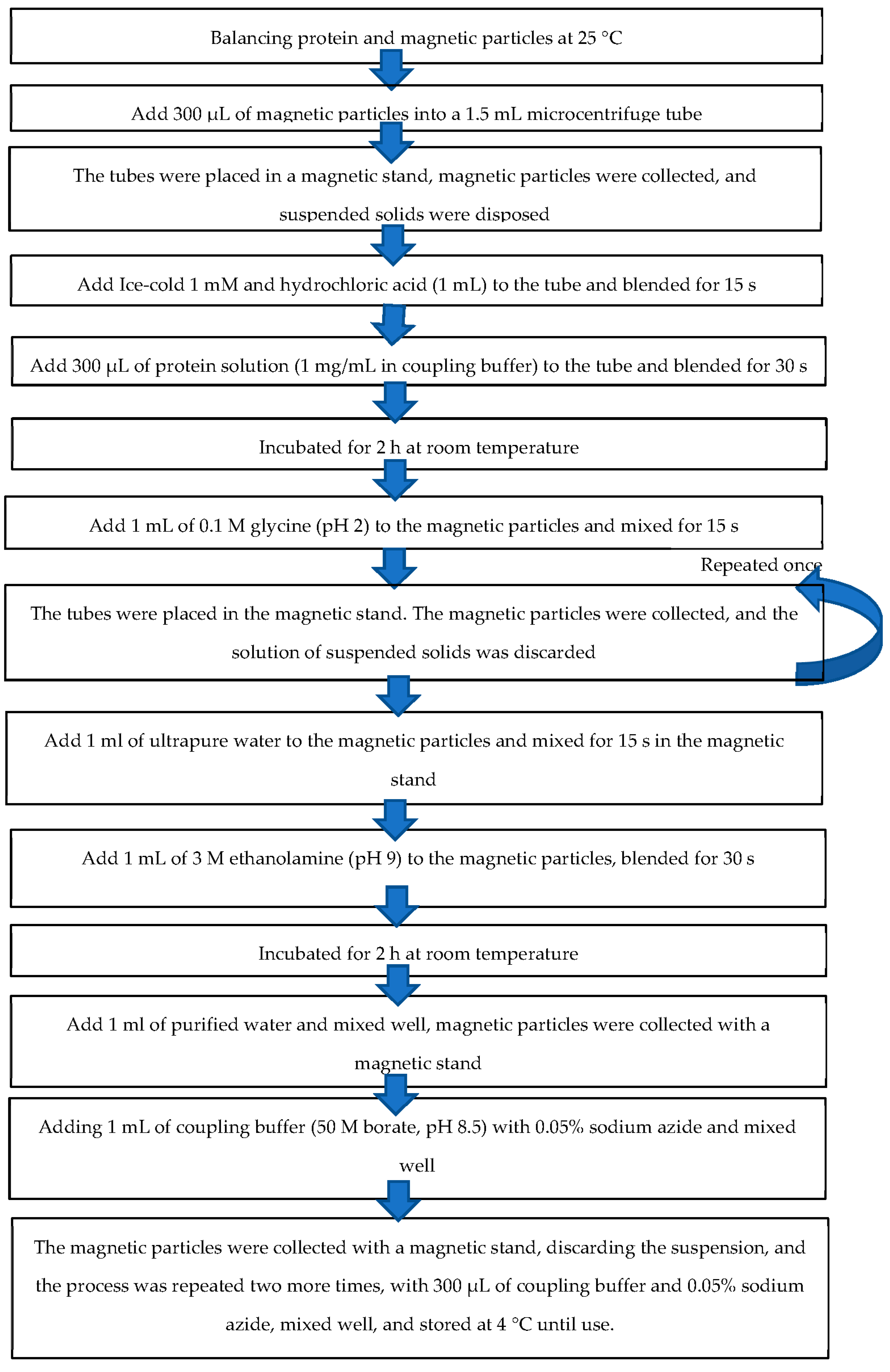
2.5. Preparation of Monoclonal Antibodies Conjugated with Magnetic Particles
2.6. Monoclonal Antibodies Conjugated with Magnetic Particles and Sperm
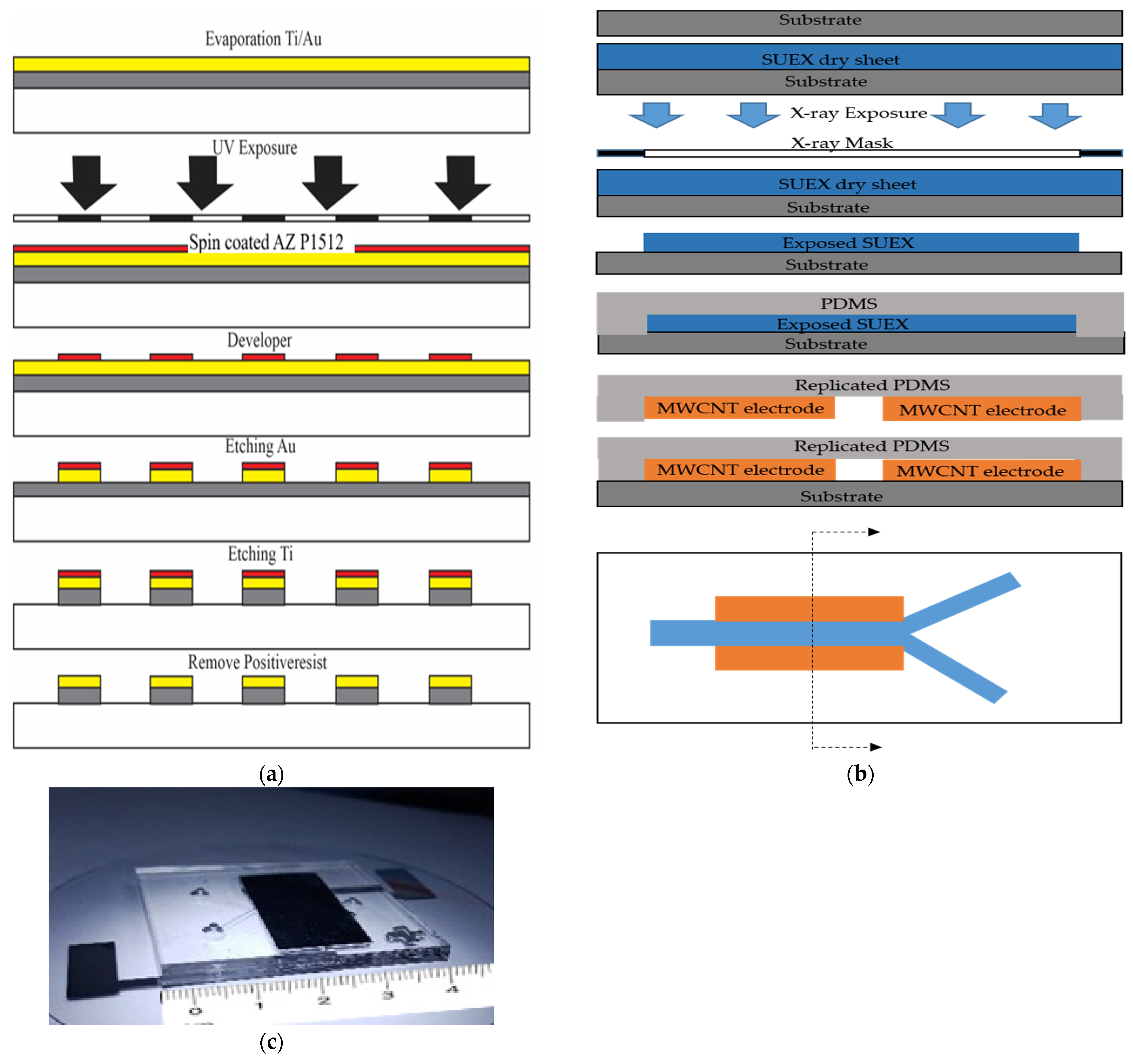
2.7. Microfluidic Device Fabrication
2.7.1. Fabrication of Microfluidic Chip for Thin Film Electrode
2.7.2. Fabrication of Microfluidic Chip for MWCNT Electrode
3. Results
3.1. Testing of Monoclonal Antibodies Conjugated with Sperm
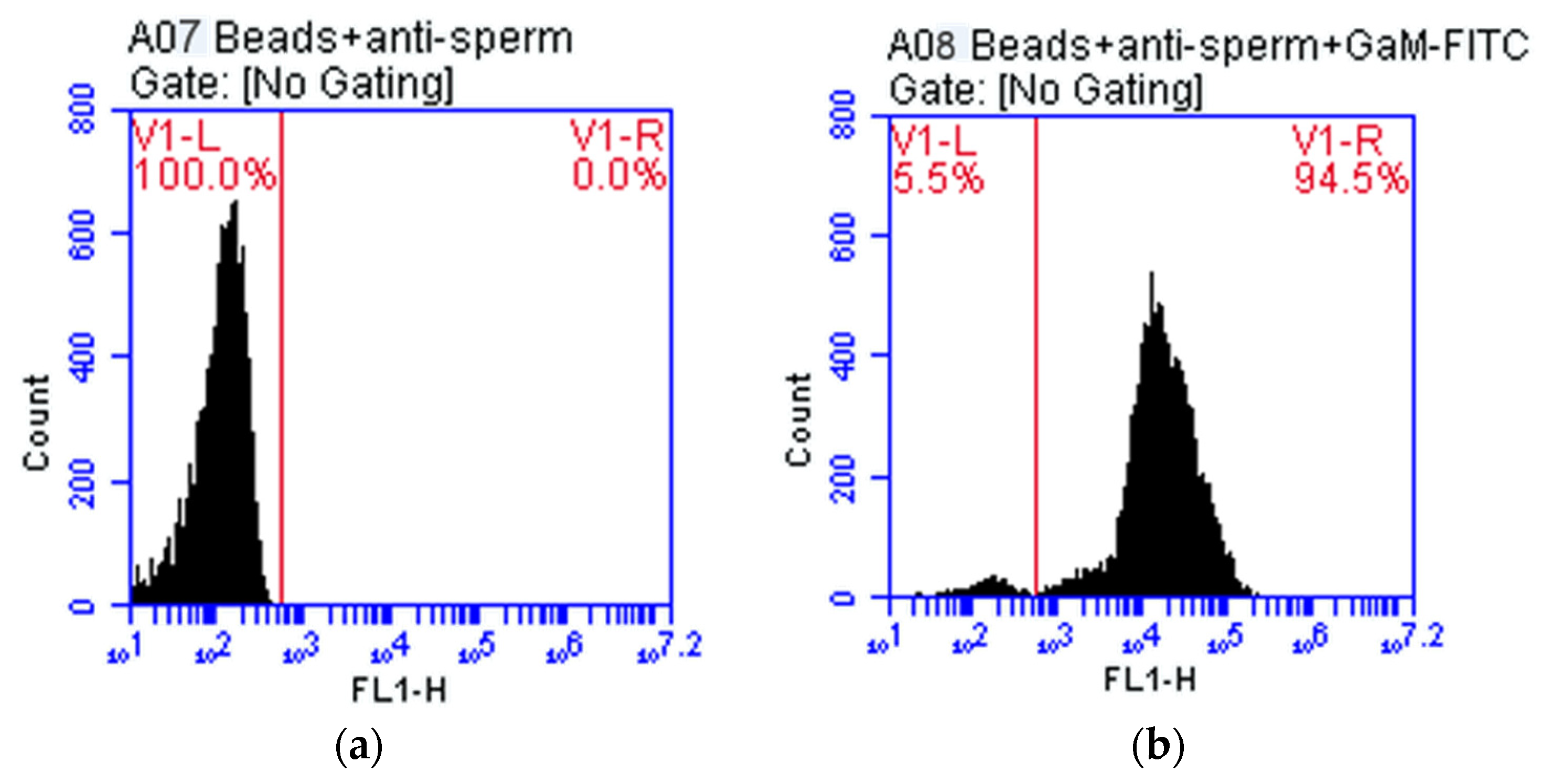
3.2. Testing of Monoclonal Antibodies Conjugated with Magnetic Beads
3.3. Testing the Magnetic Particle Beads in a Microfluidic Chip
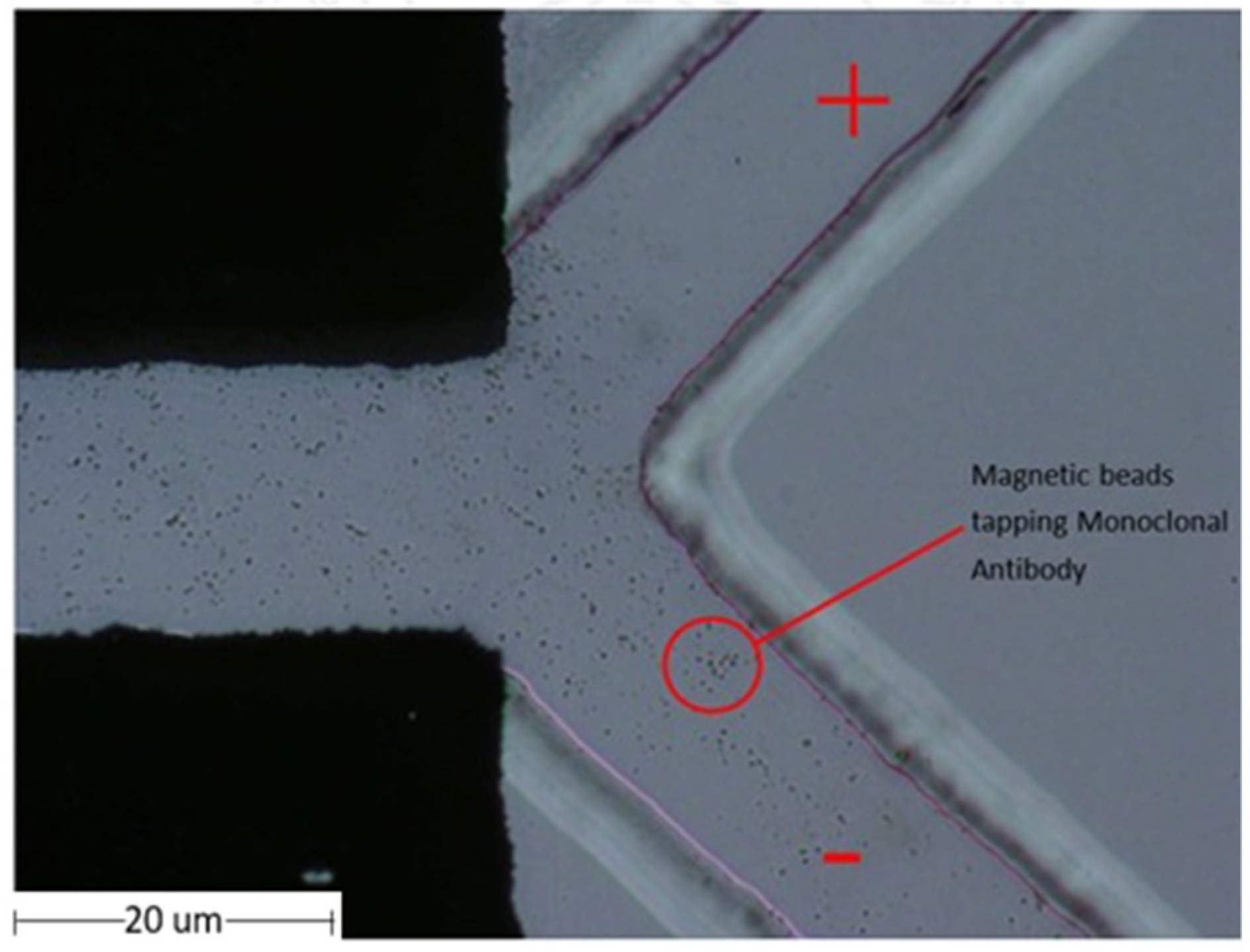
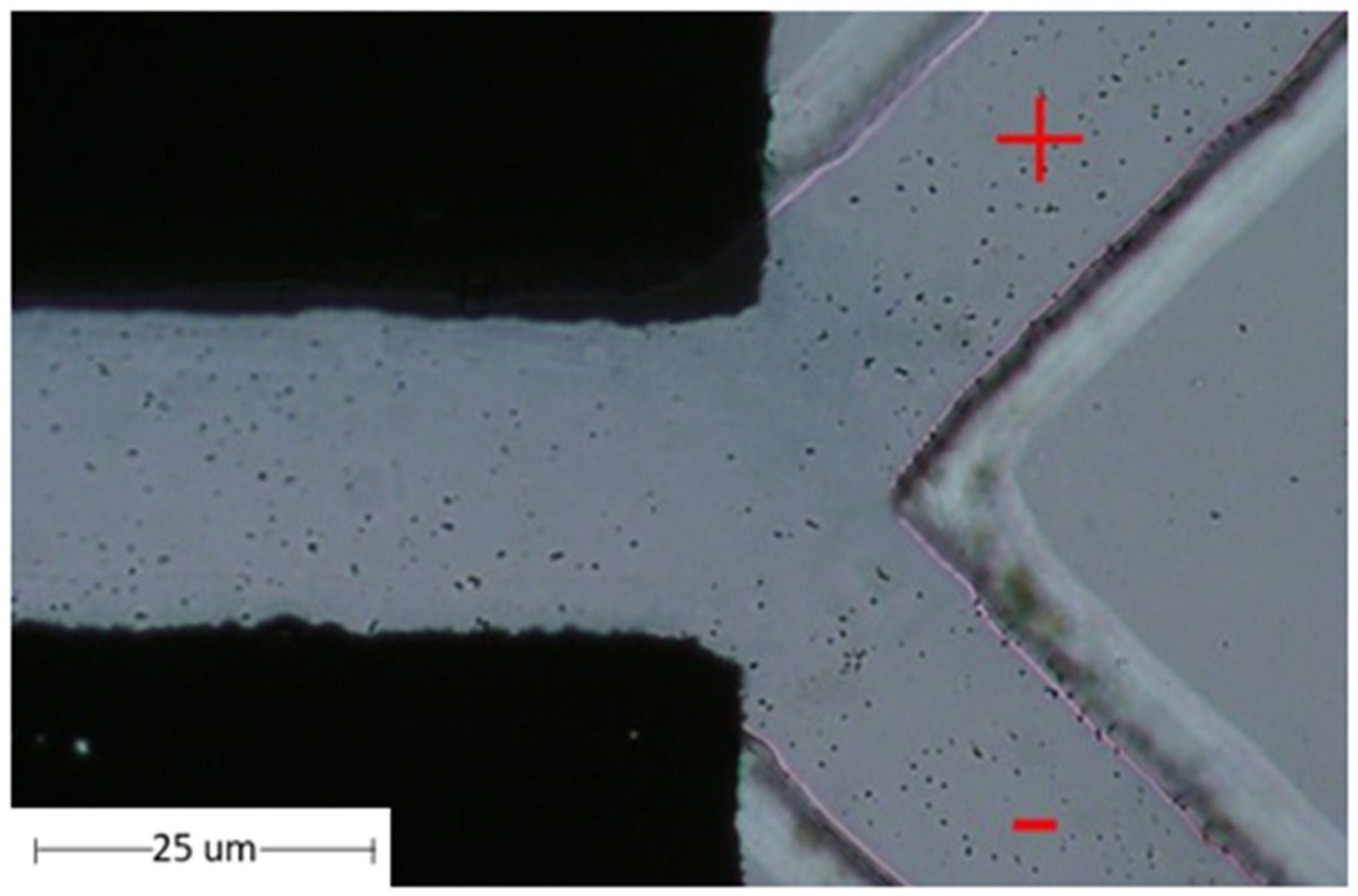
3.4. Testing the Monoclonal Antibodies Conjugated with Magnetic Beads in a Microfluidic Chip
3.5. Testing Sperm and Sperm with Monoclonal Antibodies Conjugated Magnetic Particle Beads in Microfluidic Chip
4. Discussion
4.1. Magnetic Particles Beads in Microfluidic System
4.2. Monoclonal Antibodies Conjugated with Magnetic Particle Beads
4.3. Sperm and Sperm with Monoclonal Antibodies Conjugated Magnetic Particle Beads
4.4. Magnetic Beads and Electrophoresis Sperm Separation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidel, G.E., Jr. Overview of sexing sperm. Theriogenology 2007, 68, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, U.; Krassnigg, F.; Schatz, H.; Schill, W.B. Separation of human X and Y spermatozoa by free-flow electrophoresis. Gamete Res. 1998, 19, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ishijima, S.A.; Okuno, M.; Mohri, H. Zeta potential of human X- and Y-bearing sperm. Int. J. Androl. 1991, 14, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Iizuka, R.; Oshiro, S.; Nakajima, H.; Oshio, S.; Mohri, H. Separation of human X- and Y-bearing sperm using free-flow electrophoresis. Proc. Jpn. Acad. Ser. B 1983, 59, 276–279. [Google Scholar] [CrossRef][Green Version]
- Wannaluk, T.; Surat, H.; Marninphan, T.; Supamit, M.; Wiwat, P.; Korawan, S. Production of single-chain fragment variable (scFv) antibodies specific to plasma membrane epitopes on bull Y-bearing sperm. Anim. Biotechnol. 2020, 1–11. [Google Scholar] [CrossRef]
- Quelhas, J.; Santiago, J.; Matos, B.; Rocha, A.; Lopes, G.; Fardilha, M. Bovine semen sexing: Sperm embrane proteomics as candidates for immunological selection of X- and Ychromosome-bearing sperm. Vet. Med. Sci. 2021, 7, 1613–1641. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Keeler, K.D.; Noakes, D.E.; Mackenzie, N.M.; Dresser, D.W. Sexing of sperm by flow cytometry. Vet. Rec. 1988, 122, 322–324. [Google Scholar] [CrossRef]
- Garner, D.L.; Seidel, G.E., Jr. Past, present and future perspectives on sexing sperm. Can. J. Anim. Sci. 2003, 83, 375–384. [Google Scholar] [CrossRef]
- Zhang, H.; Xuan, X.; Yang, S.; Li, X.; Xu, C.; Gao, X. Selection of viable human spermatozoa with low levels of DNA fragmentation from an immotile population using density gradient centrifugation and magnetic-activated cell sorting. Andrologia 2018, 50, e12821. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.; Feng, H.; Jafek, A.; Despain, D.; Jenkins, T.; Gale, B. Microfluidic-Based sperm sorting & analysis for treatment of male infertility. Transl. Androl. Urol. 2018, 7, S336–S347. [Google Scholar] [PubMed]
- Bonner, W.A.; Hulett, H.R.; Sweet, R.G.; Herzenberg, L.A. Fluorescence activated cell sorting. Rev. Sci. Instrum. 1972, 43, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Welch, G.R.; Keyvanfar, K.; Dorfmann, A.; Fugger, E.F.; Schulman, J.D. Gender preselection in humans Flow cytometric separation of X and Y spermatozoa for the prevention of X-linked diseases. Hum. Reprod. 1993, 8, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, D.; Kalams, S.A.; Eid, J.E. DC-dielectrophoretic separation of biological cells by size. Biomed. Microdevices 2008, 10, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Aitken, R.J. The Physical, Insemination, and Reproductive Quality of Honey Bee Queens (Apis mellifera L.); Springer: New York, NY, USA, 2011; pp. 423–429. [Google Scholar]
- Solomon, D.H.; Caulfield, M.J.; Purss, H.K. Polymerization Process and Polymers Produced Thereby. U.S. Patent US4581429A, 8 April 1986. [Google Scholar]
- Phiphatanaphiphop, C.; Leksakul, K.; Phatthanakun, R.; Busayaporn, W.; Saiyasombat, C.; Phothongkam, P.; Rana, M.M.; Suzuki, H. Multiwalled carbon nanotubes in microfluidic chip for the separation of X- and Y-sperm based on a photolithography technique. J. Microelectromech. Syst. 2020, 29, 1264–1277. [Google Scholar] [CrossRef]
- Rappa, K.L.; Rodriguez, H.F.; Hakkarainen, G.C.; Anchan, R.M.; Mutter, G.L.; Asghar, W. Sperm processing for advanced reproductive technologies: Where are we today? Biotechnol. Adv. 2016, 34, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Miska, W.; Miska, G.; Rasch, M.; Reinhardt, M.; Glander, H.-J.; Paasch, U. Molecular glass wool filtration as a new tool for sperm preparation. Hum. Reprod. 2007, 22, 1405–1412. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Zborowski, M.; Grunewald, S.; Glander, H.-J.; Paasch, U. Utility of magnetic cell separation as a molecular sperm preparation technique. J. Androl. 2008, 29, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Phiphattanaphiphop, C.; Leksakul, K.; Phatthanakun, R.; Khamlor, T. A novel microfluidic chip-based sperm-sorting device constructed using design of experiment method. Sci. Rep. 2020, 10, 17143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Khimji, I.; Gurkan, U.A.; Safaee, H.; Catalano, P.N.; Keles, H.O.; Kayaalp, E.; Demirci, U. Lensless imaging for simultaneous microfluidic sperm monitoring and sorting. Lab Chip 2011, 11, 2535–2540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, R.R.; Yin, T.; Zou, W.; Tang, Y.; Ding, J.; Yang, J. Generation of gradients on a microfluidic device: Toward a high-throughput investigation of spermatozoa chemotaxis. PLoS ONE 2015, 10, e0142555. [Google Scholar] [CrossRef]
- Ul-Husna, A.; Awan, M.A.; Mehmood, A.; Sultana, T.; Shahzad, Q.; Ansar, M.S.; Rakha, B.A.; Naqvi, S.M.S.; Akhter, S. Sperm sexing in Nili-Ravi buffalo through modified swim up: Validation using SYBR® green real-time PCR. Anim. Reprod. Sci. 2017, 182, 69. [Google Scholar] [CrossRef] [PubMed]
- Beernink, F.J.; Dmowski, W.P.; Ericsson, R.J. Sex preselection through albumin separation of sperm. Fertil. Steril. 1993, 59, 382–386. [Google Scholar] [CrossRef]
- Ericsson, R.J.; Langevin, C.N.; Nishino, M. Isolation of fractions rich in human Y sperm. Nature 1973, 246, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E. Sperm Sexing Mediated by Magnetic Nanoparticles in Donkeys, a Preliminary In Vitro Study. J. Equine Vet. Sci. 2018, 65, 123–127. [Google Scholar] [CrossRef]
- Hashimoto, H.; Eto, T.; Suemizu, H.; Ito, M. Application of a new convenience gender sorting method for mouse spermatozoa to mouse reproductive engineering technology. J. Vet. Med. Sci. 2013, 75, 231–235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xuan, X.; Li, D. Focused electrophoretic motion and selected electrokinetic dispensing of particles and cells in cross-microchannels. Electrophoresis 2005, 26, 3552–3560. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Natl. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-B.; Agca, Y.; Feng, Z.C.; Critser, J.K. Development of sorting, aligning, and orienting motile sperm using microfluidic device operated by hydrostatic pressure. Microfluid. Nanofluid. 2007, 3, 561–570. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semenl; World Health Organization: Geneva, Switzerland, 2010.
- Alvarez, J.G.; Lasso, J.L.; Blasco, L.; Nuñez, R.C.; Heyner, S.; Caballero, P.P.; Storey, B.T. Centrifugation of human spermatozoa induces sublethal damage; separation of human spermatozoa from seminal plasma by a dextran swim-up procedure without centrifugation extends their motile lifetime. Hum. Reprod. 1993, 8, 1087–1092. [Google Scholar] [CrossRef]
- Barroso, G.; Morshedi, M.; Oehninger, S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum. Reprod. 2000, 15, 1338–1344. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J. Androl. 1988, 9, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.; Lewis, S.E.; McKelvey-Martin, V.J.; Thompson, W. The effects of antioxidant supplementation during percoll preparation on human sperm DNA integrity. Hum. Reprod. 1998, 13, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Kashaninejad, N.; Shiddiky, M.J.A.; Nguyen, N.-T. Advances in microfluidics-based assisted reproductive technology: From sperm sorter to reproductive system-on-a-chip. Adv. Biosyst. 2018, 2, 1700197. [Google Scholar] [CrossRef]
- Saar, K.L.; Peter, Q.; Müller, T.; Challa, P.K.; Herling, T.W.; Knowles, T.P.J. Rapid two-dimensional characterisation of proteins in solution. Microsyst. Nanoeng. 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed]



| Material | MWCNTs | MWCNTs + PDMS 0.5 g:10 g | MWCNTs + PDMS 1 g:10 g |
|---|---|---|---|
| Voltage (V) | Current (A) | Current (A) | Current (A) |
| 0.1 | 1.44 × 10−3 | No Value | No Value |
| 0.2 | 2.91 × 10−3 | No Value | No Value |
| 0.3 | 4.45 × 10−3 | No Value | No Value |
| 0.4 | 6.08 × 10−3 | No Value | No Value |
| 0.5 | 7.85 × 10−3 | 1.85 × 10−9 | 6.62 × 10−8 |
| Parameter | Preparation of Sample |
|---|---|
| A01 | 50 μL of FACS buffer and 50 μL of bull sperm |
| A02 | 1 μL of ST28A antibody, 50 μL of FACS buffer, and 50 μL of bull sperm. |
| A03 | 50 μL of 100 μg/mL MAb, with a final 1F9 MAb concentration of 50 μg/mL |
| A04 | 50 μL of 50 μg/mL MAb and 50 μL of bull sperm, with a final 1F9 MAb concentration of 25 μg/mL. |
| A05 | 50 µL of 25 µg/mL MAb and 50 µL cow sperm, with a final 1F9 MAb concentration of 12.5 µg/mL |
| A06 | 50 µL of 12.5 µg/mL MAb and 50 µL of cow sperm, with a final 1F9 MAb concentration of 6.25 µg/mL |
| Plot 2: A08 Beads + Anti-Sperm + GαM-FITC | Count | Events/µL | % of This Plot | % of All | Mean FL1-H | CV FL1-H |
|---|---|---|---|---|---|---|
| All | 11,931 | 5966 | 100.00% | 100.00% | 25,833.83 | 177.21% |
| V1-L (1.0/528.0) | 652 | 326 | 5.46% | 5.46% | 144.73 | 78.39% |
| V1-R (528.0/16,777,215.0) | 11,279 | 5640 | 94.54% | 94.54% | 27,318.83 | 170.78% |
| Type of Electrode | Voltage (V) | Sorting of Magnetic Particle Beads in Microfluidic Capacity (%) |
|---|---|---|
| Microfluidic Thin film electron | 2.5 | 100 |
| MCNT-Microfluidic chip | 2.5 | 100 |
| Type of Electrode | Voltage (V) | Sorting Magnetic Particles on Monoclonal Antibody Capacity (%) |
|---|---|---|
| Microfluidic Thin film electron | 2.5 | 95.42 |
| MCNT-Microfluidic chip | 2.5 | 98.84 |
| Type of Electrode | Sorting Sperm Y/X Capacity (%) without Magnetic Beads (Bottom) | Sorting Sperm Y/X Capacity (%) without Magnetic Beads (Top) | Sorting Antibody + Magnetic Beads + Sperm Y/X (Bottom) | Sorting Antibody + Magnetic Beads + Sperm Y/X (Top) |
|---|---|---|---|---|
| Thin film electrodes microfluidic chip | 53.99/46.01 | 50.39/49.61 | 61.11/38.89 | 31.33/68.67 |
| MCNTs electrodes microfluidic chip | 74.62/25.38 | 51.29/48.71 | 80.12/19.88 | 28.56/71.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiphattanaphiphop, C.; Leksakul, K.; Wanta, T.; Khamlor, T.; Phattanakun, R. Antibody-Conjugated Magnetic Beads for Sperm Sexing Using a Multi-Wall Carbon Nanotube Microfluidic Device. Micromachines 2022, 13, 426. https://doi.org/10.3390/mi13030426
Phiphattanaphiphop C, Leksakul K, Wanta T, Khamlor T, Phattanakun R. Antibody-Conjugated Magnetic Beads for Sperm Sexing Using a Multi-Wall Carbon Nanotube Microfluidic Device. Micromachines. 2022; 13(3):426. https://doi.org/10.3390/mi13030426
Chicago/Turabian StylePhiphattanaphiphop, Chalinee, Komgrit Leksakul, Thananut Wanta, Trisadee Khamlor, and Rungrueang Phattanakun. 2022. "Antibody-Conjugated Magnetic Beads for Sperm Sexing Using a Multi-Wall Carbon Nanotube Microfluidic Device" Micromachines 13, no. 3: 426. https://doi.org/10.3390/mi13030426
APA StylePhiphattanaphiphop, C., Leksakul, K., Wanta, T., Khamlor, T., & Phattanakun, R. (2022). Antibody-Conjugated Magnetic Beads for Sperm Sexing Using a Multi-Wall Carbon Nanotube Microfluidic Device. Micromachines, 13(3), 426. https://doi.org/10.3390/mi13030426







