The Effect of Bacteria on the Stability of Microfluidic-Generated Water-in-Oil Droplet
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Cultures
2.2. Microfluidic Device Fabrication
2.3. Bacterial Cells Preparation
2.4. Bacterial Encapsulation in Single Water-in-Oil Emulsion
2.5. Determination of Bacterial Viability
2.6. Optical Microscopy for Determining Droplet Stability Changes during Storage
2.7. Fluorescence Microscopy for Bacterial Response Observation
2.8. Observation of Bacterial Clustering with Confocal Microscopy
2.9. Bacterial Hydrophobicity Test
2.10. Interfacial Tension Determination between Bacteria and Mineral Oil
2.11. Bacterial Surface Zeta Potential Determination
2.12. Statistical Analysis
3. Results and Discussion
3.1. The Effect of Bacteria on Droplet Stability
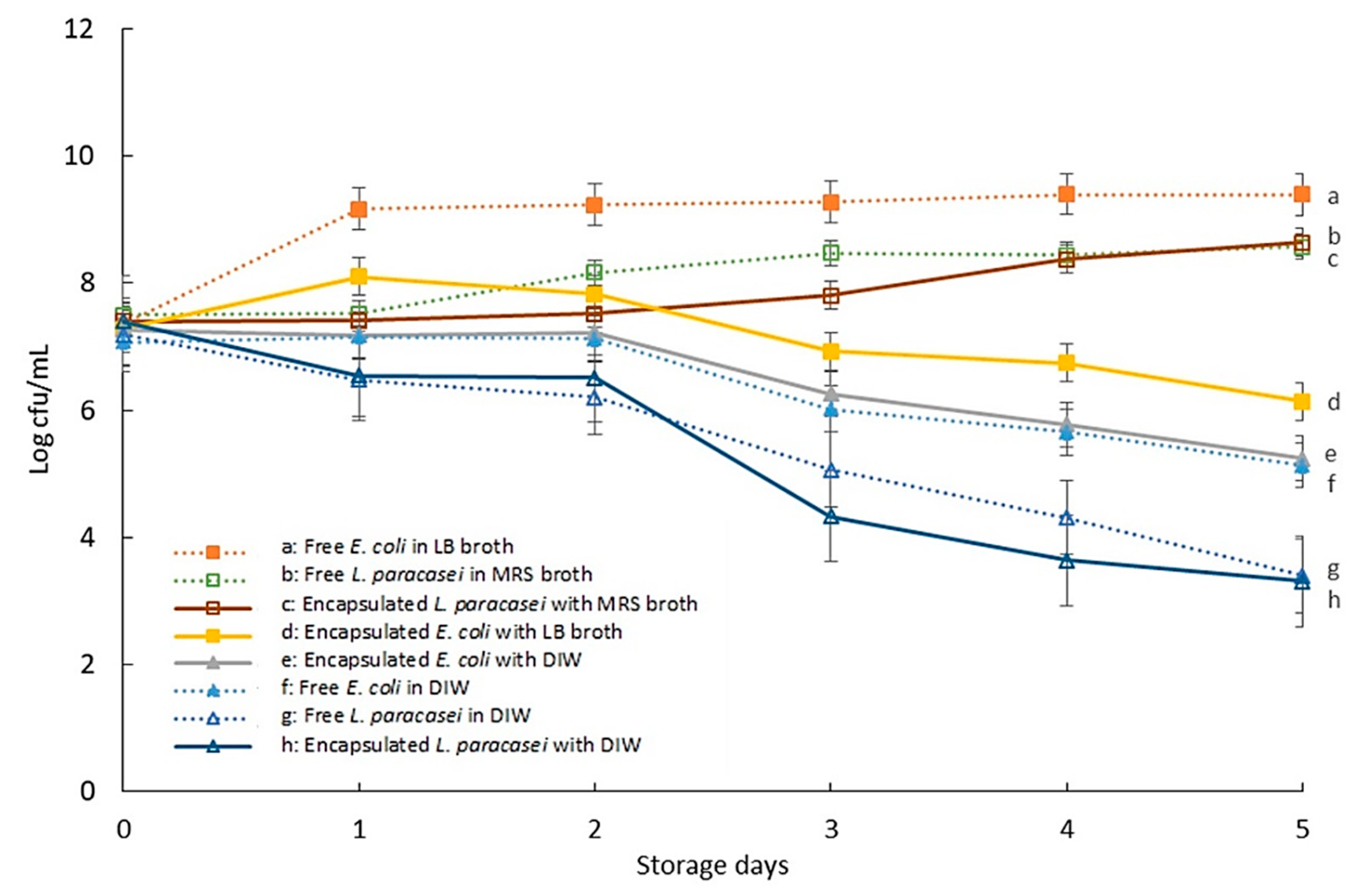
3.2. The Viability of Encapsulated Bacteria during Storage
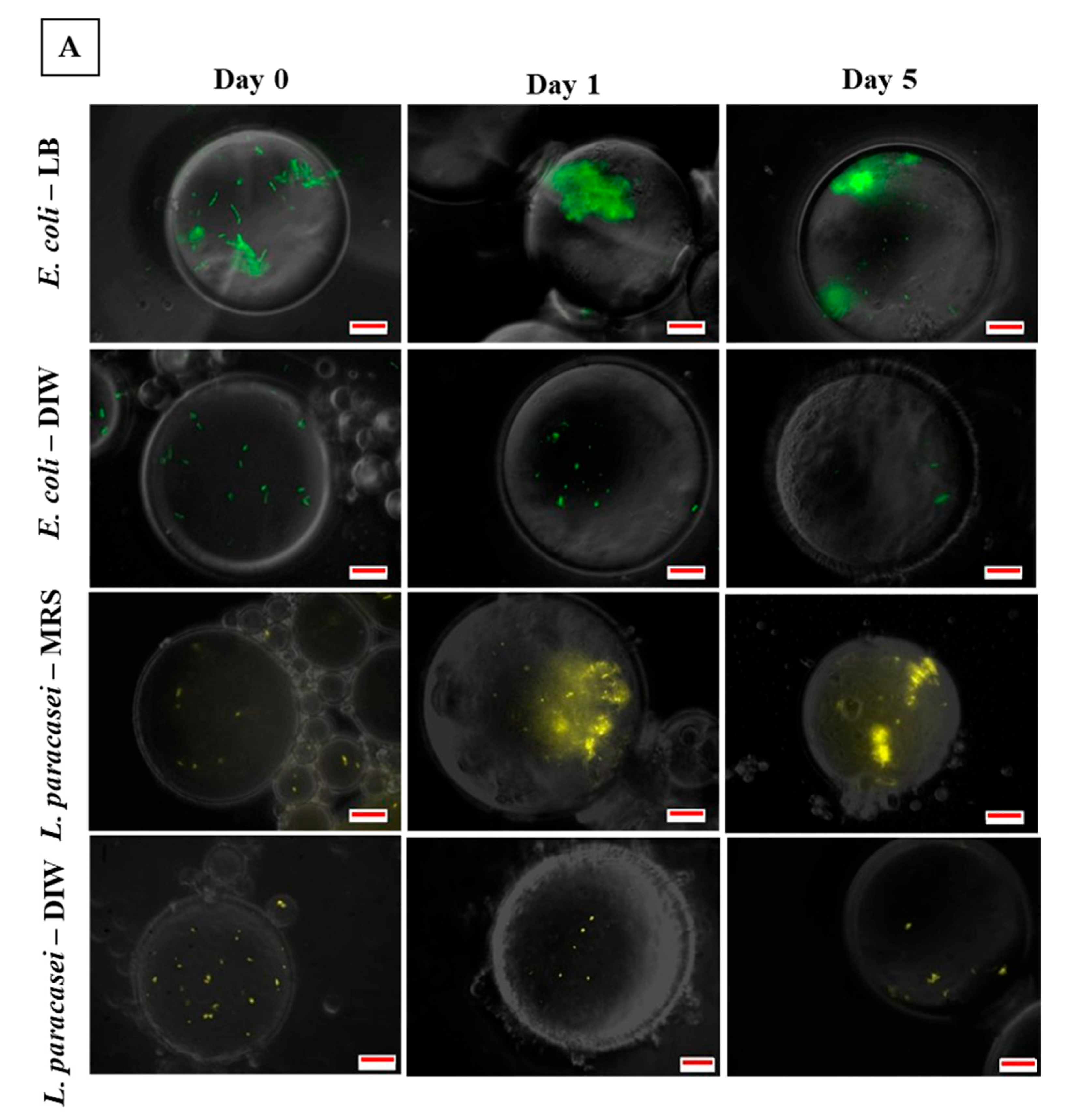
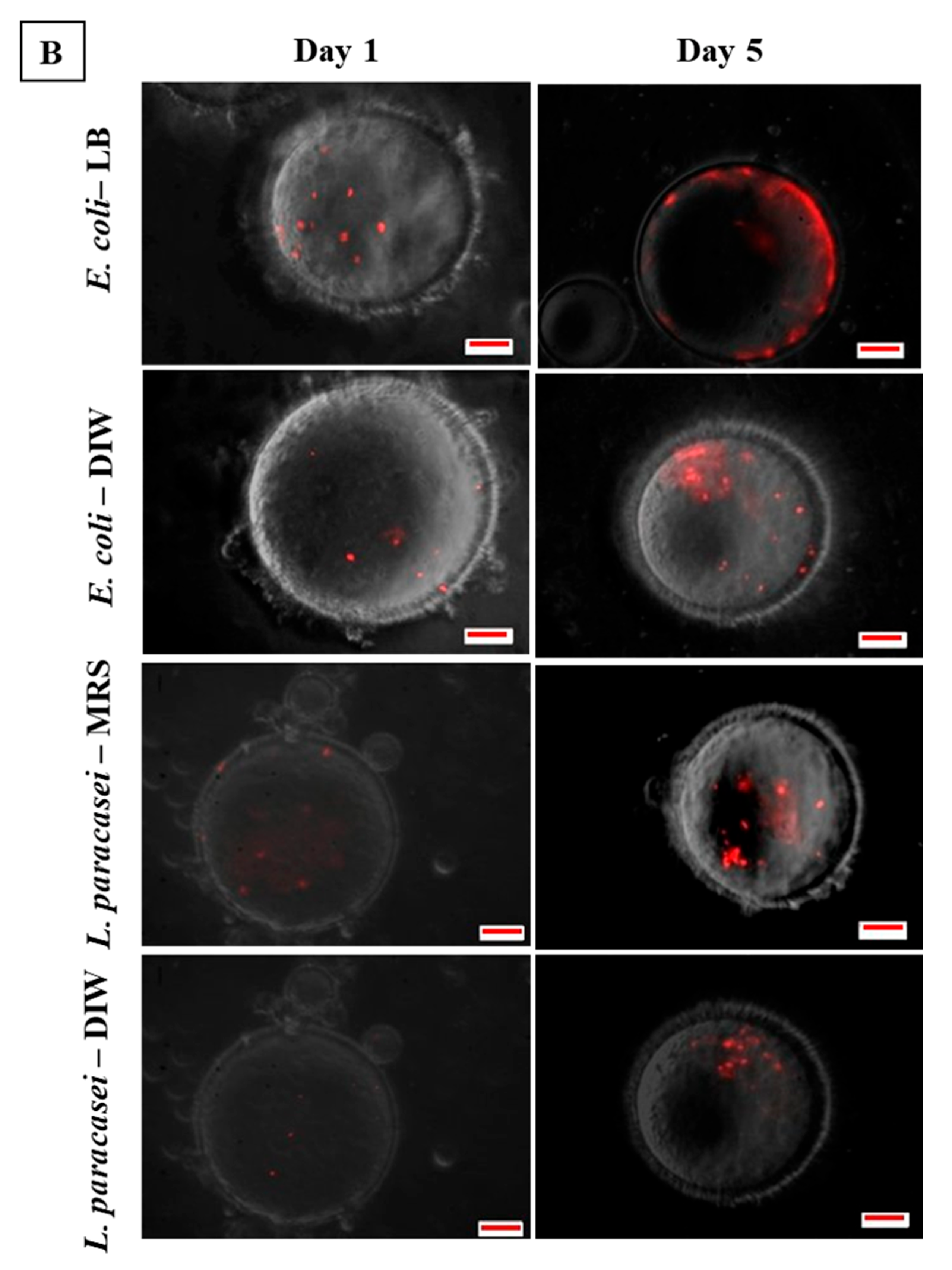
3.3. Microscopic Observation of Bacterial Response in W/O Droplet
3.4. Characterisation of Bacterial Hydrophobicity
3.5. Changes in the Interfacial Tension of Droplets in the Presence of Bacteria
3.6. Colloidal Stability of Bacterial Suspension
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lalou, S.; Kadri, H.E.; Gkatzionis, K. Incorporation of water-in-oil-in-water (W1/O/W2) double emulsion in a set-type yogurt model. Food Res. Int. 2017, 100, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Klojdová, I.; Štětina, J.; Horáčková, Š. W/O/W Multiple emulsions as the functional component of dairy products. Chem. Eng. Technol. 2019, 42, 1–14. [Google Scholar] [CrossRef]
- Shima, M.; Morita, Y.; Yamashita, M.; Adachi, S. Protection of Lactobacillus acidophilus from the low pH of a model gastric juice by incorporation in a W/O/W emulsion. Food Hydrocoll. 2006, 20, 1164–1169. [Google Scholar] [CrossRef]
- Pimentel-González, D.J.; Campos-Montiel, R.G.; Lobato-Calleros, C.; Pedroza-Islas, R.; Vernon-Carter, E.J. Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Res. Int. 2009, 42, 292–297. [Google Scholar] [CrossRef]
- Marcoux, P.; Dupoy, M.; Mathey, R.; Novelli-Rousseau, A.; Héran, V.; Morales, S.; Rivera, F.; Joly, P.; Moy, J.-P.; Mallard, F. Micro-confinement of bacteria into W/O emulsion droplets for rapid detection and enumeration. Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 54–62. [Google Scholar] [CrossRef]
- Boedicker, J.Q.; Vincent, M.E.; Ismagilov, R.F. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew. Chem. Int. Ed. 2009, 48, 5908–5911. [Google Scholar] [CrossRef]
- Hou, R.C.W.; Lin, M.Y.; Wang, M.M.C.; Tzen, J.T.C. Increase of viability of entrapped cells of Lactobacillus delbrueckii ssp. bulgaricus in artificial sesame oil emulsions. J. Dairy Sci. 2003, 86, 424–428. [Google Scholar] [CrossRef]
- Isa, N.S.M.; Kadri, H.E.; Vigolo, D.; Gkatzionis, K. Optimisation of bacterial release from a stable microfluidic-generated water-in-oil-in-water emulsion. RSC Adv. 2021, 11, 7738–7749. [Google Scholar]
- Kadri, H.E.; Overton, T.; Bakalis, S.; Gkatzionis, K. Understanding and controlling the release mechanism of Escherichia coli in double W1/O/W2 emulsion globules in the presence of NaCl in the W2 phase. RSC Adv. 2015, 5, 105098–105110. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Kadri, H.E.; Bowden, A.; Spyropoulos, F.; Gkatzionis, K. Segregation of Tetragenococcus halophilus and Zygosaccharomyces rouxii using W1/O/W2 double emulsion for use in mixed culture fermentation. Food Res. Int. 2018, 105, 333–343. [Google Scholar] [CrossRef]
- Bernath, K.; Hai, M.; Mastrobattista, E.; Griffiths, A.D.; Magdassi, S.; Tawfik, D.S. In vitro compartmentalization by double emulsions: Sorting and gene enrichment by fluorescence activated cell sorting. Anal. Biochem. 2004, 325, 151–157. [Google Scholar] [CrossRef]
- Dorobantu, L.S.; Yeung, A.K.C.; Foght, J.M.; Gray, M.R. Stabilization of oil-water emulsions by hydrophobic bacteria. Appl. Environ. Microbiol. 2004, 70, 6333–6336. [Google Scholar] [CrossRef] [PubMed]
- Wongkongkatep, P.; Manopwisedjaroen, K.; Tiposoth, P.; Archakunakorn, S.; Pongtharangkul, T.; Suphantharika, M.; Honda, K.; Hamachi, I.; Wongkongkatep, J. Bacteria Interface Pickering Emulsions Stabilized by Self-assembled Bacteria − Chitosan Network. Langmuir 2012, 28, 5729–5736. [Google Scholar] [CrossRef]
- Firoozmand, H.; Rousseau, D. Microbial cells as colloidal particles: Pickering oil-in-water emulsions stabilized by bacteria and yeast. FRIN 2016, 81, 66–73. [Google Scholar] [CrossRef]
- Idalia, V.N.; Bernardo, F. Escherichia coli as a Model Organism and Its Application in Biotechnology; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/53916 (accessed on 8 April 2022).
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft lithography for microfluidics: A review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Bauer, W.A.C.; Fischlechner, M.; Abell, C.; Huck, W.T.S. Hydrophilic PDMS microchannels for high-throughput formation of oil-in-water microdroplets and water-in-oil-in-water double emulsions. Lab Chip 2010, 10, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal treatments for fruit and vegetable juices and beverages: A literature overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef]
- Miles, A.; Misra, S.; Irwin, O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- The MathWorks, Inc. Find Circles Using Circular Hough Transform MATLAB Imfindcircles. Available online: https://www.mathworks.com/help/images/ref/imfindcircles.html (accessed on 8 April 2022).
- Muijlwijk, K.; Berton-Carabin, C.; Schroën, K. Cross-flow microfluidic emulsification from a food perspective. Trends Food Sci. Technol. 2016, 49, 51–63. [Google Scholar] [CrossRef]
- Maan, A.A.; Schroën, K.; Boom, R. Monodispersed water-in-oil emulsions prepared with semi-metal microfluidic EDGE systems. Microfluid. Nanofluid. 2013, 14, 187–196. [Google Scholar] [CrossRef]
- Romoscanu, A.I.; Fenollosa, A.; Acquistapace, S.; Gunes, D.; Martins-Deuchande, T.; Clausen, P.; Mezzenga, R.; Nydén, M.; Zick, K.; Hughes, E. Structure, diffusion, and permeability of protein-stabilized monodispersed oil in water emulsions and their gels: A self-diffusion NMR study. Langmuir 2010, 26, 6184–6192. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Chang, C.B.; Wilking, J.N.; Kim, S.H.; Shum, H.C.; Weitz, D.A. Monodisperse emulsion drop microenvironments for bacterial biofilm growth. Small 2015, 11, 3954–3961. [Google Scholar] [CrossRef] [PubMed]
- Boitard, L.; Cottinet, D.; Kleinschmitt, C.; Bremond, N.; Baudry, J.; Yvert, G.; Bibette, J. Monitoring single-cell bioenergetics via the coarsening of emulsion droplets. Proc. Natl. Acad. Sci. USA 2012, 109, 7181–7186. [Google Scholar] [CrossRef] [PubMed]
- Geersens, É.; Vuilleumier, S.; Ryckelynck, M. Growth-associated droplet shrinkage for bacterial quantification, growth monitoring, and separation by ultrahigh-throughput microfluidics. ACS Omega 2022, 7, 12039–12047. [Google Scholar] [CrossRef] [PubMed]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, M.A.; Serrano, P.; Ferez, Y. Studies on probiotics properties of two Lactobacillus strains. Braz. Arch. Biol. Technol. 2001, 44, 95–99. [Google Scholar] [CrossRef]
- de Mesquita, A.R.C.; Costa, C.R.R.; Frutuoso, J.; Pinheiro, I.O.; Mota, A.; Franchitti, A.A.; Ximenes, E.A. Activity of metabolites produced by new strains of Lactobacillus in modified de Man, Rogosa and Sharpe (MRS) medium against multidrug-resistant bacteria. Afr. J. Microbiol. Res. 2017, 11, 345–355. [Google Scholar]
- Rezvani, F.; Ardestani, F.; Najafpour, G. Growth kinetic models of five species of Lactobacilli and lactose consumption in batch submerged culture. Braz. J. Microbiol. 2017, 48, 251–258. [Google Scholar] [CrossRef]
- Parker, M.L.; Brocklehurst, T.F.; Gunning, P.A.; Coleman, H.P.; Robins, M.M. Growth of food-borne pathogenic bacteria in oil-in-water emulsions: I--Methods for investigating the form of growth. J. Appl. Bacteriol. 1995, 78, 601–608. [Google Scholar] [CrossRef]
- Barlow, J.; Gozzi, K.; Kelley, C.P.; Geilich, B.M.; Webster, T.J.; Chai, Y.; Sridhar, S.; van de Ven, A.L. High throughput microencapsulation of Bacillus subtilis in semi-permeable biodegradable polymersomes for selenium remediation. Appl. Microbiol. Biotechnol. 2017, 101, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Bang, W.; Drake, M.A. Stress response of Escherichia coli. Compr. Rev. Food Sci. Food Saf. 2006, 5, 52–64. [Google Scholar] [CrossRef]
- Rowlett, V.W.; Mallampalli, V.W.K.P.S.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef]
- Lebeer, S.; Verhoeven, T.L.A.; Vélez, M.P.; Vanderleyden, J.; de Keersmaecker, S.C.J. Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2007, 73, 6768–6775. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Wilson, S.; Hall-Stoodley, L.; Boyle, J.D.; Lappin-Scott, H.M.; Costerton, J.W. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl. Environ. Microbiol. 2001, 67, 5608–5613. [Google Scholar] [CrossRef] [PubMed]
- Seltmann, G.; Holst, O. The Bacterial Cell Wall; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Chen, Y.Y.; Wang, H.Y. Fabrication and characterization of polyelectrolyte microcarriers for microorganism cultivation through a microfluidic droplet system. Biomicrofluidics 2016, 10, 0140126. [Google Scholar] [CrossRef]
- Soni, K.A.; Balasubramanian, A.K.; Beskok, A.; Pillai, S.D. Zeta potential of selected bacteria in drinking water when dead, starved, or exposed to minimal and rich culture media. Curr. Microbiol. 2008, 56, 93–97. [Google Scholar] [CrossRef]
- Larsen, N.; Barbosa, T.; Marta, S.; Saad, I.; Blennow, A. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Liu, H.; McClements, D.J.; Cheng, C.; Zou, L.; Liu, W.; Liu, W. Probiotic encapsulation in water-in-oil high internal phase emulsions: Enhancement of viability under food and gastrointestinal conditions. LWT 2022, 163, 113499. [Google Scholar] [CrossRef]

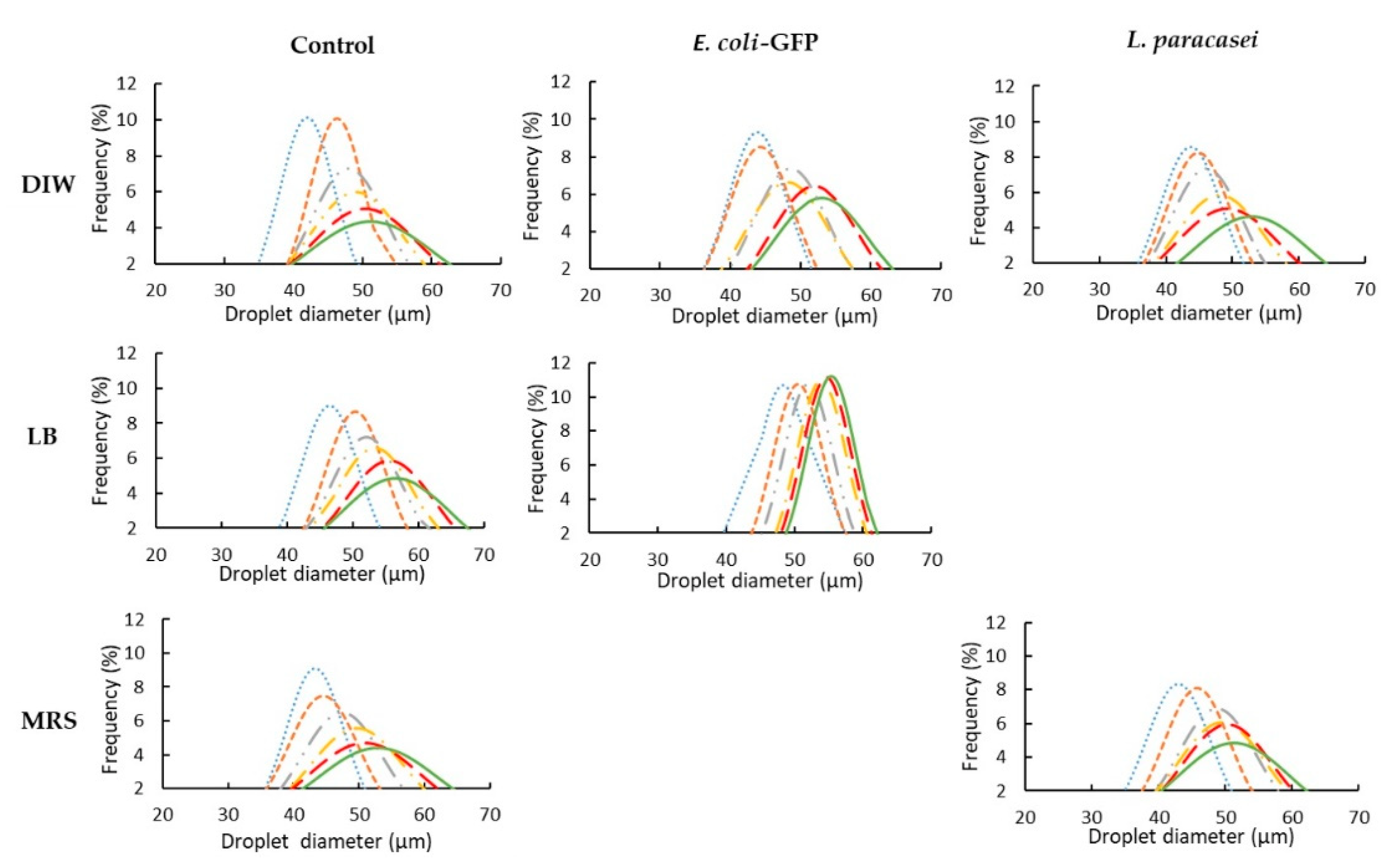

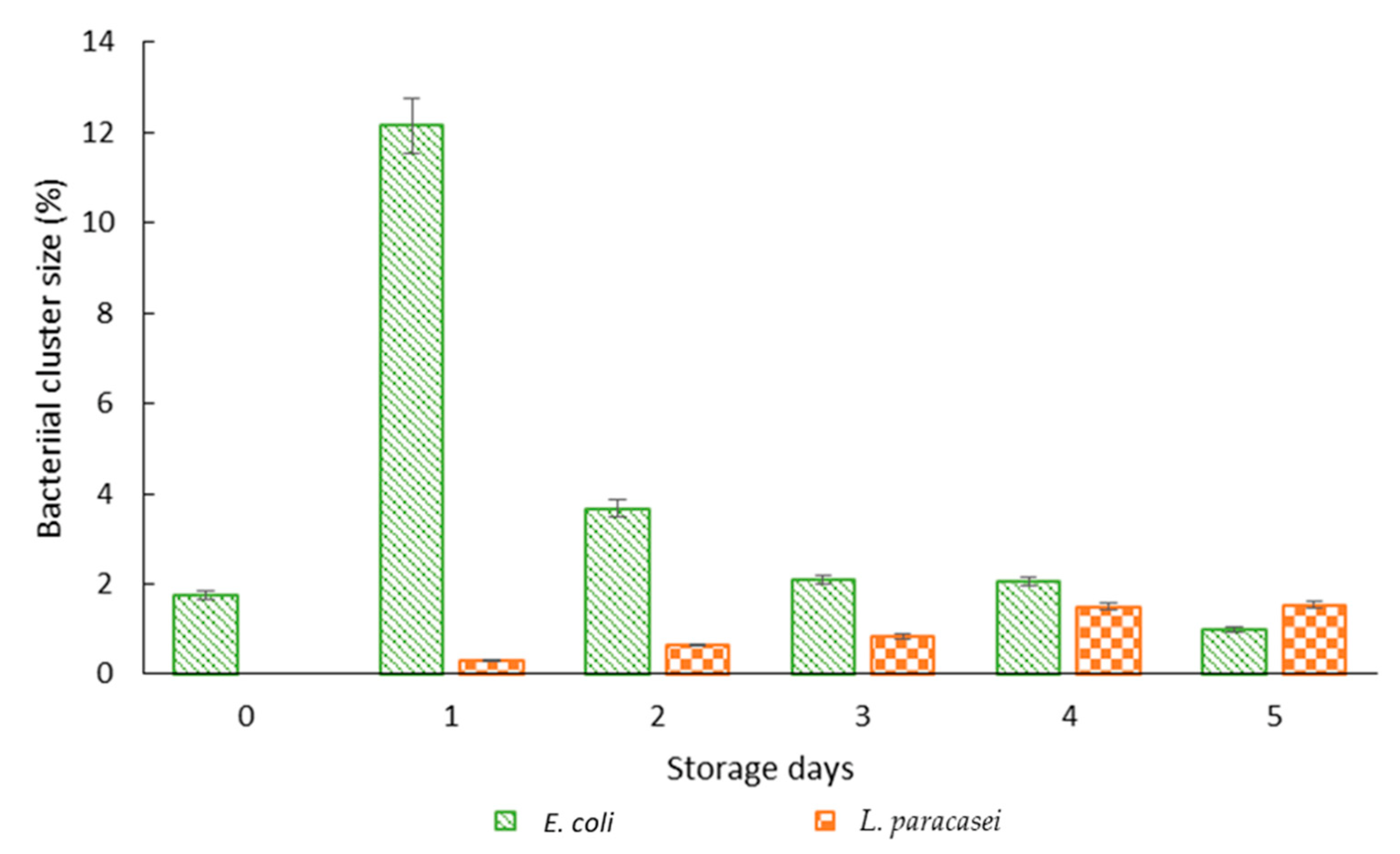
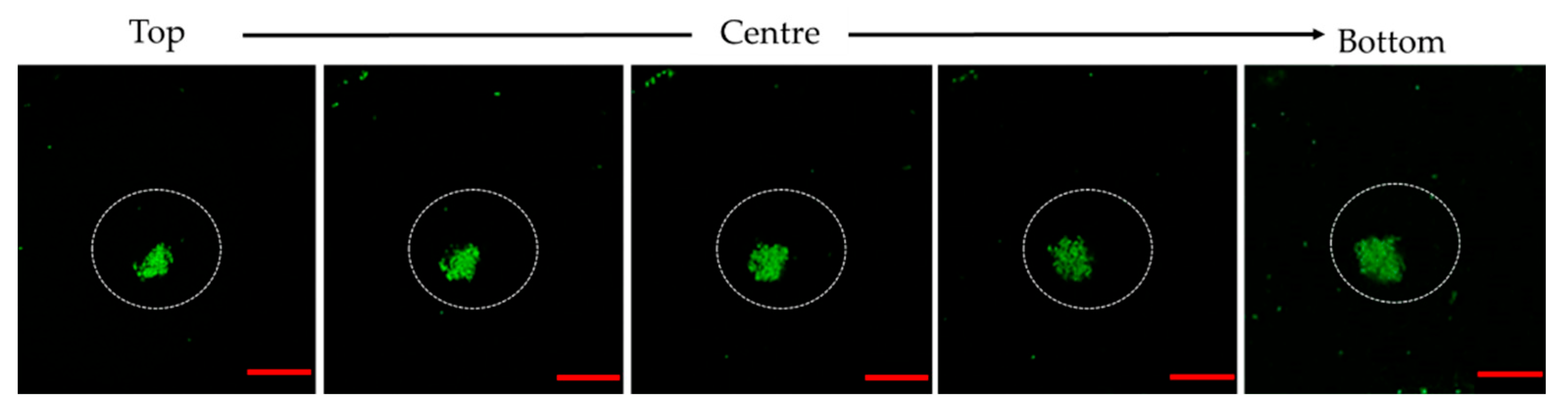
| Samples | Days | Average Diameter (µm) | Overall Diameter Changes (%) | Coefficient of Variance (%) |
|---|---|---|---|---|
| Empty DIW | 0 | 42.0 ± 3.9 a | 21.2 ± 10.7 a | 9.3 |
| 1 | 46.3 ± 4.0 b | 8.6 | ||
| 2 | 48.0 ± 5.5 bc | 11.5 | ||
| 3 | 49.0 ± 6.7 cd | 13.6 | ||
| 4 | 50.4 ± 7.9 d | 15.7 | ||
| 5 | 51.2 ± 9.2 d | 18.0 | ||
| Empty LB broth | 0 | 46.5 ± 4.4 a | 21.3 ± 6.2 a | 9.5 |
| 1 | 50.4 ± 4.6 b | 9.1 | ||
| 2 | 52.1 ± 5.5 c | 10.6 | ||
| 3 | 53.4 ± 6.0 cd | 11.2 | ||
| 4 | 55.6 ± 6.8 de | 12.2 | ||
| 5 | 56.6 ± 8.2 e | 14.5 | ||
| E. coli in DIW | 0 | 43.8 ± 4.3 a | 20.6 ± 3.9 a | 9.8 |
| 1 | 44.3 ± 4.7 a | 10.6 | ||
| 2 | 48.5 ± 5.4 b | 11.1 | ||
| 3 | 48.1 ± 6.0 b | 12.5 | ||
| 4 | 51.9 ± 6.2 c | 11.9 | ||
| 5 | 53.0 ± 6.9 c | 13.0 | ||
| E. coli in LB broth | 0 | 48.3 ± 3.7 a | 14.8 ± 1.4 b | 7.7 |
| 1 | 50.6 ± 3.7 ab | 7.3 | ||
| 2 | 52.0 ± 3.7 bc | 7.1 | ||
| 3 | 53.9 ± 3.6 cd | 6.7 | ||
| 4 | 54.7 ± 3.6 d | 6.6 | ||
| 5 | 55.4 ± 3.6 d | 6.4 | ||
| Empty MRS broth | 0 | 43.3 ± 4.4 a | 21.6 ± 8.7 a | 10.2 |
| 1 | 44.6 ± 5.3 b | 11.5 | ||
| 2 | 47.4 ± 6.1 b | 12.7 | ||
| 3 | 49.7 ± 7.2 c | 14.5 | ||
| 4 | 50.9 ± 8.5 c | 16.7 | ||
| 5 | 52.9 ± 9.1 d | 17.3 | ||
| L. paracasei in DIW | 0 | 43.7 ± 4.7 a | 20.6 ± 6.8 ac | 10.8 |
| 1 | 44.8 ± 4.8 ab | 10.7 | ||
| 2 | 46.0 ± 5.4 b | 11.7 | ||
| 3 | 48.1 ± 6.8 c | 14.1 | ||
| 4 | 49.2 ± 7.9 c | 16.1 | ||
| 5 | 52.9 ± 8.6 d | 16.3 | ||
| L. paracasei in MRS broth | 0 | 42.9 ± 4.8 a | 19.1 ± 6.1 c | 11.2 |
| 1 | 45.8 ± 4.9 a | 11.0 | ||
| 2 | 48.8 ± 5.8 b | 11.9 | ||
| 3 | 49.5 ± 6.6 b | 13.3 | ||
| 4 | 50.3 ± 6.7 bd | 13.3 | ||
| 5 | 51.3 ± 8.3 d | 16.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Isa, N.S.; El Kadri, H.; Vigolo, D.; Gkatzionis, K. The Effect of Bacteria on the Stability of Microfluidic-Generated Water-in-Oil Droplet. Micromachines 2022, 13, 2067. https://doi.org/10.3390/mi13122067
Mohd Isa NS, El Kadri H, Vigolo D, Gkatzionis K. The Effect of Bacteria on the Stability of Microfluidic-Generated Water-in-Oil Droplet. Micromachines. 2022; 13(12):2067. https://doi.org/10.3390/mi13122067
Chicago/Turabian StyleMohd Isa, Nur Suaidah, Hani El Kadri, Daniele Vigolo, and Konstantinos Gkatzionis. 2022. "The Effect of Bacteria on the Stability of Microfluidic-Generated Water-in-Oil Droplet" Micromachines 13, no. 12: 2067. https://doi.org/10.3390/mi13122067
APA StyleMohd Isa, N. S., El Kadri, H., Vigolo, D., & Gkatzionis, K. (2022). The Effect of Bacteria on the Stability of Microfluidic-Generated Water-in-Oil Droplet. Micromachines, 13(12), 2067. https://doi.org/10.3390/mi13122067







