Phase Formation Behavior and Thermoelectric Transport Properties of S-Doped FeSe2−xSx Polycrystalline Alloys
Abstract
1. Introduction
2. Experimental Section
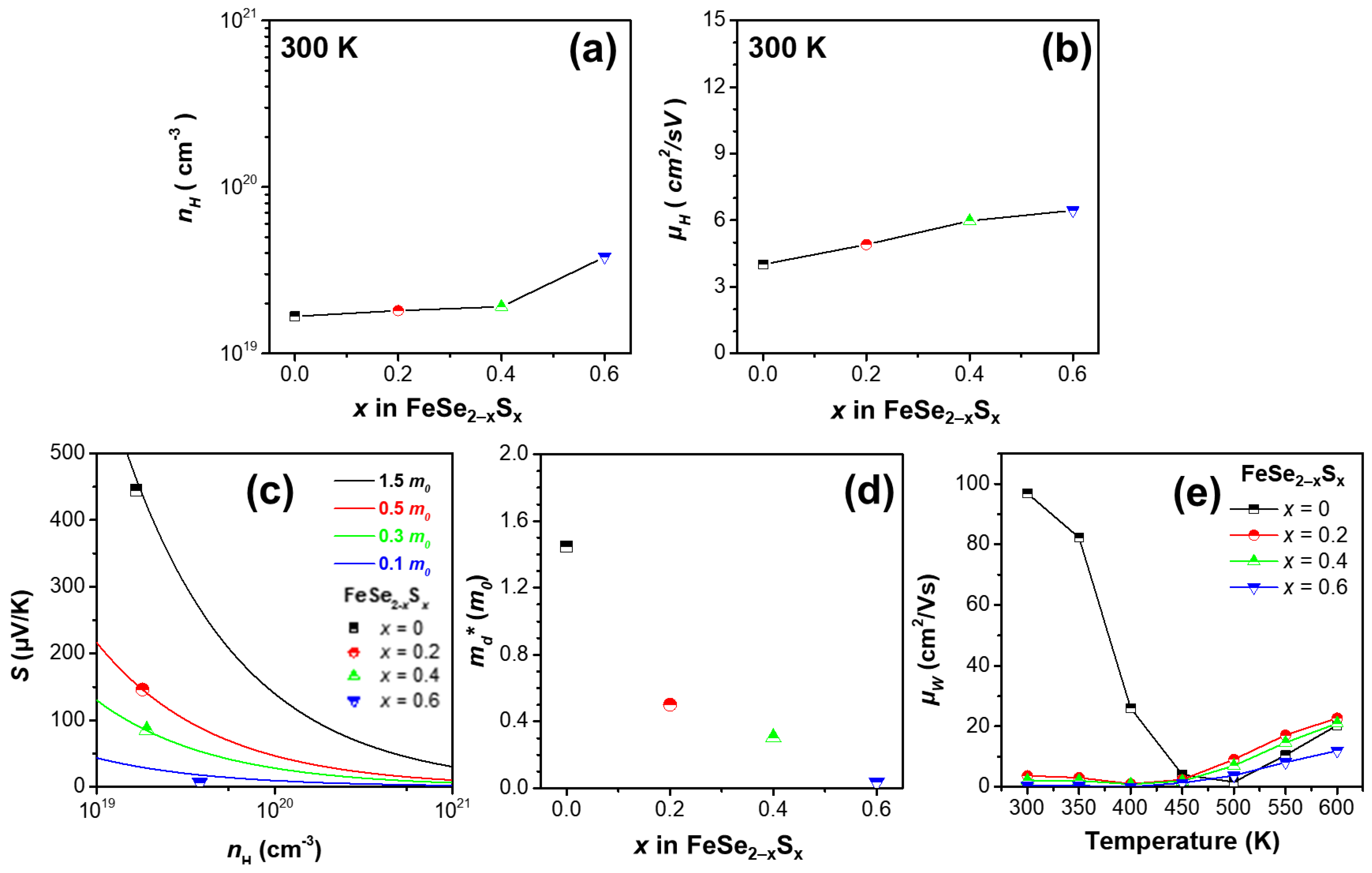
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flipse, J.; Bakker, F.L.; Slachter, A.; Dejene, F.K.; van Wees, B.J. Direct observation of the spin-dependent Peltier effect. Nat. Nanotechnol. 2012, 7, 166–168. [Google Scholar]
- Tritt, T.M.; Subramanian, M.A. Thermoelectric Materials, Phenomena, and Applications: A Bird’s Eye View. MRS Bull. 2006, 31, 188–198. [Google Scholar] [CrossRef]
- Gaultois, M.W.; Sparks, T.D.; Borg, C.K.H.; Seshadri, R.; Bonificio, W.D.; Clarke, D.R. Data-Driven Review of Thermoelectric Materials: Performance and Resource Considerations BT—Chemistry of Materials. Chem. Mater. 2013, 25, 2911–2920. [Google Scholar] [CrossRef]
- Zhao, K.; Guan, M.; Qiu, P.; Blichfeld, A.B.; Eikeland, E.; Zhu, C.; Ren, D.; Xu, F.; Iversen, B.B.; Shi, X.; et al. Thermoelectric Properties of Cu2Se1−xTex Solid Solutions. J. Mater. Chem. A 2018, 6, 6977–6986. [Google Scholar] [CrossRef]
- Skoug, E.J.; Cain, J.D.; Morelli, D.T. High thermoelectric figure of merit in the Cu3SbSe4-Cu3SbS4 solid solution. Appl. Phys. Lett. 2011, 98, 261911. [Google Scholar] [CrossRef]
- Xing, T.; Zhu, C.; Song, Q.; Huang, H.; Xiao, J.; Ren, D.; Shi, M.; Qiu, P.; Shi, X.; Xu, F.; et al. Ultralow Lattice Thermal Conductivity and Superhigh Thermoelectric Figure-of-Merit in (Mg, Bi) Co-Doped GeTe. Adv. Mater. 2021, 33, 2008773. [Google Scholar] [CrossRef]
- Asfandiyar, T.-R.W.; Li, Z.; Sun, F.-H.; Pan, Y.; Wu, C.-F.; Farooq, M.U.; Tang, H.; Li, F.; Li, B.; Li, J.-F. Thermoelectric SnS and SnS-SnSe solid solutions prepared by mechanical alloying and spark plasma sintering: Anisotropic thermoelectric properties. Sci. Rep. 2017, 7, 43262. [Google Scholar] [CrossRef]
- Bang, J.; Kim, H.-S.; Kim, D.H.; Lee, S.W.; Park, O.; Kim, S.-I. Phase formation behavior and electronic transport properties of HfSe2-HfTe2 solid solution system. J. Alloys Compd. 2022, 920, 166028. [Google Scholar] [CrossRef]
- Song, H.-Y.; Sun, J.-J.; Li, M. Enhancement of monolayer HfSe2 thermoelectric performance by strain engineering: A DFT calculation. Chem. Phys. Lett. 2021, 784, 139109. [Google Scholar] [CrossRef]
- Abdollah, H.M.; Mohammad, Y.; Mojtaba, Y.; Alireza, A. Investigation into thermoelectric properties of M (M = Hf, Zr) X2 (X = S, Se, Te) nanotubes using first-principles calculation. Solid State Commun. 2021, 336, 114289. [Google Scholar] [CrossRef]
- Kumar, S.; Schwingenschlögl, U. Thermoelectric Response of Bulk and Monolayer MoSe2 and WSe2. Chem. Mater. 2015, 27, 1278–1284. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Luo, Z.; Hao, S.; Du, C.; Liang, Q.; Li, Z.; Khor, K.A.; Hippalgaonkar, K.; Xu, J.; et al. n-Type SnSe2 Oriented-Nanoplate-Based Pellets for High Thermoelectric Performance. Adv. Energy Mater. 2018, 8, 1702167. [Google Scholar] [CrossRef]
- Kim, S.-I.; Bang, J.; An, J.; Hong, S.; Bang, G.; Shin, W.H.; Kim, T.; Lee, K. Effect of Br substitution on thermoelectric transport properties in layered SnSe2. J. Alloys Compd. 2021, 868, 159161. [Google Scholar] [CrossRef]
- Mavrokefalos, A.; Nguyen, N.T.; Pettes, M.T.; Johnson, D.C.; Shi, L. In-plane thermal conductivity of disordered layered WSe2 and (W)x(WSe2)y superlattice films. Appl. Phys. Lett. 2007, 91, 171912. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, R.; Yan, S.; Xiong, W.; Zhu, J.; Lu, K.; Wang, X. Atomic Layer Deposition of FeSe2, CoSe2, and NiSe2. Chem. Mater. 2021, 33, 2478–2487. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Rao, J.; Herranz Gonzalez, D.; Blake, G.R.; de Groot, R.A.; Palstra, T.T. Effect of vacancies on magnetism, electrical transport, and thermoelectric performance of marcasite FeSe2−δ (δ = 0.05). Chem. Mat. 2015, 27, 8220. [Google Scholar] [CrossRef]
- Gudelli, V.K.; Kanchana, V.; Vaitheeswaran, G.; Valsakumar, M.C.; Mahanti, S.D. Thermoelectric properties of marcasite and pyrite FeX2 (X = Se, Te): A first principle study. RSC Adv. 2014, 4, 9424. [Google Scholar] [CrossRef][Green Version]
- Park, O.; Kim, T.; Lee, S.W.; Kim, H.-S.; Shin, W.H.; Rahman, J.U.; Kim, S.-I. Study of Phase Formation Behavior and Electronic Transport Properties in the FeSe2-FeTe2 System. Korean J. Met. Mater. 2022, 60, 315–320. [Google Scholar] [CrossRef]
- Zuniga-Puelles, E.; Cardoso-Gil, R.; Bobnar, M.; Veremchuk, I.; Himcinschi, C.; Hennig, C.; Kortus, J.; Heide, G.; Gumeniuk, R. Structural stability and thermoelectric performance of high quality synthetic and natural pyrites (FeS2). Dalton Trans. 2019, 48, 10703–10713. [Google Scholar] [CrossRef]
- Harran, I.; Li, Y.; Wang, H.; Chen, Y.; Ni, Y. Iron Disulfide Compound: A Promising Thermoelectric Material. Mater. Res. Express 2017, 4, 105907. [Google Scholar] [CrossRef]
- Gronvold, F.; Westrum, E.F., Jr. Heat capacities of iron disulfides Thermodynamics of marcasite from 5 to 700 K, pyrite from 300 to 780 K, and the transformation of marcasite to pyrite. J. Chem. Thermodyn. 1976, 8, 1039–1048. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, J.-I.; Cho, H.-J.; Choo, S.-S.; Kim, S.-I.; Lee, K.; Shin, W.H.; Kim, H.-S.; Roh, J.W. Electronic and Thermal Properties of Si-doped InSe Layered Chalcogenides. J. Korean Phys. Soc. 2018, 72, 775–779. [Google Scholar] [CrossRef]
- Ioffe, A.F. Physics of Semiconductors; Academic Press: New York, NY, USA, 1960. [Google Scholar]
- Snyder, G.J.; Snyder, A.H.; Wood, M.; Gurunathan, R.; Snyder, B.H.; Niu, C. Weighted mobility. Adv. Mater. 2020, 32, 2001537. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.-I.; Kim, S.W.; Kim, H.-S.; Lee, K.H. Weighted mobility ratio engineering for high-performance Bi-Te-based thermoelectric materials via suppression of minority carrier transport. Adv. Mater. 2021, 33, 2005931. [Google Scholar] [CrossRef]
- Lorenz, L. Determination of the degree of heat in absolute measure. Ann. Phys. Chem. 1872, 147, 429–451. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Sharp, J.W. Estimation of the thermal band gap of a semiconductor from seebeck measurements. J. Electron. Mater. 1999, 28, 869–872. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, O.; Lee, S.W.; Park, S.J.; Kim, S.-i. Phase Formation Behavior and Thermoelectric Transport Properties of S-Doped FeSe2−xSx Polycrystalline Alloys. Micromachines 2022, 13, 2066. https://doi.org/10.3390/mi13122066
Park O, Lee SW, Park SJ, Kim S-i. Phase Formation Behavior and Thermoelectric Transport Properties of S-Doped FeSe2−xSx Polycrystalline Alloys. Micromachines. 2022; 13(12):2066. https://doi.org/10.3390/mi13122066
Chicago/Turabian StylePark, Okmin, Se Woong Lee, Sang Jeong Park, and Sang-il Kim. 2022. "Phase Formation Behavior and Thermoelectric Transport Properties of S-Doped FeSe2−xSx Polycrystalline Alloys" Micromachines 13, no. 12: 2066. https://doi.org/10.3390/mi13122066
APA StylePark, O., Lee, S. W., Park, S. J., & Kim, S.-i. (2022). Phase Formation Behavior and Thermoelectric Transport Properties of S-Doped FeSe2−xSx Polycrystalline Alloys. Micromachines, 13(12), 2066. https://doi.org/10.3390/mi13122066






