Facile Synthesis of 3D Printed Tailored Electrode for 3-Monochloropropane-1,2-Diol (3-MCPD) Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Methods
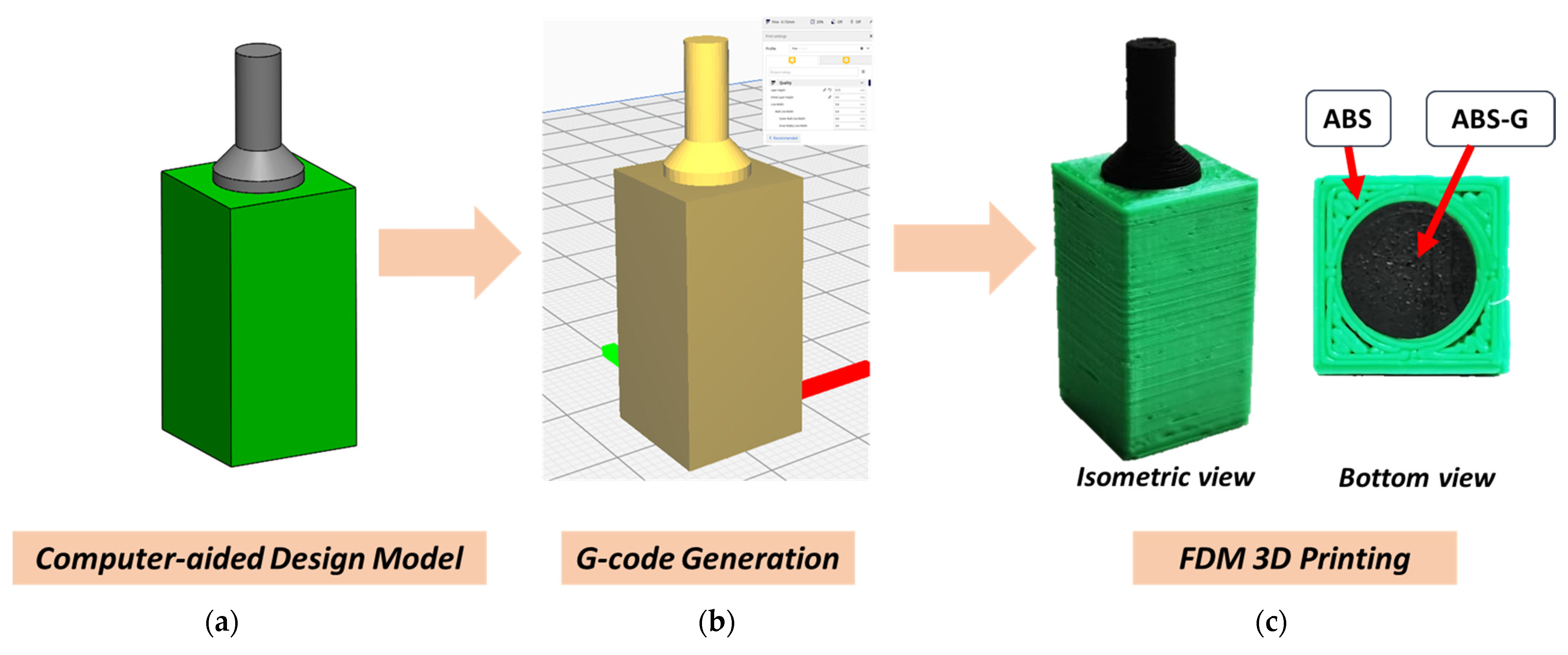
2.3. Fabrication of 3D Printed ABS-G Electrode
2.4. Preparation and Deposition of Zero-Valent Iron (ZVI)
2.5. Electrochemical Characterization and Calibration of ABS-G and ABS-G/ZVI
3. Results and Discussion
3.1. 3D Printing of Electrochemically Conductive Electrode
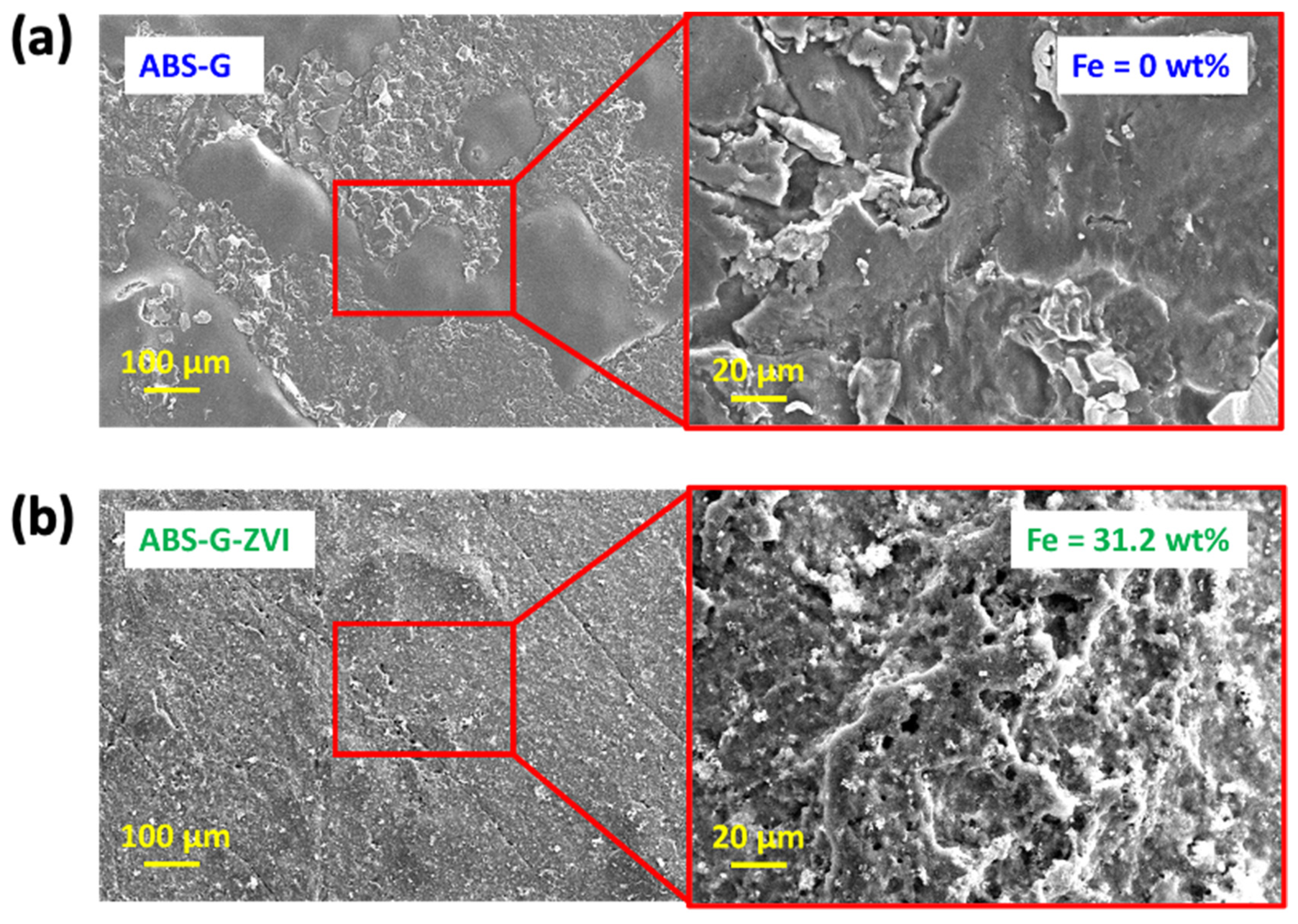
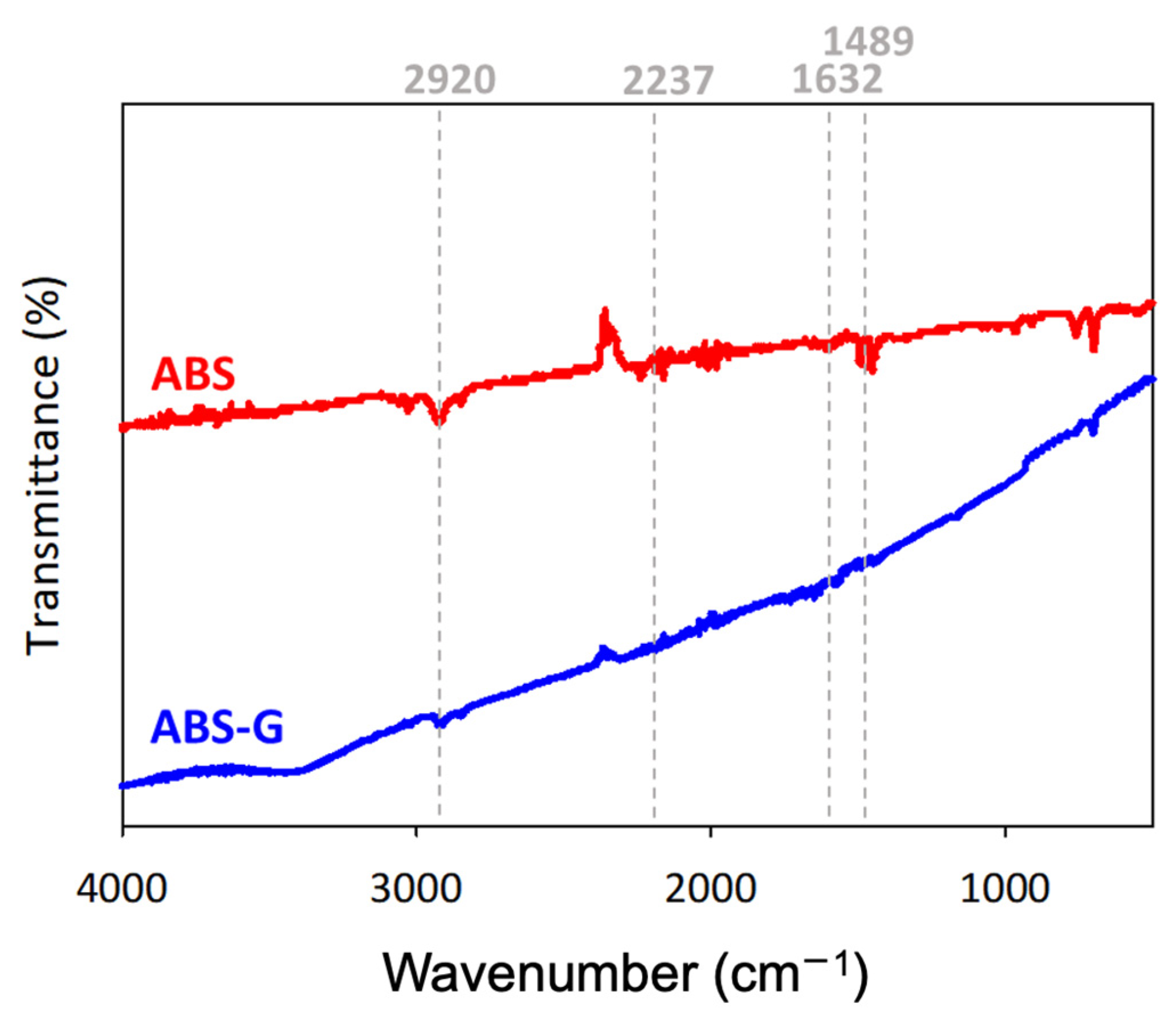
3.2. Characterization of 3D Printed ABS-G and ABS-G/ZVI Electrodes
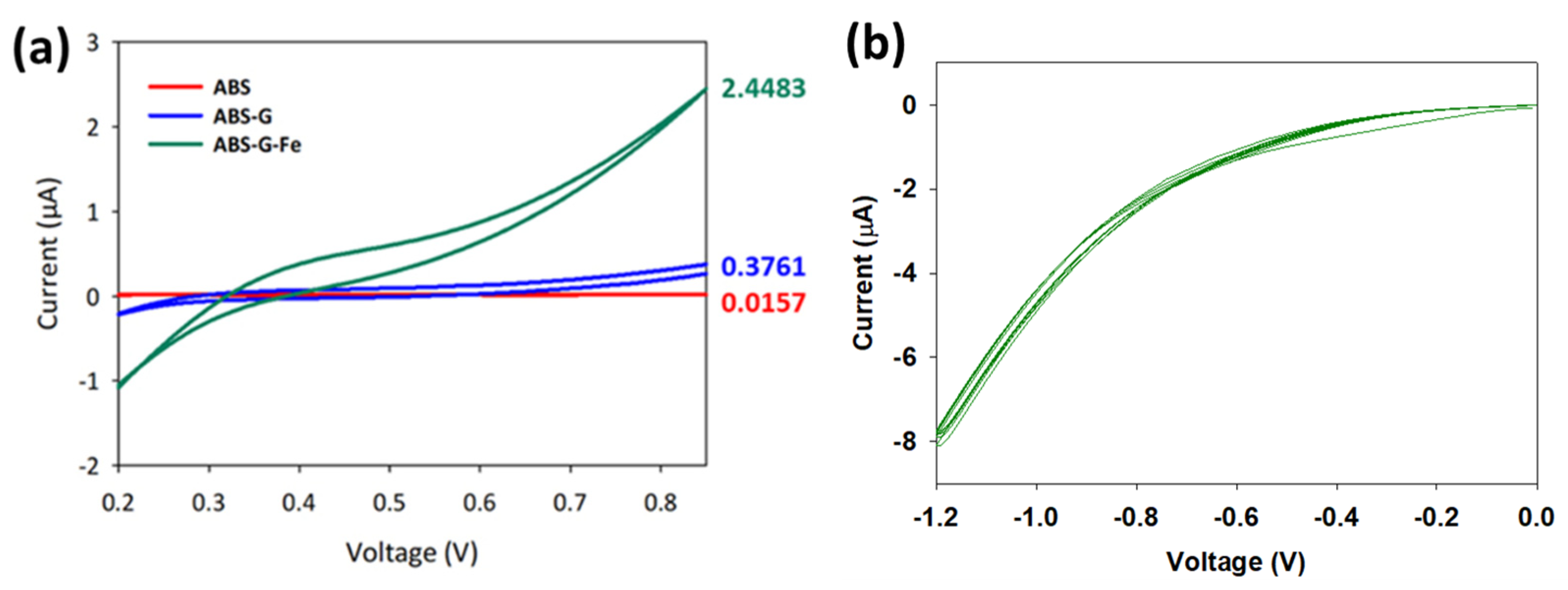
3.3. Electrochemical Characterization of 3D Printed ABS-G and ABS-G/ZVI Electrodes
3.4. Calibration in 3-MCPD Using ZVI-3D Printed ABS-G Electrode
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohan, D.; Sajab, M.S.; Bakarudin, S.B.; Bin Roslan, R.; Kaco, H. 3D Printed Polyurethane Reinforced Graphene Nanoplatelets. Mater. Sci. Forum 2021, 1025, 47–52. [Google Scholar] [CrossRef]
- Mohan, D.; Sajab, M.S.; Kaco, H.; Bakarudin, S.B.; Noor, A.M. 3D Printing of UV-Curable Polyurethane Incorporated with Surface-Grafted Nanocellulose. Nanomaterials 2019, 9, 1726. [Google Scholar] [CrossRef] [Green Version]
- Mohan, D.; Khairullah, N.F.; How, Y.P.; Sajab, M.S.; Kaco, H. 3D Printed Laminated CaCO3-Nanocellulose Films as Controlled-Release 5-Fluorouracil. Polymers 2020, 12, 986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sandhu, G.S.; Penna, R.; Farina, I. Investigations for thermal and electrical conductivity of ABS-graphene blended prototypes. Materials 2017, 10, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, C.Y.; Lim, H.N.; Mahdi, M.A.; Wahid, M.H.; Huang, N.M. Three-dimensional printed electrode and its novel applications in electronic devices. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Jayanth, N.; Senthil, P. Application of 3D printed ABS based conductive carbon black composite sensor in void fraction measurement. Compos. Part B Eng. 2019, 159, 224–230. [Google Scholar]
- Wirth, D.M.; Sheaff, M.J.; Waldman, J.V.; Symcox, M.P.; Whitehead, H.D.; Sharp, J.D.; Doerfler, J.R.; Lamar, A.A.; Leblanc, G. Electrolysis activation of fused-filament-fabrication 3D-printed electrodes for electrochemical and spectroelectrochemical analysis. Anal. Chem. 2019, 91, 5553–5557. [Google Scholar] [CrossRef]
- Flowers, P.F.; Reyes, C.; Ye, S.; Kim, M.J.; Wiley, B.J. 3D printing electronic components and circuits with conductive thermoplastic filament. Addit. Manuf. 2017, 18, 156–163. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Novotný, F.; Webster, R.D.; Pumera, M. 3D-printed Ag/AgCl pseudo-reference electrodes. Electrochem. Commun. 2019, 103, 104–108. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Mendonça, D.M.H.; Silva, W.P.; Silva, M.N.T.; Nossol, E.; da Silva, R.A.B.; Richter, E.M.; Muñoz, R.A.A. 3D printing for electroanalysis: From multiuse electrochemical cells to sensors. Anal. Chim. Acta 2018, 1033, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.Z.M.; Novotný, F.; Alduhaish, O.; Pumera, M. 3D-printed electrodes for the detection of mycotoxins in food. Electrochem. Commun. 2020, 115, 106735. [Google Scholar] [CrossRef]
- European Food Safety Authority. Analysis of occurrence of 3-monochloropropane-1, 2-diol (3-MCPD) in food in Europe in the years 2009–2011 and preliminary exposure assessment. EFSA J. 2013, 11, 3381. [Google Scholar] [CrossRef]
- Abraham, K.; Appel, K.E.; Berger-Preiss, E.; Apel, E.; Gerling, S.; Mielke, H.; Creutzenberg, O.; Lampen, A. Relative oral bioavailability of 3-MCPD from 3-MCPD fatty acid esters in rats. Arch. Toxicol. 2013, 87, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Arisseto, A.P.; Marcolino, P.F.C.; Vicente, E. Determination of 3-monochloropropane-1,2-diol fatty acid esters in Brazilian vegetable oils and fats by an in-house validated method. Food Addit. Contam. A 2014, 31, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Pudel, F.; Benecke, P.; Fehling, P.; Freudenstein, A.; Matthäus, B.; Schwaf, A. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur. J. Lipid Sci. Technol. 2011, 113, 368–373. [Google Scholar] [CrossRef]
- Arris, F.A.; Thai, V.T.S.; Manan, W.N.; Sajab, M.S. A Revisit to the Formation and Mitigation of 3-Chloropropane-1,2-Diol in Palm Oil Production. Foods 2020, 9, 1769. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Craft, B.D.; Sandoz, L.; Nagy, K. Formation mechanisms of Monochloropropanediol (MCPD) fatty acid diesters in refined palm (Elaeis guineensis) oil and related fractions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2012, 29, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Sandoz, L.; Craft, B.D.; Destaillats, F. Mass-defect filtering of isotope signatures to reveal the source of chlorinated palm oil contaminants. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2011, 28, 1492–1500. [Google Scholar] [CrossRef]
- Šmidrkal, J.; Tesărová, M.; Hrádková, I.; Berčíková, M.; Adamčíková, A.; Filip, V. Mechanism of formation of 3-chloropropan-1,2-diol (3-MCPD) esters under conditions of the vegetable oil refining. Food Chem. 2016, 211, 124–129. [Google Scholar] [CrossRef]
- Freudenstein, A.; Weking, J.; Matthäus, B. Influence of precursors on the formation of 3-MCPD and glycidyl esters in a model oil under simulated deodorization conditions. Eur. J. Lipid Sci. Technol. 2013, 115, 286–294. [Google Scholar] [CrossRef]
- Shimizu, M.; Weitkamp, P.; Vosmann, K.; Matthäus, B. Influence of chloride and glycidyl-ester on the generation of 3-MCPD- and glycidyl-esters. Eur. J. Lipid Sci. Technol. 2013, 115, 735–739. [Google Scholar] [CrossRef]
- Tiong, S.H.; Saparin, N.; Teh, H.F.; Ng, T.L.M.; bin Zain, M.M.Z.; Neoh, B.K.; Md Noor, A.; Tan, C.P.; Lai, O.M.; Appleton, D.R. Natural Organochlorines as Precursors of 3-Monochloropropanediol Esters in Vegetable Oils. J. Agric. Food Chem. 2018, 66, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Weißhaar, R. Fatty acid esters of 3-MCPD: Overview of occurrence and exposure estimates. Eur. J. Lipid Sci. Technol. 2011, 113, 304–308. [Google Scholar] [CrossRef]
- Dininova, V.; Svejkovska, B.; Dolezal, M.; Velisek, J. Determination of free and bound 3-chloropropane-1,2-diol by gas chromatography with mass spectrometric detection using deuterated 3-chloropropane-1,2-diol as internal standard. Czech. J. Food Sci. 2005, 22, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Wang, C.; Li, X.Z. Electron efficiency of zero-valent iron for groundwater remediation and wastewater treatment. Chem. Eng. J. 2013, 215, 90–95. [Google Scholar] [CrossRef]
- Cook, S.M. Assessing the Use and Application of Zero-Valent Iron Nanoparticle Technology for Remediation at Contaminated Sites; Jackson State University: Washington, DC, USA, 2009. [Google Scholar]
- Li, C.; Iqbal, M.; Lin, J.; Luo, X.; Jiang, B.; Malgras, V.; Wu, K.C.W.; Kim, J. Electrochemical deposition: An advanced approach for templated synthesis of nanoporous metal architectures. Acc. Chem. Res. 2018, 51, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Bakir, A.N.; Sajab, M.S.; Bakarudin, S.B.; Mansor, N.N.; Roslan, R.; Kaco, H. Homogeneous distribution of lignin/graphene fillers with enhanced interlayer adhesion for 3D printing filament. Polym. Compos. 2021, 42, 2408–2421. [Google Scholar] [CrossRef]
- Arris, F.A.; Manan, W.N.; Kaco, H.; Shaffie, A.H.; Sajab, M.S. Electrochemical Characterization of Graphite-Zero-Valent Iron for 3-Monochloropropane-1,2-Diol (3-MCPD) Detection. Mater. Sci. Forum 2021, 1025, 20–25. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, T.; Xu, J.; Tao, D.; Luo, C.; Wang, C.; Dong, Q.; Wang, Y. Investigation into hydroxypropyl-methylcellulose-reinforced polylactide composites for fused deposition modelling. Ind. Crops Prod. 2020, 146, 112174. [Google Scholar] [CrossRef]
- Heo, C.; Moon, H.G.; Yoon, C.S.; Chang, J.H. ABS nanocomposite films based on functionalized-graphene sheets. J. Appl. Polym. Sci. 2012, 124, 4663–4670. [Google Scholar] [CrossRef]
- Fahmy, T.; Sarhan, A.; Elsayed, I.A.; Abdelwahed, H.G. Optical Properties of Poly (Vinyl Chloride-co-Vinyl Acetate-co-2-Hydroxypropyl Acrylate)/(Acrylonitrile-Butadiene-Styrene) Blends. Int. J. Eng. Res. 2018, 11, 1405–1415. [Google Scholar]
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.S.; Kubo, M. Degradation of Bioplastics in Soil and Their Degradation Effects on Environmental Microorganisms. J. Agric. Chem. Environ. 2016, 05, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; van-Eerten Jansen, M.C.A.A.; Acharya, B. Biodegradation of bioplastic using anaerobic digestion at retention time as per industrial biogas plant and international norms. Sustainability 2020, 12, 4231. [Google Scholar] [CrossRef]
- Han, S.; Ding, Y.; Teng, F.; Yao, A.; Leng, Q. Determination of chloropropanol with an imprinted electrochemical sensor based on multi-walled carbon nanotubes/metal-organic framework composites. RSC Adv. 2021, 11, 18468–18475. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Zhang, H.; Qian, H.; Zhang, Y.; Tang, L.; Li, Z. Development and application of 3-chloro-1,2-propandiol electrochemical sensor based on a polyaminothiophenol modified molecularly imprinted film. J. Agric. Food Chem. 2014, 62, 4552–4557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, Q.; Wu, D.; Yang, Y.; Zhang, Y.; Tang, X. A facile electrochemical method for rapid determination of 3-chloropropane-1,2-diol in soy sauce based on nanoporous gold capped with molecularly imprinted polymer. Food Control 2022, 134, 108750. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Ni, X.; Cao, Y. A biosensor based on hemoglobin immobilized with magnetic molecularly imprinted nanoparticles and modified on a magnetic electrode for direct electrochemical determination of 3-chloro-1, 2-propandiol. J. Electroanal. Chem. 2019, 834, 233–240. [Google Scholar] [CrossRef]
- Martin, A.A.; Fodjo, E.K.; Eric-Simon, Z.V.; Gu, Z.; Yang, G.; Albert, T.; Kong, C.; Wang, H.F. Cys-AgNPs modified gold electrode as an ultrasensitive electrochemical sensor for the detection of 3-chloropropane-1, 2-diol. Arab. J. Chem. 2021, 14, 103319. [Google Scholar] [CrossRef]

| Electrode | Method of Detection | Linear Range | Lower Detection Limit | Reference |
|---|---|---|---|---|
| Glassy carbon electrode (GCE)/nanoporous gold (NPG)/molecularly imprinted polymer (MIP) | Differential pulse voltammetry (DPV) | 10−16 to 10−7 mol/L | 3.5 × 10−17 mol/L | [39] |
| Glassy carbon electrode (GCE)/hemoglobin immobilized with magnetic molecularly imprinted nanoparticles (Hb-MMIPs NPs) | Differential pulse voltammetry (DPV) | 1.0 to 160.0 mg/L | 0.25 mg/L | [40] |
| Gold (Au)/cysteine-coated silver nanoparticles (Cys-AgNPs) | Differential pulse voltammetry (DPV) | 2.5 to 200 ng/mL | 2.4 ng/mL | [41] |
| Glassy carbon electrode (GCE)/carboxylated multi-wall carbon nanotubes cMWCNT)/metal–organic framework (MOF-199) | Differential pulse voltammetry (DPV) | 1.0 x 10−9 to 1.0 × 10−5 mol/L | 4.3 × 10−10 mol/L | [37] |
| GCE/AuN/p-ATP | Differential pulse voltammetry (DPV) | 1.0 x 10−17 to 1.0 × 10−13 mol/L | 3.8 × 10−18 mol/L | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arris, F.A.; Mohan, D.; Sajab, M.S. Facile Synthesis of 3D Printed Tailored Electrode for 3-Monochloropropane-1,2-Diol (3-MCPD) Sensing. Micromachines 2022, 13, 383. https://doi.org/10.3390/mi13030383
Arris FA, Mohan D, Sajab MS. Facile Synthesis of 3D Printed Tailored Electrode for 3-Monochloropropane-1,2-Diol (3-MCPD) Sensing. Micromachines. 2022; 13(3):383. https://doi.org/10.3390/mi13030383
Chicago/Turabian StyleArris, Farrah Aida, Denesh Mohan, and Mohd Shaiful Sajab. 2022. "Facile Synthesis of 3D Printed Tailored Electrode for 3-Monochloropropane-1,2-Diol (3-MCPD) Sensing" Micromachines 13, no. 3: 383. https://doi.org/10.3390/mi13030383
APA StyleArris, F. A., Mohan, D., & Sajab, M. S. (2022). Facile Synthesis of 3D Printed Tailored Electrode for 3-Monochloropropane-1,2-Diol (3-MCPD) Sensing. Micromachines, 13(3), 383. https://doi.org/10.3390/mi13030383






