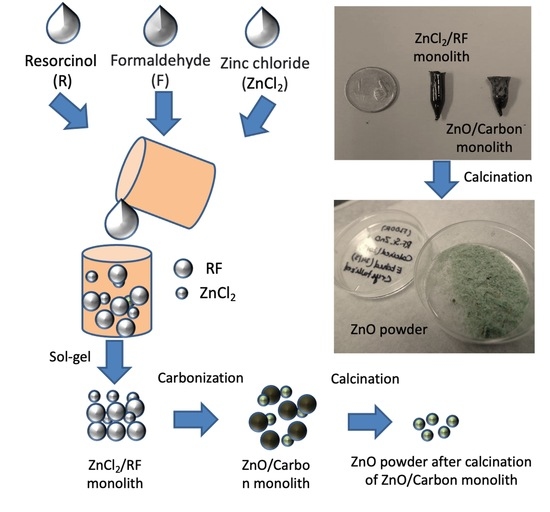
Fabrication of High Surface Area Microporous ZnO from ZnO/Carbon Sacrificial Composite Monolith Template
Abstract
1. Introduction
2. Experimental Section
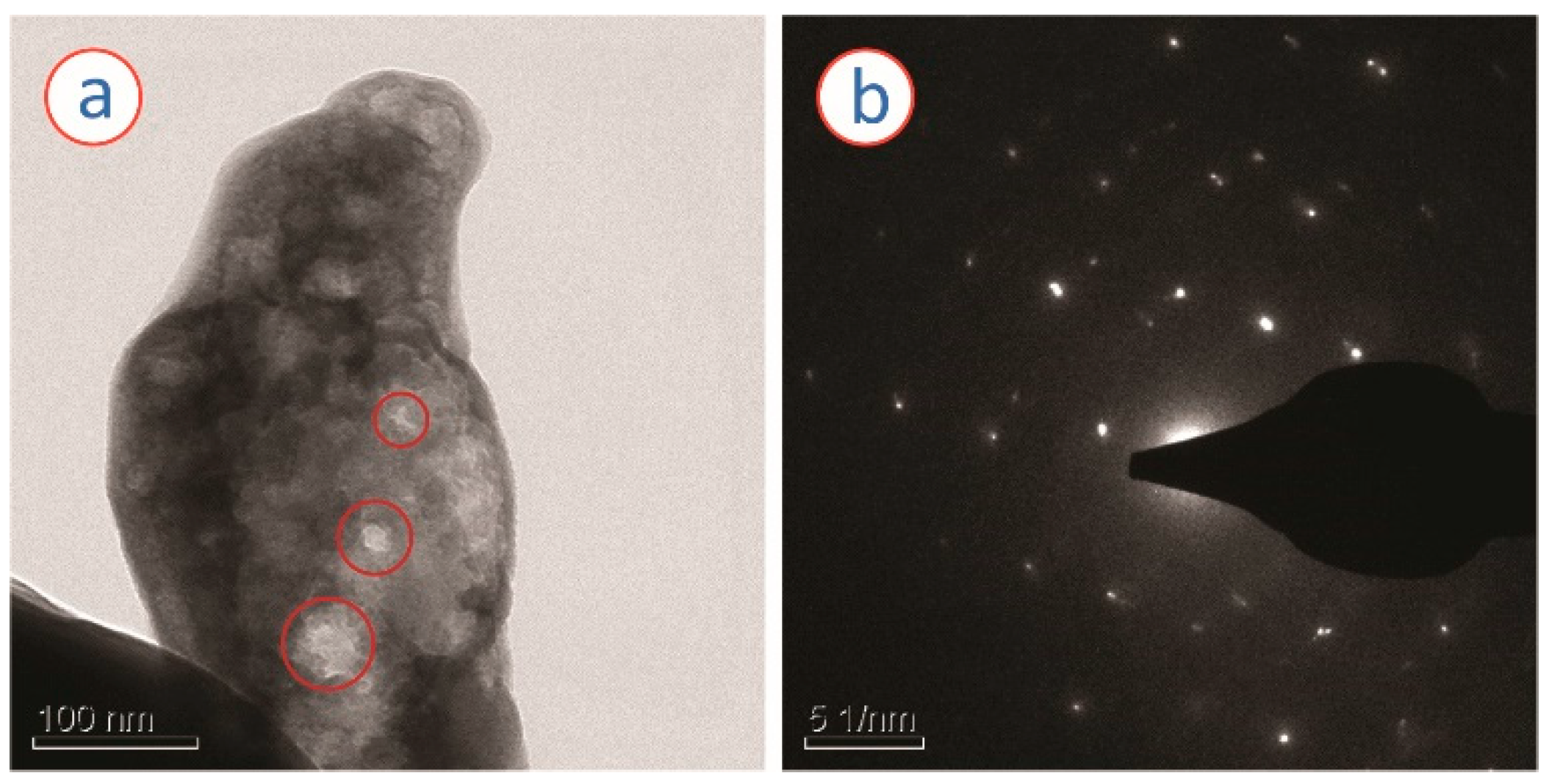
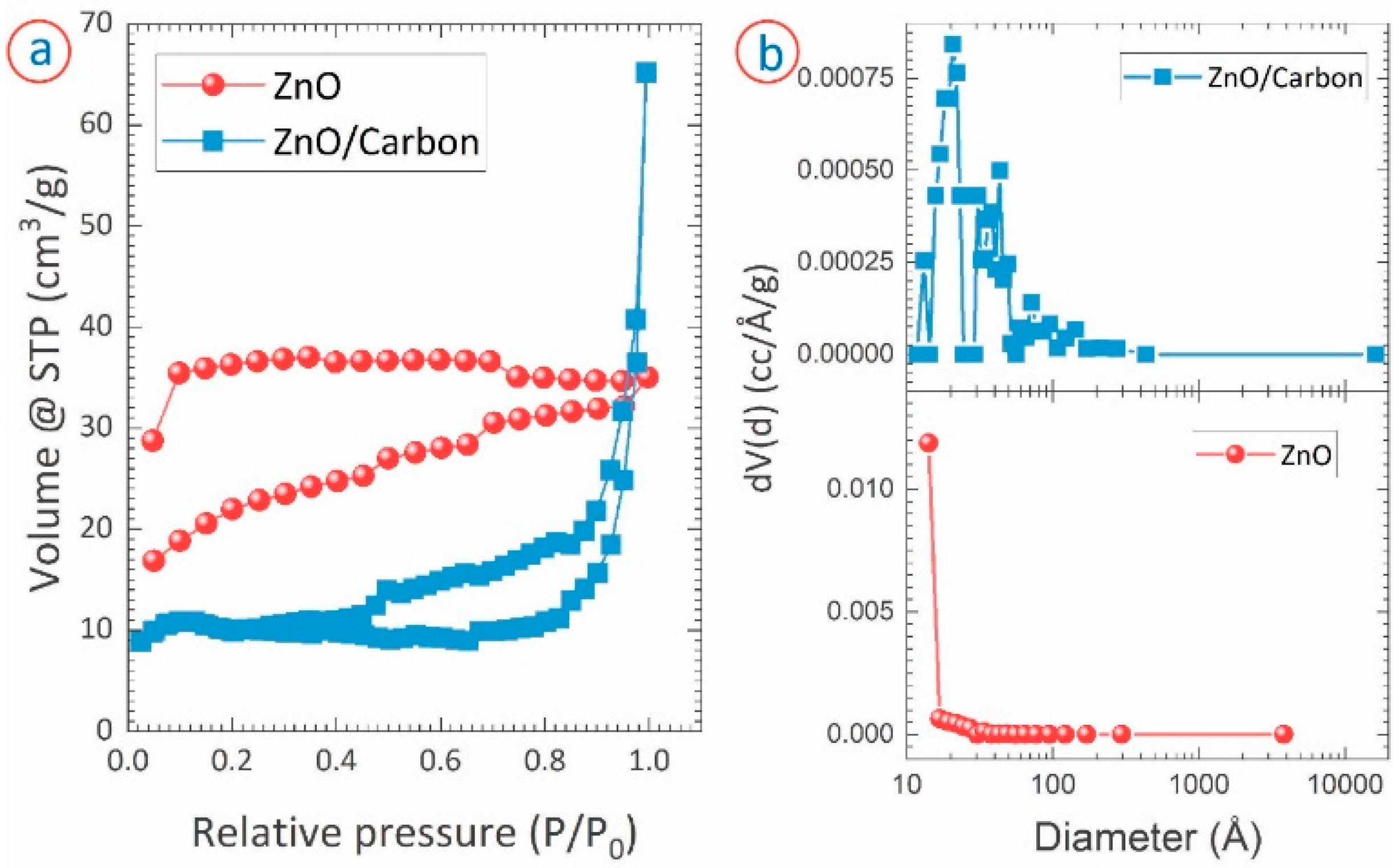
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Islam, M.; Sadaf, A.; Mager, D.; Korvink, J.G. Recent progress on titanium oxide nanostructures for biosensing applications. In Metal Oxides for Biomedical and Biosensor Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–470. [Google Scholar]
- Ali, M.A.; Mondal, K.; Singh, C.; Dhar Malhotra, B.; Sharma, A. Anti-epidermal growth factor receptor conjugated mesoporous zinc oxide nanofibers for breast cancer diagnostics. Nanoscale 2015, 7, 7234–7245. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Saputro, A.G.; Kaneti, Y.V.; Maulana, A.L.; Fathurrahman, F.; Lim, H.; Yuliarto, B.; Nugraha; Dipojono, H.K.; Golberg, D.; et al. Hollow Zinc Oxide Microsphere–Multiwalled Carbon Nanotube Composites for Selective Detection of Sulfur Dioxide. ACS Appl. Nano Mater. 2020, 3, 8982–8996. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Late, D.; Sumesh, C.K. Photosensitive WS2/ZnO Nano-Heterostructure-Based Electrocatalysts for Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 755–762. [Google Scholar] [CrossRef]
- Wang, Z.; Zhi, M.; Xu, M.; Guo, C.; Man, Z.; Zhang, Z.; Li, Q.; Lv, Y.; Zhao, W.; Yan, J.; et al. Ultrasensitive NO2 gas sensor based on Sb-doped SnO2 covered ZnO nano-heterojunction. J. Mater. Sci. 2021, 56, 7348–7356. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Moriceau, J.; Liu, M.; Yuan, Y.; Zakaria, Q.; Jiang, X.; Yu, A. Hydrothermal synthesis of ternary α-Fe2O3–ZnO–Au nanocomposites with high gas-sensing performance. Sens. Actuators B Chem. 2015, 209, 889–897. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Du, L.; Yao, H.; Wang, J.; Luo, G. Precipitation Preparation of High Surface Area and Porous Nanosized ZnO by Continuous Gas-Based Impinging Streams in Unconfined Space. Ind. Eng. Chem. Res. 2016, 55, 11943–11949. [Google Scholar] [CrossRef]
- Singh, P.; Mondal, K.; Sharma, A. Reusable electrospun mesoporous ZnO nanofiber mats for photocatalytic degradation of polycyclic aromatic hydrocarbon dyes in wastewater. J. Colloid Interface Sci. 2013, 394, 208–215. [Google Scholar] [CrossRef]
- Srinivasu, P.; Singh, S.P.; Islam, A.; Han, L. Dye-Sensitized Solar Cells Based on High Surface Area Nanocrystalline Zinc Oxide Spheres. Adv. Optoelectron. 2011, 2011, 194508. [Google Scholar] [CrossRef][Green Version]
- Mondal, K.; Sharma, A. Recent advances in the synthesis and application of photocatalytic metal–metal oxide core–shell nanoparticles for environmental remediation and their recycling process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, M.A.; Agrawal, V.V.; Malhotra, B.D.; Sharma, A. Highly Sensitive Biofunctionalized Mesoporous Electrospun TiO 2 Nanofiber Based Interface for Biosensing. ACS Appl. Mater. Interfaces 2014, 6, 2516–2527. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent advances in electrospun metal-oxide nanofiber based interfaces for electrochemical biosensing. RSC Adv. 2016, 6, 94595–94616. [Google Scholar] [CrossRef]
- Ali, M.A.; Mondal, K.; Jiao, Y.; Oren, S.; Xu, Z.; Sharma, A.; Dong, L. Microfluidic Immuno-Biochip for Detection of Breast Cancer Biomarkers Using Hierarchical Composite of Porous Graphene and Titanium Dioxide Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 20570–20582. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-S.; Zhu, Z.-Y.; Zhao, X.; Song, T.-B.; Huang, J.-H.; Zhang, Y.-X.; Liu, X.; Chen, L.; Niu, X.-Q.; Wang, Y.-G.; et al. Self-assembled ZnO-carbon dots anode materials for high performance nickel-zinc alkaline batteries. Chem. Eng. J. 2021, 425, 130660. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Xu, X.; Wang, J. Review on porous nanomaterials for adsorption and photocatalytic conversion of CO2. Chin. J. Catal. 2017, 38, 1956–1969. [Google Scholar] [CrossRef]
- Bhanja, P.; Bhaumik, A. Porous nanomaterials as green catalyst for the conversion of biomass to bioenergy. Fuel 2016, 185, 432–441. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Bhaumik, A. Supported Porous Nanomaterials as Efficient Heterogeneous Catalysts for CO2 Fixation Reactions. Chem. A Eur. J. 2018, 24, 7278–7297. [Google Scholar] [CrossRef]
- Verma, P.; Kondo, Y.; Kuwahara, Y.; Kamegawa, T.; Mori, K.; Raja, R.; Yamashita, H. Design and application of photocatalysts using porous materials. Catal. Rev. 2021, 63, 165–233. [Google Scholar] [CrossRef]
- Rabiee, N.; Fatahi, Y.; Asadnia, M.; Daneshgar, H.; Kiani, M.; Ghadiri, A.M.; Atarod, M.; Mashhadzadeh, A.H.; Akhavan, O.; Bagherzadeh, M.; et al. Green porous benzamide-like nanomembranes for hazardous cations detection, separation, and concentration adjustment. J. Hazard. Mater. 2022, 423, 127130. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Ponpandian, N.; Khan, M.A.; Siddiqui, M.R.; Ahmed, F.; Alsharaeh, E.H. Development of adsorption and electrosorption techniques for removal of organic and inorganic pollutants from wastewater using novel magnetite/porous graphene-based nanocomposites. Sep. Purif. Technol. 2017, 188, 206–218. [Google Scholar] [CrossRef]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal–organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horizons 2018, 5, 394–407. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, X.; Zhu, Y.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; Zhao, D. Ordered porous metal oxide semiconductors for gas sensing. Chin. Chem. Lett. 2018, 29, 405–416. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, B.K.; Cao, L.; Bindra, P.; Kaur, K.; Chandel, M.; Koratkar, N.; Huang, Q.; Shanmugam, V. Porous nanomaterials: Main vein of agricultural nanotechnology. Prog. Mater. Sci. 2021, 121, 100812. [Google Scholar] [CrossRef]
- Mamleyev, E.R.; Weidler, P.G.; Nefedov, A.; Szabó, D.V.; Islam, M.; Mager, D.; Korvink, J.G. Nano- and Microstructured Copper/Copper Oxide Composites on Laser-Induced Carbon for Enzyme-Free Glucose Sensors. ACS Appl. Nano Mater. 2021, 4, 13747–13760. [Google Scholar] [CrossRef]
- Alizadeh, A.; Jin, X.; Wang, M. Pore-scale Study of Ion Transport Mechanisms in Inhomogeneously Charged Nanoporous Rocks: Impacts of Interface Properties on Macroscopic Transport. J. Geophys. Res. Solid Earth 2019, 124, 5387–5407. [Google Scholar] [CrossRef]
- Allaire, G.; Brizzi, R.; Dufrêche, J.-F.; Mikelić, A.; Piatnitski, A. Ion transport in porous media: Derivation of the macroscopic equations using upscaling and properties of the effective coefficients. Comput. Geosci. 2013, 17, 479–495. [Google Scholar] [CrossRef]
- Ikonen, T.; Nissinen, T.; Pohjalainen, E.; Sorsa, O.; Kallio, T.; Lehto, V.-P. Electrochemically anodized porous silicon: Towards simple and affordable anode material for Li-ion batteries. Sci. Rep. 2017, 7, 7880. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, A.; Huber, C.; Bachmann, O.; Chopard, B. Pore-scale mass and reactant transport in multiphase porous media flows. J. Fluid Mech. 2011, 686, 40–76. [Google Scholar] [CrossRef]
- Nissan, A.; Alcolombri, U.; de Schaetzen, F.; Berkowitz, B.; Jimenez-Martinez, J. Reactive Transport with Fluid–Solid Interactions in Dual-Porosity Media. ACS ES T Water 2021, 1, 259–268. [Google Scholar] [CrossRef]
- Kubo, S.; Demir-Cakan, R.; Zhao, L.; White, R.J.; Titirici, M.-M. Porous Carbohydrate-Based Materials via Hard Templating. ChemSusChem 2010, 3, 188–194. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, L.; Liu, B.; He, J. Templated Growth of Crystalline Mesoporous Materials: From Soft/Hard Templates to Colloidal Templates. Front. Chem. 2019, 7, 22. [Google Scholar] [CrossRef]
- Yamagishi, H.; Tsunoda, M.; Iwai, K.; Hengphasatporn, K.; Shigeta, Y.; Sato, H.; Yamamoto, Y. Solvophobicity-directed assembly of microporous molecular crystals. Commun. Chem. 2021, 4, 122. [Google Scholar] [CrossRef]
- Decker, G.E.; Lorzing, G.R.; Deegan, M.M.; Bloch, E.D. MOF-mimetic molecules: Carboxylate-based supramolecular complexes as molecular metal–organic framework analogues. J. Mater. Chem. A 2020, 8, 4217–4229. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.-L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef]
- Ha, J.-H.; Oh, E.; Song, I.-H. The effect of sacrificial templates on the pore characteristics of sintered diatomite membranes. J. Ceram. Soc. Jpn. 2013, 121, 940–945. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Sung, I.; Li, X.; Kim, D. Fabrication of Porous SiC Ceramics with Special Morphologies by Sacrificing Template Method. J. Porous Mater. 2004, 11, 265–271. [Google Scholar] [CrossRef]
- Zhou, Z.; Nonnenmann, S.S. Progress in Nanoporous Templates: Beyond Anodic Aluminum Oxide and Towards Functional Complex Materials. Materials 2019, 12, 2535. [Google Scholar] [CrossRef]
- Sharma, A.; Katiyar, S.; Mondal, K. Hierarchical Porous Monoliths and Methods for Their Preparation and Use. U.S. Patent 9,908,102, 6 March 2015. [Google Scholar]
- Katiyar, S.; Mondal, K.; Sharma, A. One-step sol–gel synthesis of hierarchically porous, flow-through carbon/silica monoliths. RSC Adv. 2016, 6, 12298–12310. [Google Scholar] [CrossRef]
- Lu, A.-H.; Schüth, F. Nanocasting: A Versatile Strategy for Creating Nanostructured Porous Materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Gilmore, J.; Islam, M.; Duncan, J.; Natu, R.; Martinez-Duarte, R. Assessing the importance of the root mean square (RMS) value of different waveforms to determine the strength of a dielectrophoresis trapping force. Electrophoresis 2017, 38, 2561–2564. [Google Scholar] [CrossRef]
- Islam, M.; Lantada, A.D.; Gómez, M.R.; Mager, D.; Korvink, J.G. Microarchitectured Carbon Structures as Innovative Tissue-Engineering Scaffolds. Adv. Eng. Mater. 2020, 22, 2000083. [Google Scholar] [CrossRef]
- Islam, M.; Sadaf, A.; Gómez, M.R.; Mager, D.; Korvink, J.G.; Lantada, A.D. Carbon fiber/microlattice 3D hybrid architecture as multi-scale scaffold for tissue engineering. Mater. Sci. Eng. C 2021, 126, 112140. [Google Scholar] [CrossRef]
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Madou, M. From MEMS to NEMS with carbon. Biosens. Bioelectron. 2005, 20, 2181–2187. [Google Scholar] [CrossRef]
- Singh, S.; Mondal, K.; Sharma, A. ZnO Nanoparticle Fortified Highly Permeable Carbon/Silica Monoliths as a Flow-Through Media. Langmuir 2017, 33, 7692–7700. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Guan, Z.; Li, Z.; Liu, J.; Yu, K. Characterization and Preparation of Nano-porous Carbon Derived from Hemp Stems as Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2019, 14, 338. [Google Scholar] [CrossRef]
- Islam, M.; Weidler, P.G.; Heissler, S.; Mager, D.; Korvink, J.G. Facile template-free synthesis of multifunctional 3D cellular carbon from edible rice paper. RSC Adv. 2020, 10, 16616–16628. [Google Scholar] [CrossRef]
- Kakunuri, M.; Vennamalla, S.; Sharma, C.S. Synthesis of carbon xerogel nanoparticles by inverse emulsion polymerization of resorcinol–formaldehyde and their use as anode materials for lithium-ion battery. RSC Adv. 2015, 5, 4747–4753. [Google Scholar] [CrossRef]
- Mitra, J.; Jain, S.; Sharma, A.; Basu, B. Patterned growth and differentiation of neural cells on polymer derived carbon substrates with micro/nano structures in vitro. Carbon N. Y. 2013, 65, 140–155. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Seo, H.K.; Kim, G.S.; Khang, G.; Shin, H.-S. Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater. Res. Bull. 2007, 42, 1640–1648. [Google Scholar] [CrossRef]
- Marie, M.; Mandal, S.; Manasreh, O. An Electrochemical Glucose Sensor Based on Zinc Oxide Nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef]
- Hadžić, B.; Romčević, N.; Sibera, D.; Narkiewicz, U.; Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Romčević, M. Laser power influence on Raman spectra of ZnO(Co) nanoparticles. J. Phys. Chem. Solids 2016, 91, 80–85. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; He, R.; Lei, H.; Fang, D. Carbon Aerogel for Insulation Applications: A Review. Int. J. Thermophys. 2019, 40, 39. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and Properties of Resorcinol-Formaldehyde Organic and Carbon Gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Horikawa, T.; Hayashi, J.; Muroyama, K. Controllability of pore characteristics of resorcinol–formaldehyde carbon aerogel. Carbon N. Y. 2004, 42, 1625–1633. [Google Scholar] [CrossRef]
- Siyasukh, A.; Maneeprom, P.; Larpkiattaworn, S.; Tonanon, N.; Tanthapanichakoon, W.; Tamon, H.; Charinpanitkul, T. Preparation of a carbon monolith with hierarchical porous structure by ultrasonic irradiation followed by carbonization, physical and chemical activation. Carbon N. Y. 2008, 46, 1309–1315. [Google Scholar] [CrossRef]
- Schulz, D.; Ganschow, S.; Klimm, D.; Struve, K. Inductively heated Bridgman method for the growth of zinc oxide single crystals. J. Cryst. Growth 2008, 310, 1832–1835. [Google Scholar] [CrossRef]
- Kato, H.; Sano, M.; Miyamoto, K.; Yao, T. Homoepitaxial Growth of High-Quality Zn-Polar ZnO Films by Plasma-Assisted Molecular Beam Epitaxy. Jpn. J. Appl. Phys. 2003, 42, L1002–L1005. [Google Scholar] [CrossRef]
- Ismail, M.A.; Taha, K.K.; Modwi, A.; Khezami, L. ZnO nanoparticles: Surface and X-ray profile analysis. J. Ovonic Res. 2018, 14, 381–393. [Google Scholar]
- Polarz, S.; Orlov, A.V.; Schüth, F.; Lu, A.-H. Preparation of High-Surface-Area Zinc Oxide with Ordered Porosity, Different Pore Sizes, and Nanocrystalline Walls. Chem. A Eur. J. 2007, 13, 592–597. [Google Scholar] [CrossRef]
- Sulciute, A.; Nishimura, K.; Gilshtein, E.; Cesano, F.; Viscardi, G.; Nasibulin, A.G.; Ohno, Y.; Rackauskas, S. ZnO Nanostructures Application in Electrochemistry: Influence of Morphology. J. Phys. Chem. C 2021, 125, 1472–1482. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, N.; Li, L.; Li, R.; Ji, G.; Gan, S. Preparation of porous 3D Ce-doped ZnO microflowers with enhanced photocatalytic performance. RSC Adv. 2015, 5, 59887–59894. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Nair, K.G.; Dhakshinamoorthy, J.; Ravi, K.R.; Pullithadathil, B. Porous, n–p type ultra-long, ZnO@Bi2O3 heterojunction nanorods-based NO2 gas sensor: New insights towards charge transport characteristics. Phys. Chem. Chem. Phys. 2020, 22, 7524–7536. [Google Scholar] [CrossRef] [PubMed]
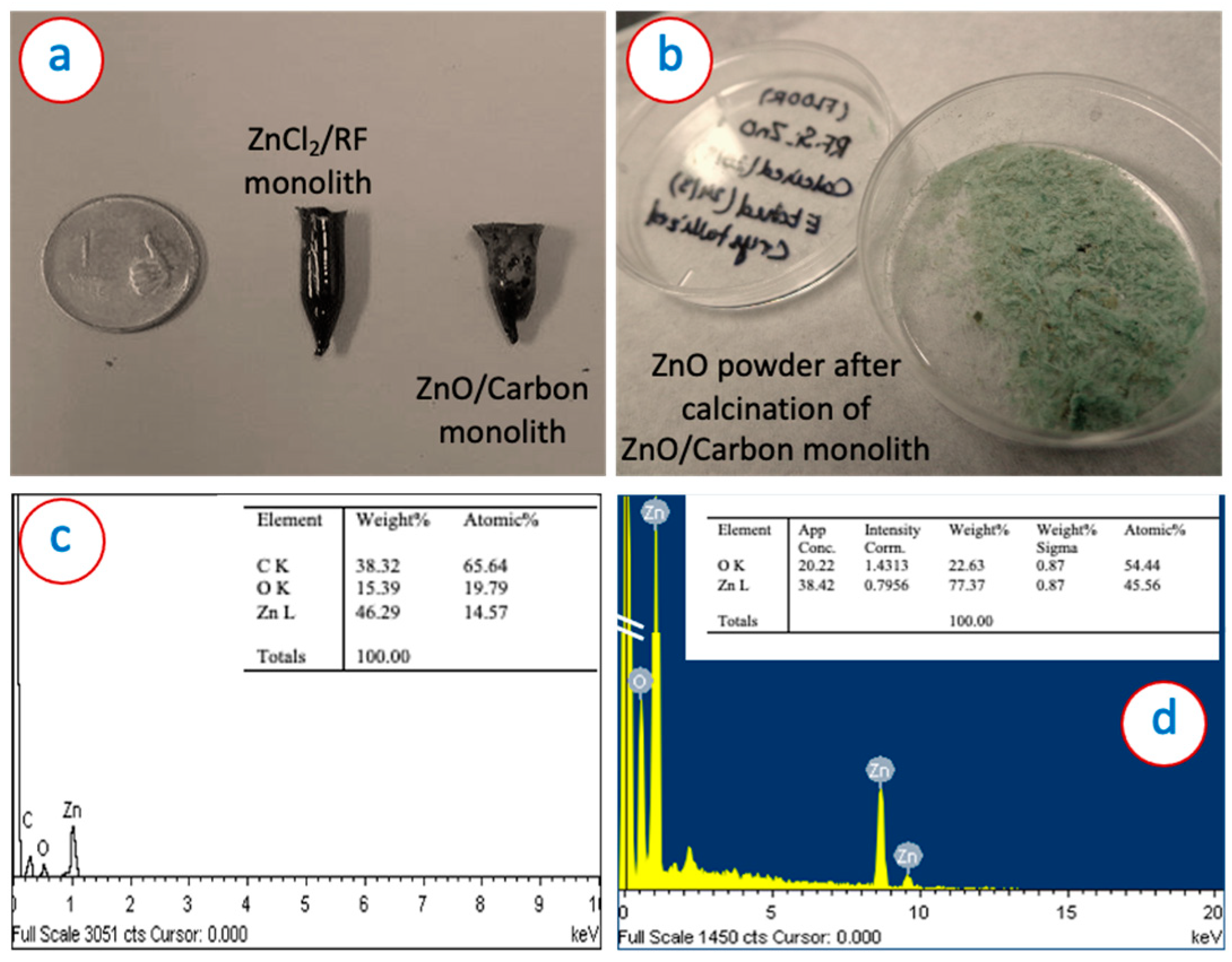
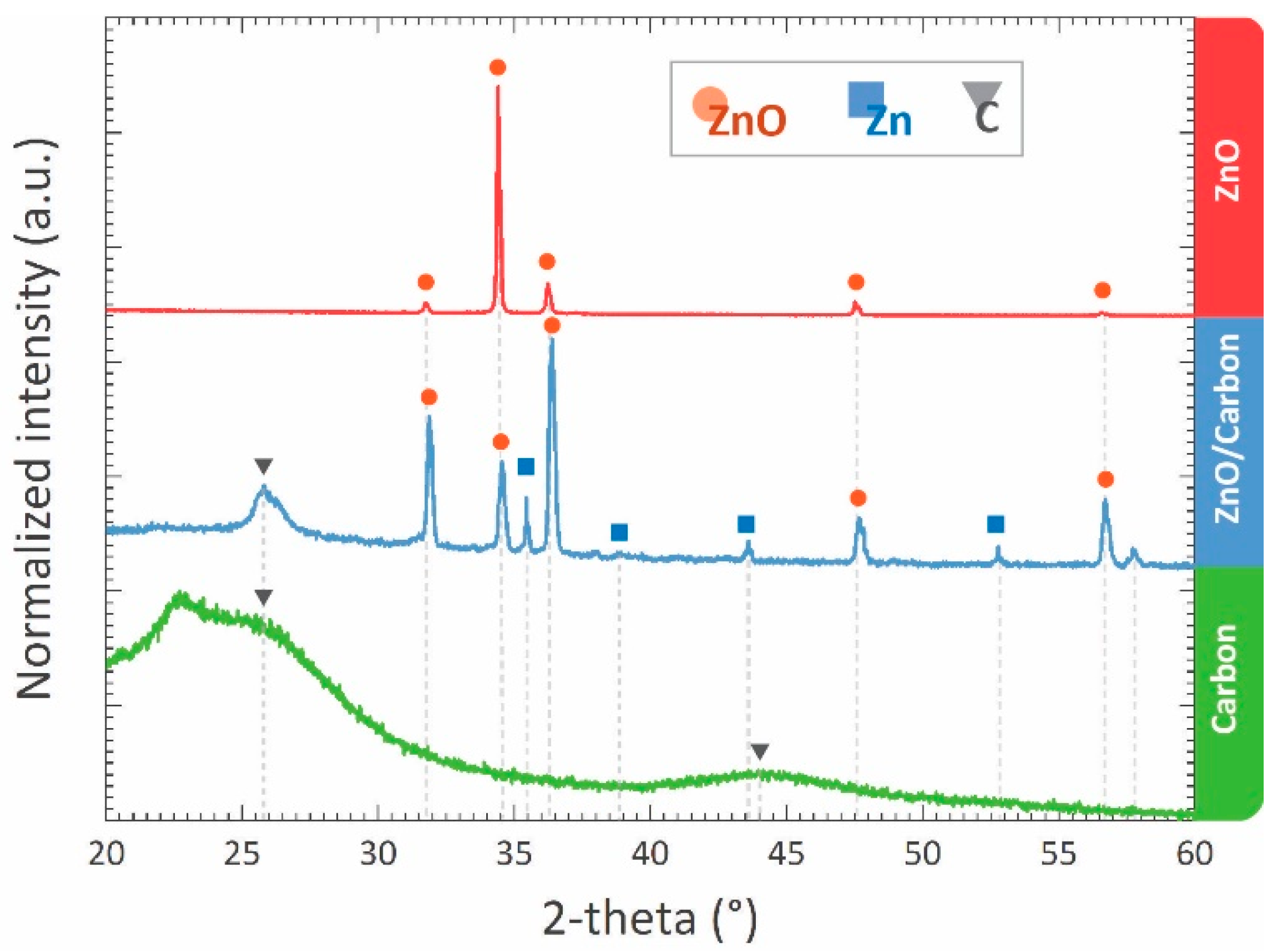
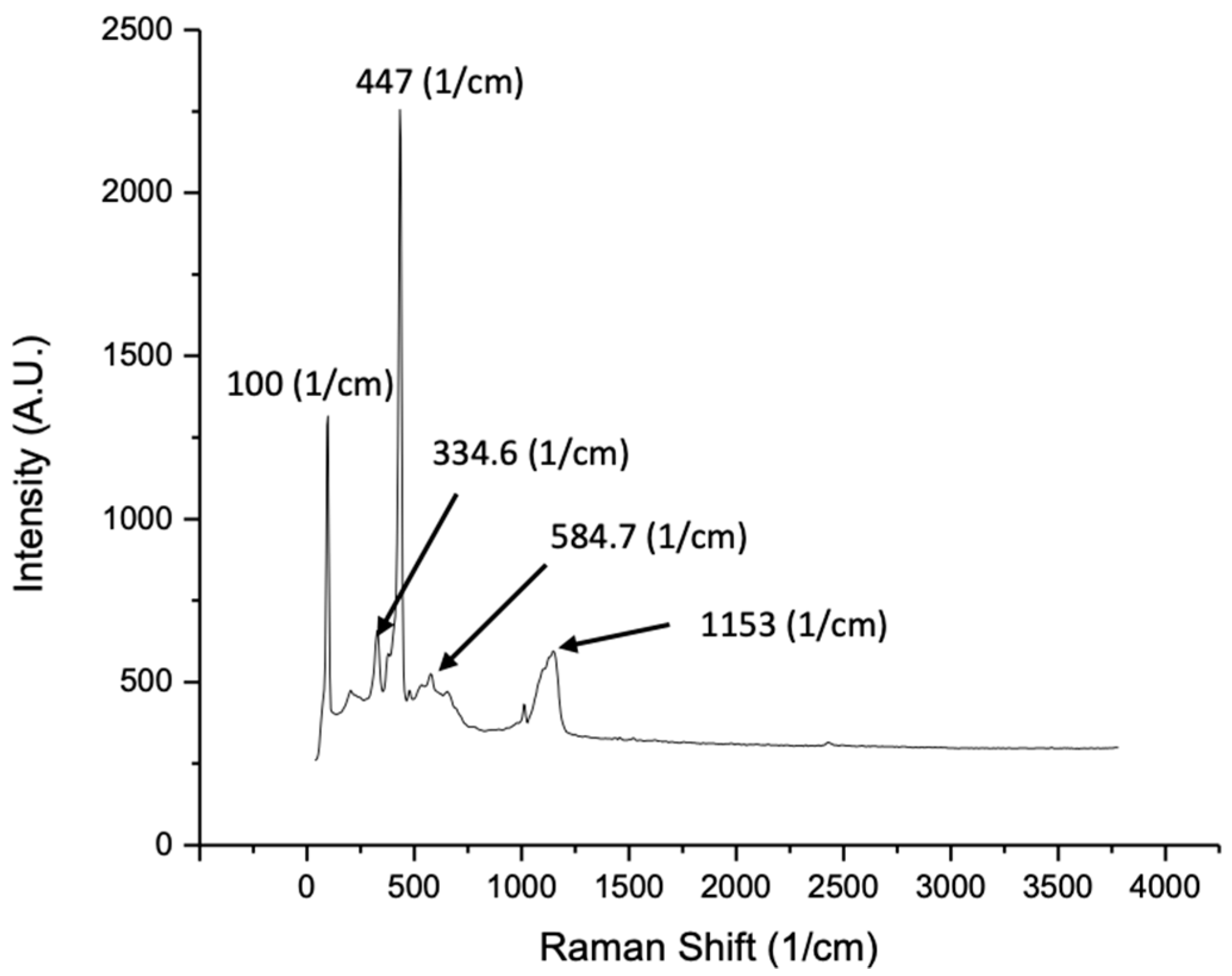

| Synthesis Method | Surface Area (m2/g) | Average Pore Size (nm) | Method Used for Surface Area | Reference |
|---|---|---|---|---|
| Precipitating method | 25.36 | 16.0 | BET nitrogen adsorption–desorption | [61] |
| Template method | 24 | 17.4 | BET nitrogen adsorption–desorption | [62] |
| Combustion method | 8–22 | - | BET nitrogen adsorption–desorption | [63] |
| Hydrothermal method | 10.5 | 18.6 | BET nitrogen adsorption–desorption | [64] |
| Solvothermal method | 10.5 | 3.5 | BET nitrogen adsorption–desorption | [65] |
| Carbon monolith-based template method | 78.1 | 1.3 | Nitrogen adsorption–desorption | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, K.; Islam, M.; Singh, S.; Sharma, A. Fabrication of High Surface Area Microporous ZnO from ZnO/Carbon Sacrificial Composite Monolith Template. Micromachines 2022, 13, 335. https://doi.org/10.3390/mi13020335
Mondal K, Islam M, Singh S, Sharma A. Fabrication of High Surface Area Microporous ZnO from ZnO/Carbon Sacrificial Composite Monolith Template. Micromachines. 2022; 13(2):335. https://doi.org/10.3390/mi13020335
Chicago/Turabian StyleMondal, Kunal, Monsur Islam, Srujan Singh, and Ashutosh Sharma. 2022. "Fabrication of High Surface Area Microporous ZnO from ZnO/Carbon Sacrificial Composite Monolith Template" Micromachines 13, no. 2: 335. https://doi.org/10.3390/mi13020335
APA StyleMondal, K., Islam, M., Singh, S., & Sharma, A. (2022). Fabrication of High Surface Area Microporous ZnO from ZnO/Carbon Sacrificial Composite Monolith Template. Micromachines, 13(2), 335. https://doi.org/10.3390/mi13020335