Enhancement of the Optical and Dielectric Properties at Low Frequency of (Sr1−xCax)5Ti4O13, (0 ≤ x ≤ 0.06) Structure Ceramics
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
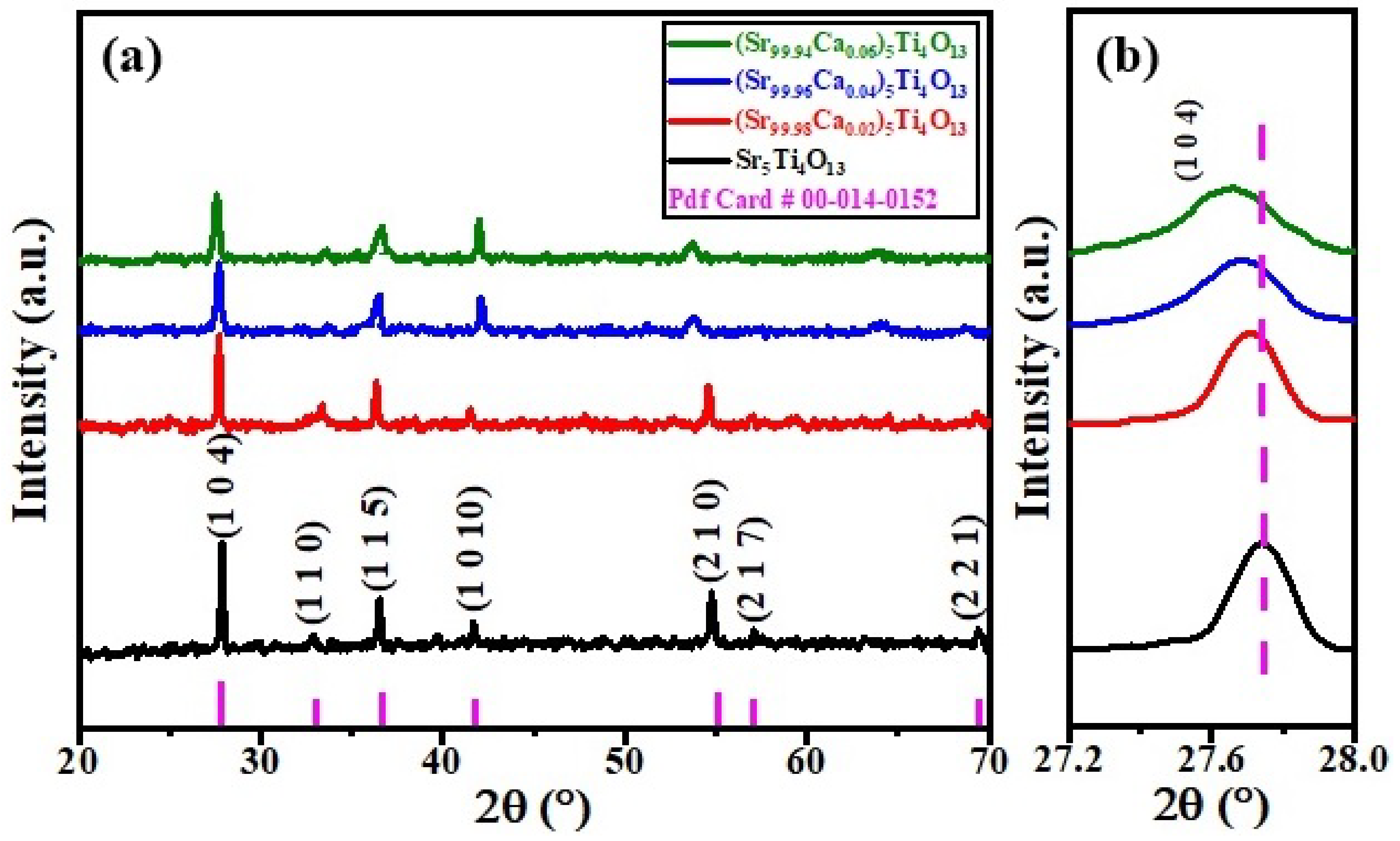
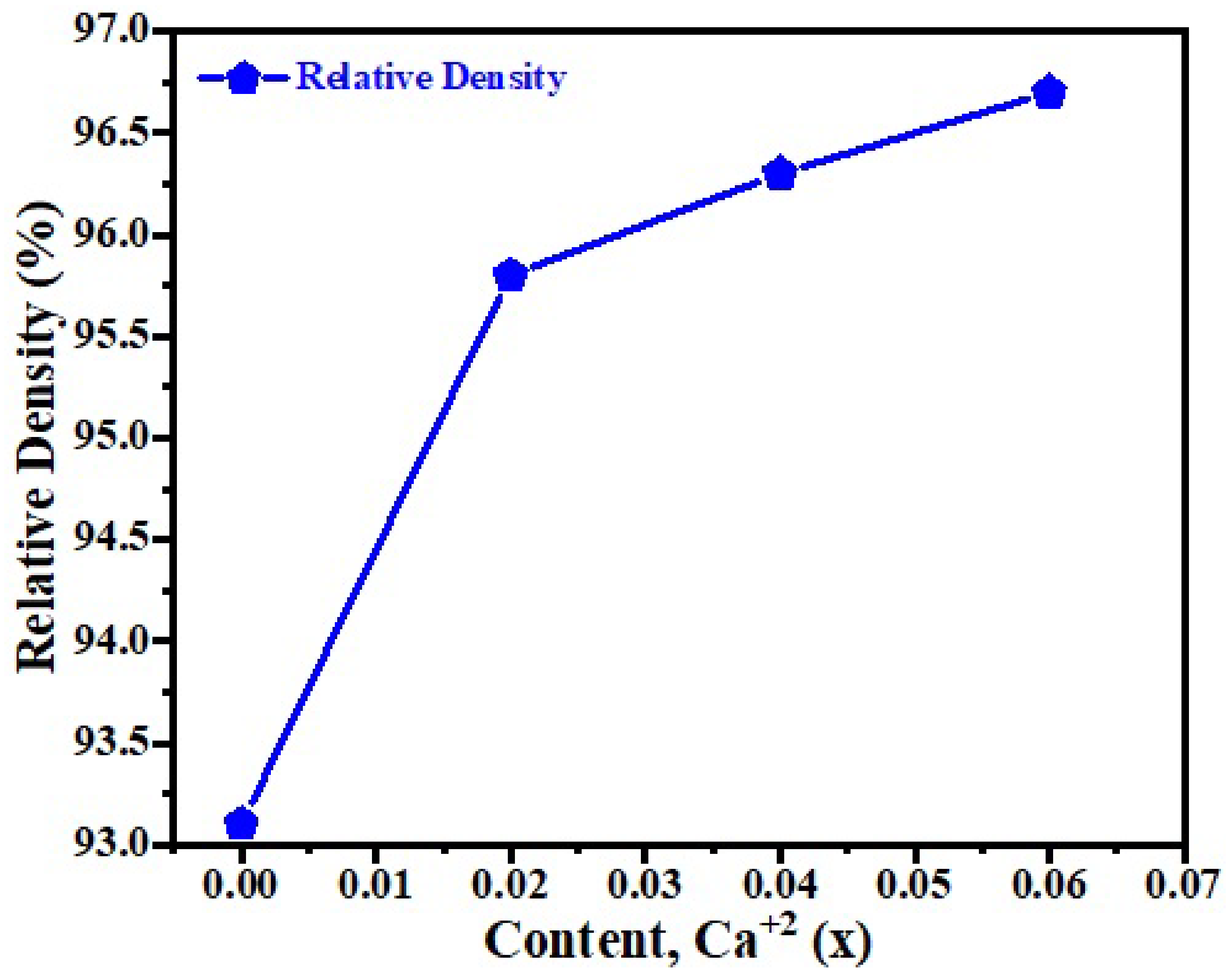
3.1. Phase Analysis
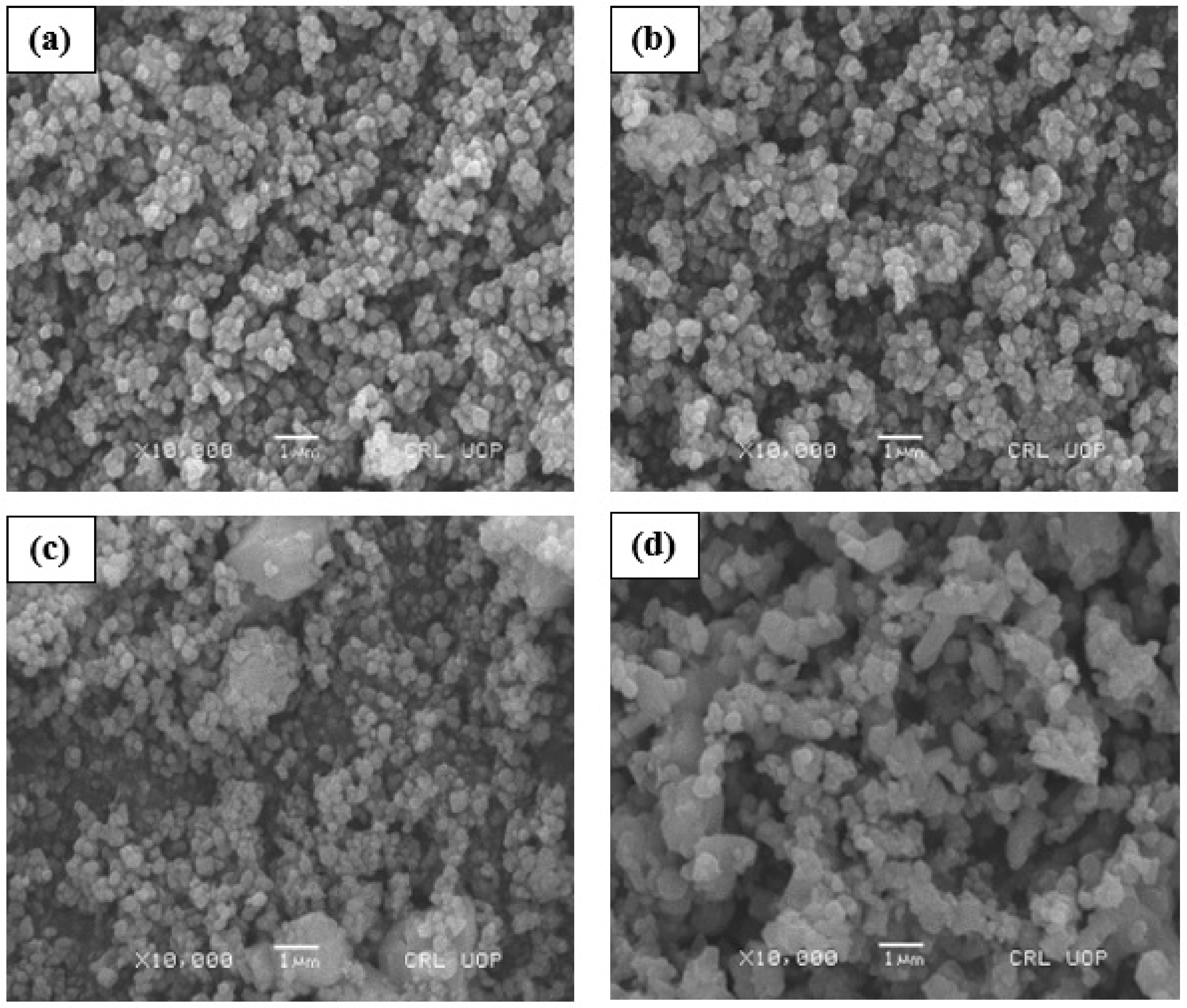
3.2. Surface Morphology
3.3. Fourier Transform Infra-Red (FTIR) Spectroscopy
3.4. UV Spectroscopy
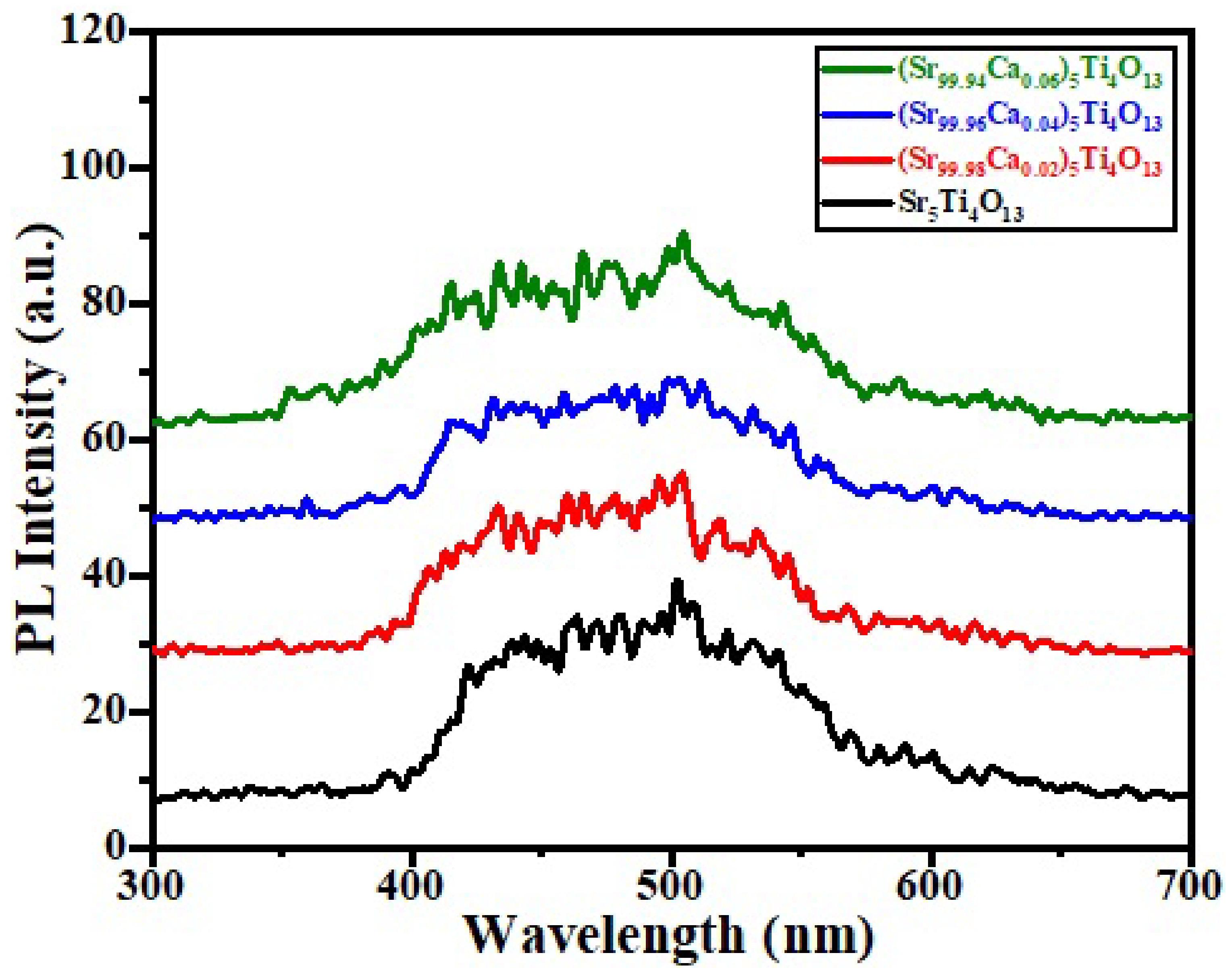
3.5. Photoluminescence (PL) Spectroscopy
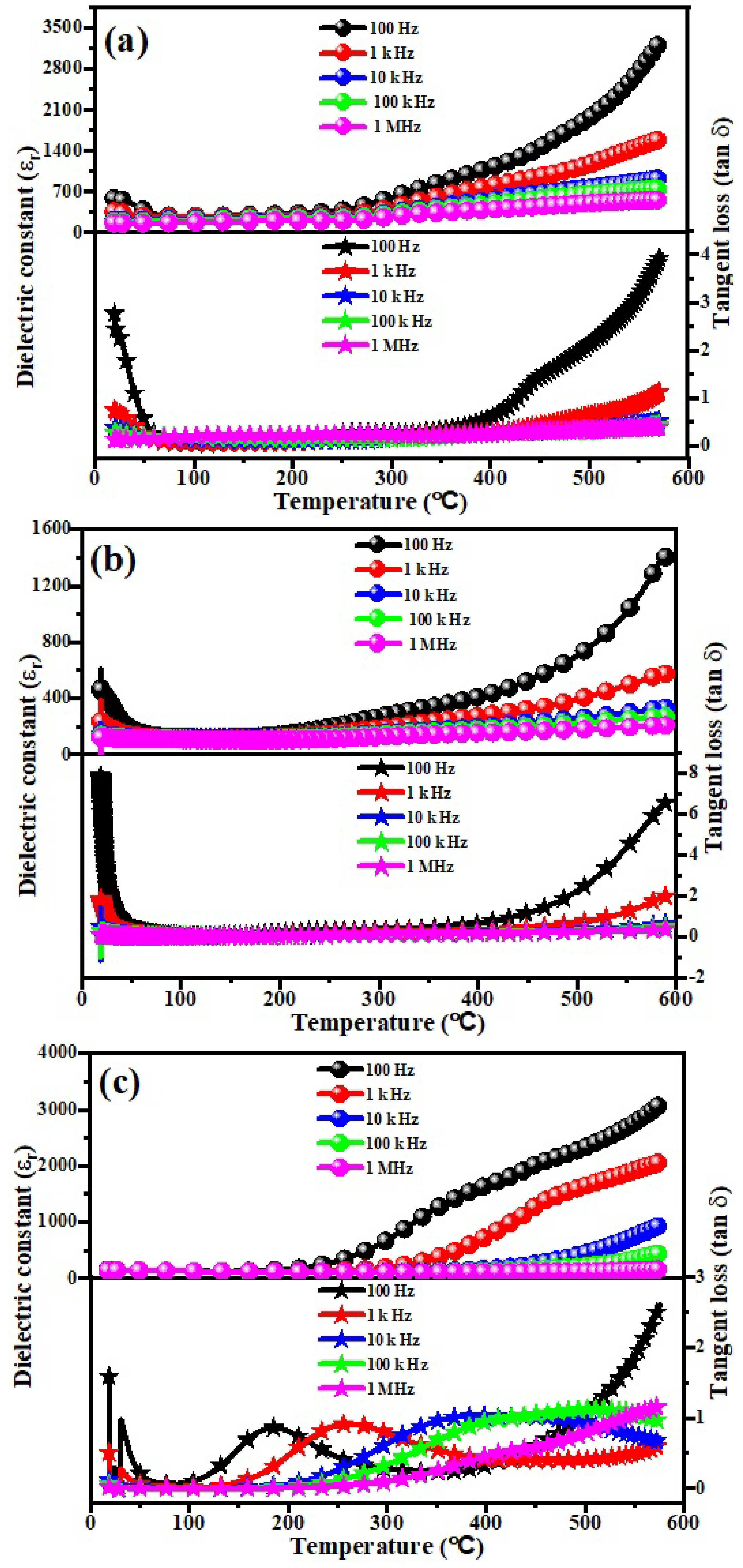
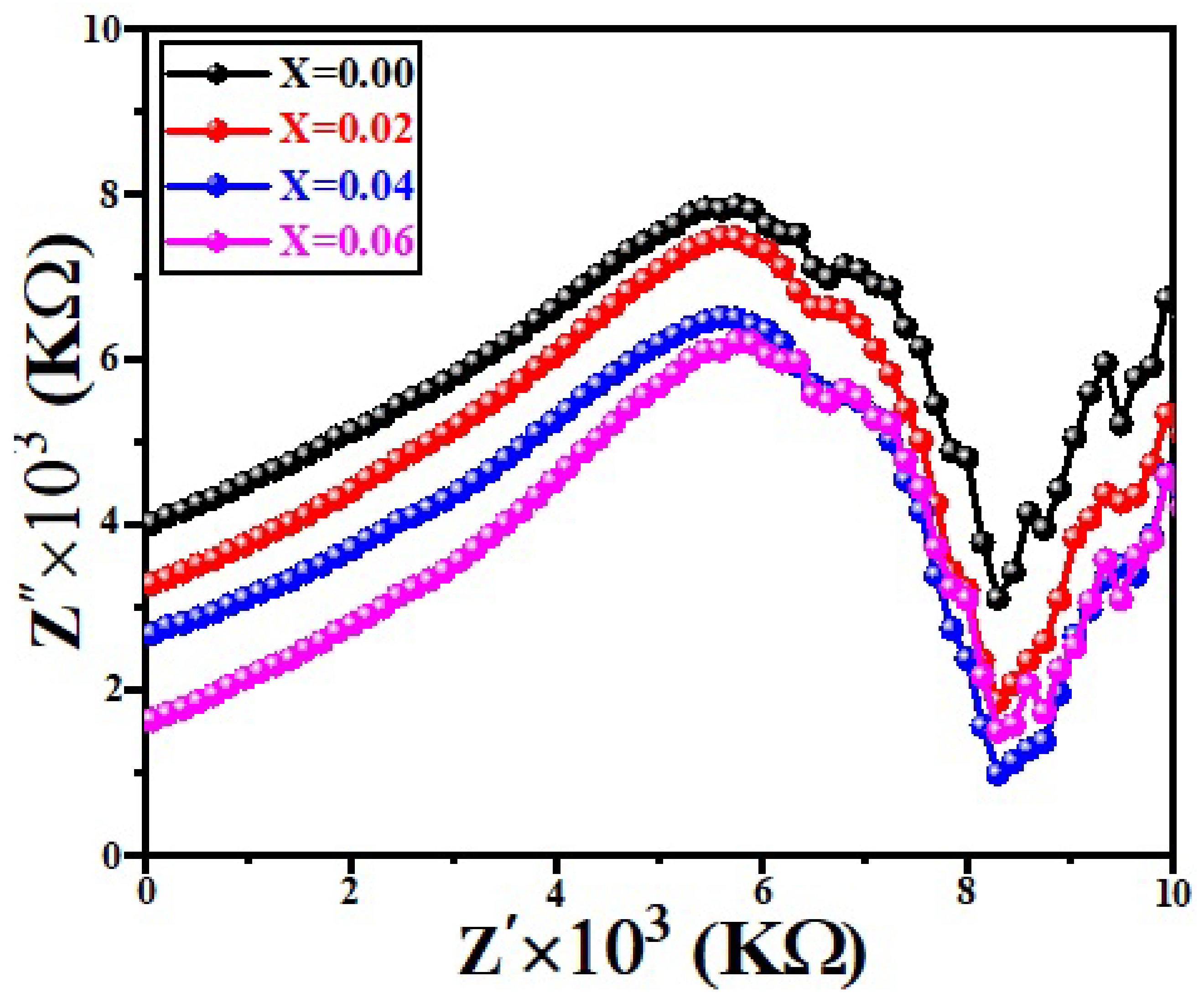
3.6. Low Frequency Dielectric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, M.D.; Cruickshank, D.B.; MacFarlane, I.A. Perspective on ceramic materials for 5G wireless communication systems. Appl. Phys. Lett. 2021, 118, 120501. [Google Scholar] [CrossRef]
- Jin, D.H.; Hu, C.C.; Liu, B. Improved sinterability and temperature stability in Zn2+/Ti4+-co-substituted CaAl2O4 ceramics and their 5G antenna applications. J. Mater. Sci. Mater. Electron. 2021, 32, 18205–18211. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yue, Z.; Li, L. Thermally stable polymer–ceramic composites for microwave antenna applications. J. Adv. Ceram. 2016, 5, 269–276. [Google Scholar] [CrossRef]
- Medeiros, J.L.G.; d’Assunção, A.G.; Mendonça, L.M. Microstrip Fractal Patch Antennas Using High Permittivity Ceramic Substrate. In Proceedings of the 2012 IEEE International Symposium on Antennas and Propagation, Chicago, IL, USA, 8–14 July 2012; pp. 1–2. [Google Scholar]
- Rhbanou, A.; El, F.A.; Jebbor, N.; Bri, S. New design of miniature C-band substrate integrated waveguide bandpass filters using ceramic material. FME Trans. 2021, 49, 103–112. [Google Scholar] [CrossRef]
- Zaman, A.; Uddin, S.; Mehboob, N.; Ali, A.; Ahmad, A.; Bashir, K. Effect of Zr4+ on the structural and microwave dielectric properties of CaTiO3 ceramics. Ferroelectrics 2021, 577, 143–152. [Google Scholar] [CrossRef]
- Yang, H.C.; Zhang, S.R.; Yang, H.Y.; Wen, Q.; Yang, Q.; Gui, L.; Zhao, Q.; Li, E. The latest process and challenges of microwave dielectric ceramics based on pseudo phase diagrams. J. Adv. Ceram. 2021, 10, 885–932. [Google Scholar] [CrossRef]
- Pei, C.; Tan, J.; Li, Y.; Yao, G.; Jia, Y.; Ren, Z.; Liu, P.; Zhang, H. Effect of Sb-site nonstoichiometry on the structure and microwave dielectric properties of Li3Mg2Sb1−xO6 ceramics. J. Adv. Ceram. 2020, 9, 588–594. [Google Scholar] [CrossRef]
- Ishizaki, T.; Fujita, M.; Kagata, H.; Uwano, T.; Miyake, H. A very small dielectric planar filter for portable telephones. IEEE Trans. Microw. Theory Tech. 1994, 42, 2017–2022. [Google Scholar] [CrossRef]
- Reaney, I.M.; Iddles, D. Microwave dielectric ceramics for resonators and filters in mobile phone networks. J. Am. Ceram. Soc. 2006, 89, 2063–2072. [Google Scholar] [CrossRef]
- Huang, C.L.; Tseng, C.F.; Yang, W.R.; Yang, T.J. High-Dielectric-Constant and Low-Loss Microwave Dielectric in the (1−x)Nd(Zn1/2Ti1/2)O3–xSrTiO3 System with a Zero Temperature Coefficient of Resonant Frequency. J. Am. Ceram. Soc. 2008, 91, 2201–2204. [Google Scholar] [CrossRef]
- Sebastian, M.T. Dielectric Materials for Wireless Communication; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Tseng, C.F. Microwave dielectric properties of a new ultra low loss pervoskite ceramic. J. Am. Ceram. Soc. 2008, 91, 4125–4128. [Google Scholar] [CrossRef]
- Xue, J.; Wu, S.; Li, J. Synthesis, microstructure, and microwave dielectric properties of spinel ZnGa2O4 ceramics. J. Am. Ceram. Soc. 2013, 96, 2481–2485. [Google Scholar] [CrossRef]
- Zheng, C.W.; Wu, S.Y.; Chen, X.M.; Song, K.X. Modification of MgAl2O4 microwave dielectric ceramics by Zn substitution. J. Am. Ceram. Soc. 2007, 90, 1483–1486. [Google Scholar]
- Nomura, S.; Toyama, K.; Kaneta, K. Ba (Mg1/3Ta2/3)O3 ceramics with temperature-stable high dielectric constant and low microwave loss. Jpn. J. Appl. Phys. 1982, 21, L624. [Google Scholar] [CrossRef]
- Hughes, H.; Iddles, D.M.; Reaney, I.M. Niobate-based microwave dielectrics suitable for third generation mobile phone base stations. Appl. Phys. Lett. 2001, 79, 2952–2954. [Google Scholar] [CrossRef]
- Yi, L.; Li, L.; Liu, X.Q.; Chen, X.M. Structure Evolution and Enhanced Microwave Dielectric Characteristics of (Sr1−xCax)La2Al2O7 Ceramics. J. Am. Ceram. Soc. 2014, 97, 3531–3536. [Google Scholar] [CrossRef]
- George, S.; Sebastian, M.T. Synthesis and Microwave Dielectric Properties of Novel Temperature Stable High Q, Li2ATi3O8 (A=Mg, Zn) Ceramics. J. Am. Ceram. Soc. 2010, 93, 2164–2166. [Google Scholar] [CrossRef]
- Liou, Y.C.; Yang, S.L. Calcium-doped MgTiO3–MgTi2O5 ceramics prepared using a reaction-sintering process. Mater. Sci. Eng. B 2007, 142, 116–120. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.Q.; Chen, X.M. Sr2LaAlTiO7: A new Ruddlesden–Popper compound with excellent microwave dielectric properties. J. Mater. Chem. C 2016, 4, 1720–1726. [Google Scholar] [CrossRef]
- Chen, X.M.; Xiao, Y.; Liu, X.Q.; Hu, X. SrLnAlO4 (Ln=Nd and Sm) Microwave Dielectric Ceramics. J. Electroceram. 2003, 10, 111–115. [Google Scholar] [CrossRef]
- Yuan, Z.Q.; Liu, B.; Liu, X.Q.; Chen, X.M. Structure and microwave dielectric characteristics of Sr(La1−xSmx)2Al2O7 ceramics. RSC Adv. 2016, 6, 96229–96236. [Google Scholar] [CrossRef]
- Guan, L.; Li, M.; Li, X.; Feng, L.; Teng, F.; Liu, B.; Musgrave, C.B. Electronic and dielectric properties of Ruddlesden–Popper type and Magnéli type SrTiO3. Comput. Mater. Sci. 2015, 96, 223–228. [Google Scholar]
- Xiao, Y.; Chen, X.M.; Liu, X.Q. Microstructures and Microwave Dielectric Characteristics of CaRAlO4 (R = Nd, Sm, Y) Ceramics with Tetragonal K2NiF4 Structure. J. Am. Ceram. Soc. 2004, 87, 2143–2146. [Google Scholar] [CrossRef]
- Yuan, H.X.; Chen, X.M.; Mao, M.M. Structure and Microwave Dielectric Characteristics of Ca1+xNd1−xAl1−xTixO4 Ceramics. J. Am. Ceram. Soc. 2009, 92, 2286–2290. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. New compounds of the K2NIF4 type. Acta Crystallogr. 1957, 10, 538–539. [Google Scholar] [CrossRef]
- Ali, A.; Zaman, A.; Khan, M.K.; Farhat, L.B.; Jabbar, A.H.; Badi, K.M.; Ullah, I.; Tirth, V.; Khan, S. Structural Evaluation, Optical, and Dielectric Properties of Ba-Doped Ca4Ti3O10-Sintered Ceramics. J. Supercond. Nov. Magn. 2022, 35, 1987–1993. [Google Scholar] [CrossRef]
- Lukaszewicz, K. Crystal structures of α-(SrO)2(TiO) and (SrO)3(TiO2)2. Rocz. Chem. 1959, 33, 239–242. [Google Scholar]
- McCarthy, G.J.; White, W.B.; Roy, R. Phase equilibria in the 1375 °C isotherm of the system Sr-Ti-O. J. Am. Ceram. Soc. 1969, 52, 463–467. [Google Scholar] [CrossRef]
- Elcombe, M.M.; Kisi, E.H.; Hawkins, K.D.; White, T.J.; Goodman, P.; Matheson, S. Structure determinations for Ca sub 3 Ti sub 2 O sub 7, Ca sub 4 Ti sub 3 O sub 10, Ca sub 3. 6 Sr sub 0. 4 Ti sub 3 O sub 10 and a refinement of Sr sub 3 Ti sub 2 O sub 7. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 305–314. [Google Scholar]
- Sugimoto, W.; Shirata, M.; Takemoto, M.; Hayami, S.; Sugahara, Y.; Kuroda, K. Synthesis and structures of carrier doped titanates with the Ruddlesden–Popper structure (Sr0. 95La0. 05) n + 1TinO3n+ 1 (n = 1, 2). Solid State Ion. 1998, 108, 315–319. [Google Scholar] [CrossRef]
- Mao, M.M.; Chen, X.M.; Liu, X.Q. Structure and microwave dielectric properties of solid solution in SrLaAlO4-Sr2TiO4 system. J. Am. Ceram. Soc. 2011, 94, 3948–3952. [Google Scholar] [CrossRef]
- Yi, L.; Liu, X.Q.; Chen, X.M. Crystal structure and infrared reflection spectra of SrLn2Al2O7 (Ln = La, Nd, Sm) microwave dielectric ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, E33–E40. [Google Scholar] [CrossRef]
- Zaman, A.; Uddin, S.; Mehboob, N.; Tirth, V.; Algahtani, A.; Abbas, M.; Mushtaq, M.; Ali, A.; Sultana, F.; Althubeiti, K.; et al. Structural Elucidation, Electronic and Microwave Dielectric Properties of Ca(SnxTi1–x)O3,(0≤ x≤ 0.8) Lead-Free Ceramics. ACS Omega 2022, 7, 4667–4676. [Google Scholar] [CrossRef]
- Singh, D.; Singh, R. Synthesis and characterization of Ruddlesden-Popper (RP) type phase LaSr2MnCrO7. J. Chem. Sci. 2010, 122, 807–811. [Google Scholar] [CrossRef]
- Kananke-Gamage, C.C.; Ramezanipour, F. Variation of the electrocatalytic activity of isostructural oxides Sr2LaFeMnO7 and Sr2LaCoMnO7 for hydrogen and oxygen-evolution reactions. Dalton Trans. 2021, 50, 14196–14206. [Google Scholar] [CrossRef]
- Sahoo, P.S.; Panigrahi, A.; Patri, S.K.; Choudhary, R.N.P. Effect of strontium concentration on structural, dielectric and electrical properties of Ba5 − xSrxDyTi3V7O30 (x = 0–5) ceramics. J. Alloys Compd. 2009, 484, 832–836. [Google Scholar] [CrossRef]
- Wise, P.L.; Reaney, I.M.; Lee, W.E.; Price, T.J.; Iddles, D.M.; Cannell, D.S. Structure-microwave property relations of Ca and Sr titanates. J. Eur. Ceram. Soc. 2001, 21, 2629–2632. [Google Scholar] [CrossRef]
- El Marssi, M.; Le Marrec, F.; Lukyanchuk, I.; Karkut, M. Ferroelectric transition in an epitaxial barium titanate thin flm: Raman spectroscopy and x-ray difraction study. J. Appl. Phys. 2003, 94, 3307–3312. [Google Scholar] [CrossRef]
- Singh, D.; Singh, R. Study of structural, electrical, and magnetic properties of layered perovskite oxides LnSr2TiFeO7 (Ln = Nd, Gd). J. Electron. Mater. 2012, 41, 540–545. [Google Scholar] [CrossRef]
- Xi, H.H.; Zhou, D.; Xie, H.D.; He, B.; Wang, Q.P. Raman spectra, infrared spectra, and microwave dielectric properties of low-temperature firing [(Li0.5Ln0.5) 1 − xCax] MoO4 (Ln = Sm and Nd) solid solution ceramics with scheelite structure. J. Am. Ceram. Soc. 2015, 98, 587–593. [Google Scholar] [CrossRef]
- Leite, E.R.; Paris, E.C.; Pontes, F.M.; Paskocimas, C.A.; Longo, E.; Sensato, F.; Pinheiro, C.D.; Varela, J.A.; Pizani, P.S.; Campos, C.E.M.; et al. The origin of photoluminescence in amorphous lead titanate. J. Mater. Sci. 2003, 38, 1175–1178. [Google Scholar] [CrossRef]
- Zaman, A.; Uddin, S.; Mehboob, N. Synthesis and Microwave Dielectric Characterization of Ca1-xSrxTiO3, Low-Loss Ceramics. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 367–371. [Google Scholar] [CrossRef]
- Ali, A.; Zaman, A.; Jabbar, A.H.; Tirth, V.; Algahtani, A.; Alhodaib, A.; Ullah, I.; JAhmed, S.; Aljohani, M. Effects of Strontium on the Structural, Optical, and Microwave Dielectric Properties of Ba2Ti9O20 Ceramics Synthesized by a Mixed Oxide Route. ACS Omega 2022, 7, 25573–25579. [Google Scholar] [CrossRef]
- Fan, X.C.; Chen, X.M.; Liu, X.Q. Structural dependence of microwave dielectric properties of SrRAlO4 (R = Sm, Nd, La) ceramics: Crystal structure refinement and infrared reflectivity study. Chem. Mater. 2008, 20, 4092–4098. [Google Scholar] [CrossRef]
- Longo, V.M.; De Figueiredo, A.T.; De Lázaro, S.; Gurgel, M.F.; Costa MG, S.; Paiva-Santos, C.O.; Franco, R.W.A. Structural conditions that leads to photoluminescence emission in SrTiO3: An experimental and theoretical approach. J. Appl. Phys. 2008, 104, 023515. [Google Scholar] [CrossRef]



| Contents | a = b (Å) | c (Å) | c/a | Error | Volume of Unit Cell (Å3) |
|---|---|---|---|---|---|
| 0.00 | 3.8512 | 28.1253 | 7.3411 | ±0.8631 | 417.1471 |
| 0.02 | 3.8518 | 28.3951 | 7.3719 | ±0.8643 | 421.2800 |
| 0.04 | 3.8523 | 28.5505 | 7.4113 | ±0.8651 | 423.6955 |
| 0.06 | 3.8615 | 28.8975 | 7.4837 | ±0.8664 | 430.8958 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.J.; Ali, A.; Zaman, A.; Alhodaib, A.; Alghtani, A.H.; Bejaoui, I.; Tirth, V.; Algahtani, A.; Khan, M.; Abdullah; et al. Enhancement of the Optical and Dielectric Properties at Low Frequency of (Sr1−xCax)5Ti4O13, (0 ≤ x ≤ 0.06) Structure Ceramics. Micromachines 2022, 13, 1824. https://doi.org/10.3390/mi13111824
Ahmed SJ, Ali A, Zaman A, Alhodaib A, Alghtani AH, Bejaoui I, Tirth V, Algahtani A, Khan M, Abdullah, et al. Enhancement of the Optical and Dielectric Properties at Low Frequency of (Sr1−xCax)5Ti4O13, (0 ≤ x ≤ 0.06) Structure Ceramics. Micromachines. 2022; 13(11):1824. https://doi.org/10.3390/mi13111824
Chicago/Turabian StyleAhmed, Sara J., Asad Ali, Abid Zaman, Aiyeshah Alhodaib, Abdulaziz H. Alghtani, Imen Bejaoui, Vineet Tirth, Ali Algahtani, Mohsin Khan, Abdullah, and et al. 2022. "Enhancement of the Optical and Dielectric Properties at Low Frequency of (Sr1−xCax)5Ti4O13, (0 ≤ x ≤ 0.06) Structure Ceramics" Micromachines 13, no. 11: 1824. https://doi.org/10.3390/mi13111824
APA StyleAhmed, S. J., Ali, A., Zaman, A., Alhodaib, A., Alghtani, A. H., Bejaoui, I., Tirth, V., Algahtani, A., Khan, M., Abdullah, & Aljohani, M. (2022). Enhancement of the Optical and Dielectric Properties at Low Frequency of (Sr1−xCax)5Ti4O13, (0 ≤ x ≤ 0.06) Structure Ceramics. Micromachines, 13(11), 1824. https://doi.org/10.3390/mi13111824






