Magnetic Forces by Permanent Magnets to Manipulate Magnetoresponsive Particles in Drug-Targeting Applications
Abstract
1. Introduction
2. Methods
2.1. Governing Equations
2.2. Hydrodynamic Force
2.3. Magnetic Force
3. Materials
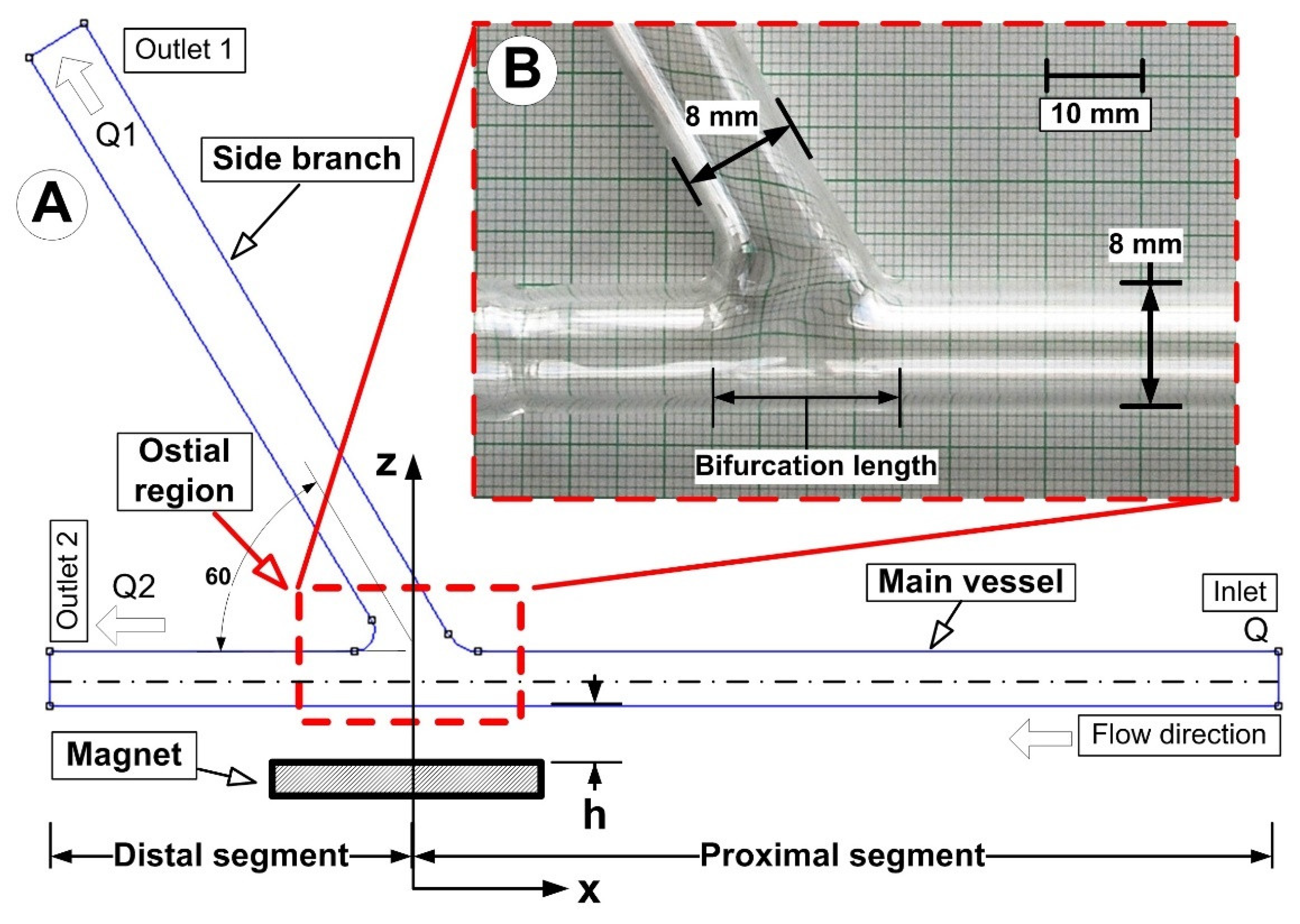
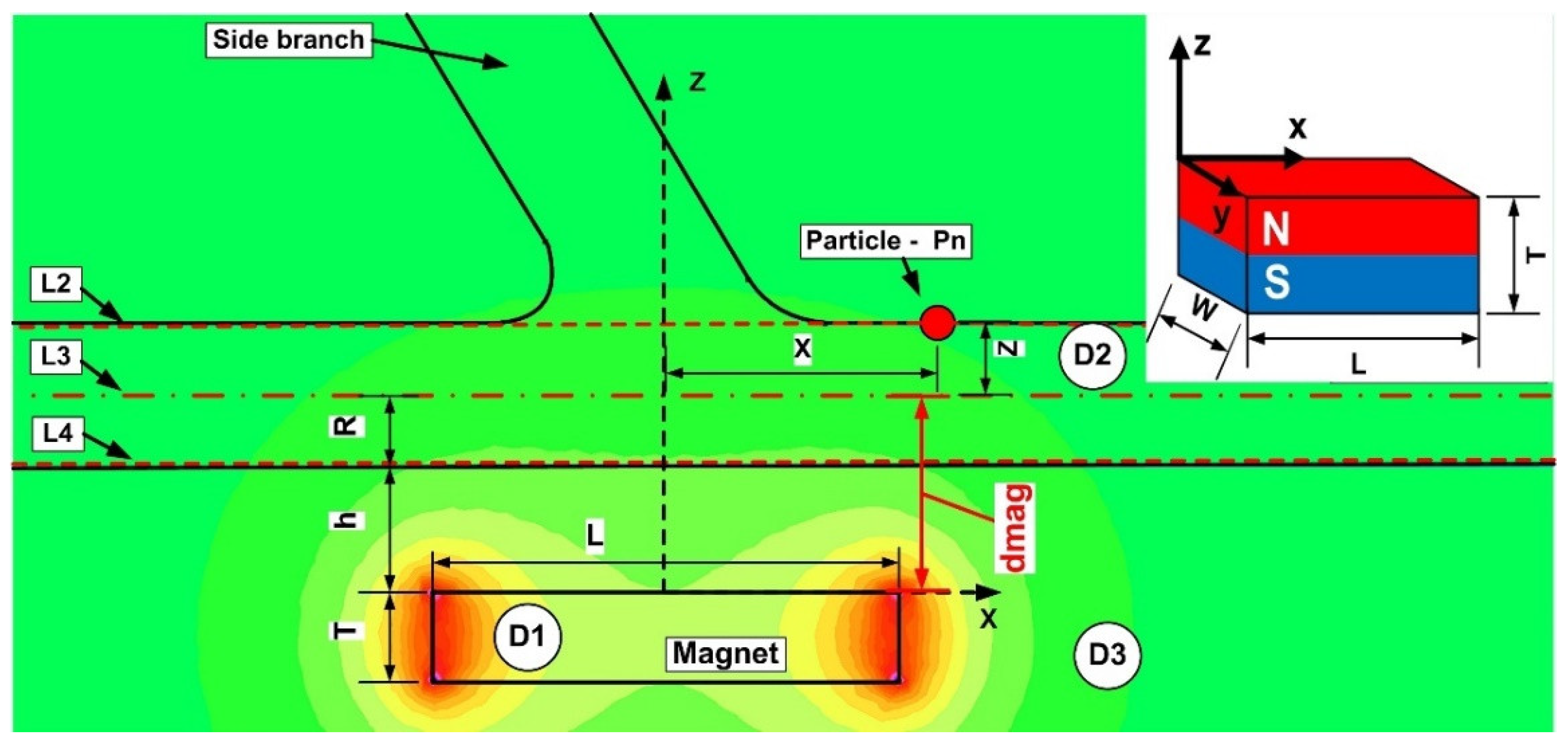
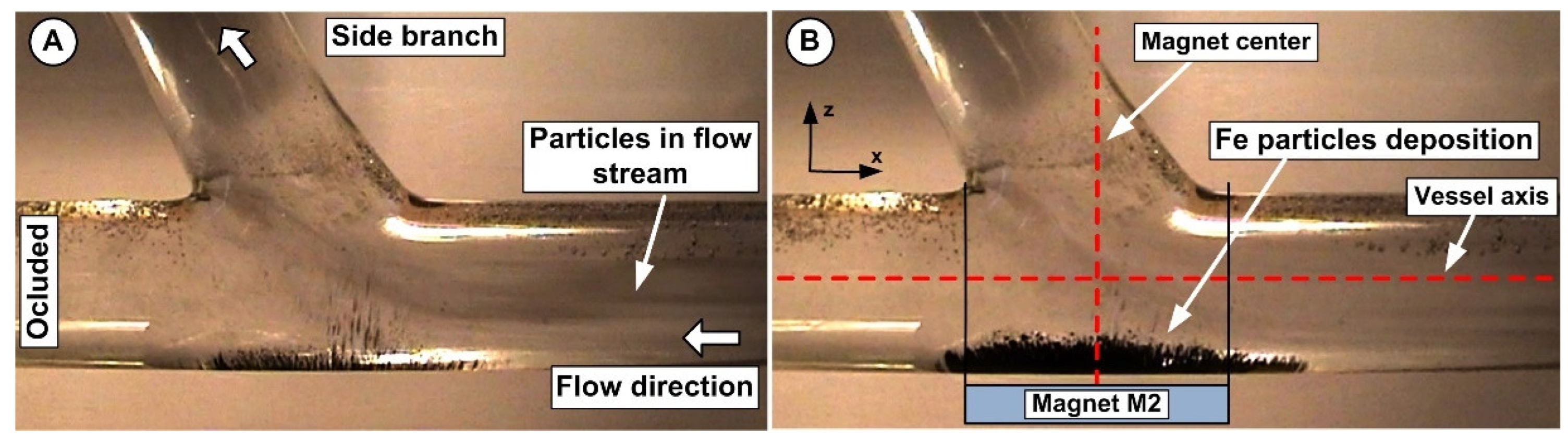
3.1. Problem Description
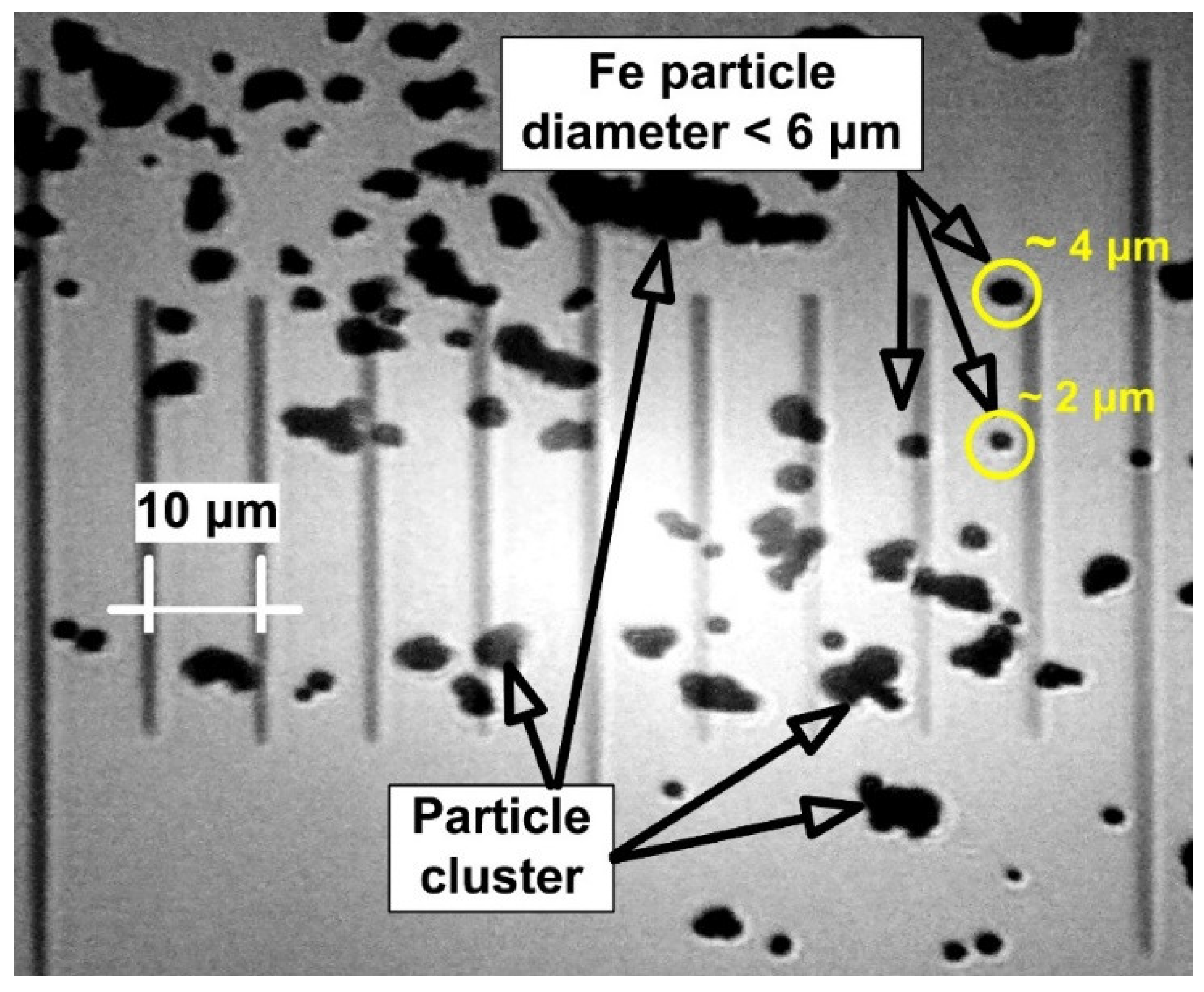
3.2. Magnetic Particle
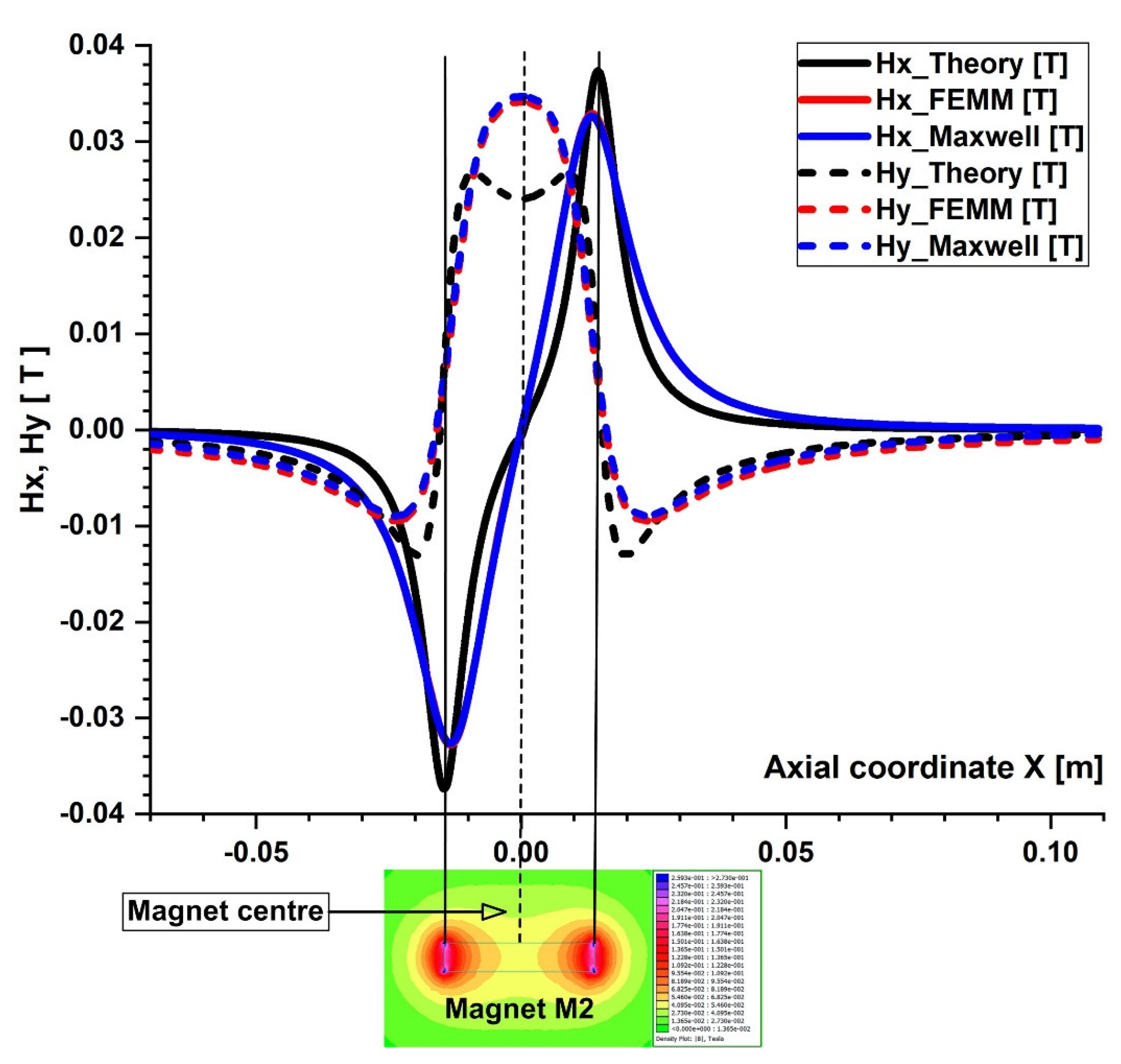
3.3. B-Field Experimental Measurement
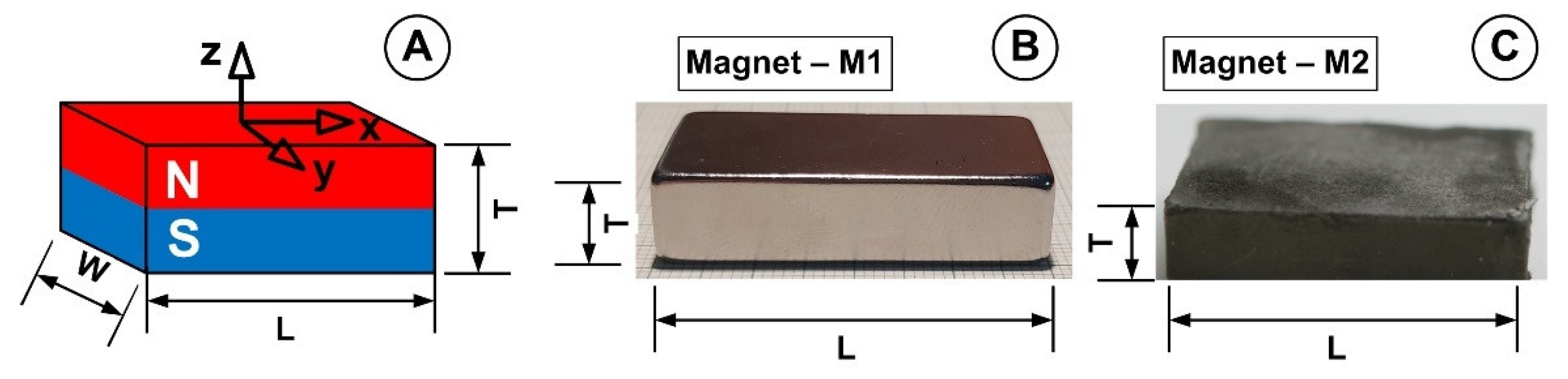
3.4. Magnets and Magnetic Field Generation
- (1)
- To have the certainty of the correctness of the numerical simulation results obtained (by comparing the results generated by the two programs);
- (2)
- To compare the accuracy of the numerical simulation solutions with the analytical and experimental results, respectively;
- (3)
- To identify the effectiveness (in terms of the computation time and computations cost) of the two numerical simulation programs for solving problems related to the properties of the magnetic field associated with different objects or magnetic equipment (in our case, two different permanent magnets).
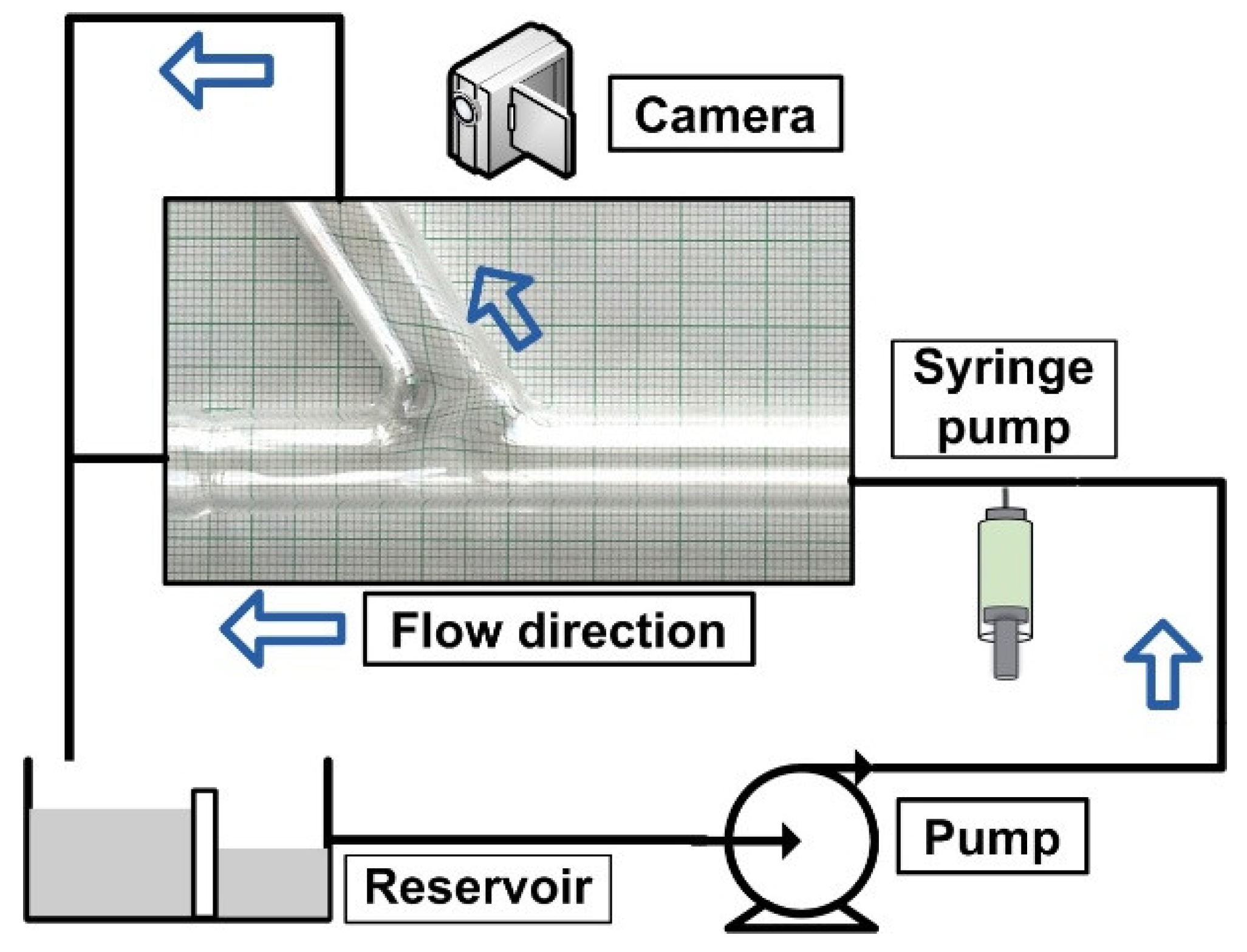
3.5. Experimental Test Rig
3.6. The Carrier Fluid
4. Results
- (1)
- Calculating the permanent magnets’ magnetic fields.
- (2)
- Calculating the magnetic force of a magnetic particle.
- (3)
- An experimental investigation of the particle deposition for both mentioned magnets.
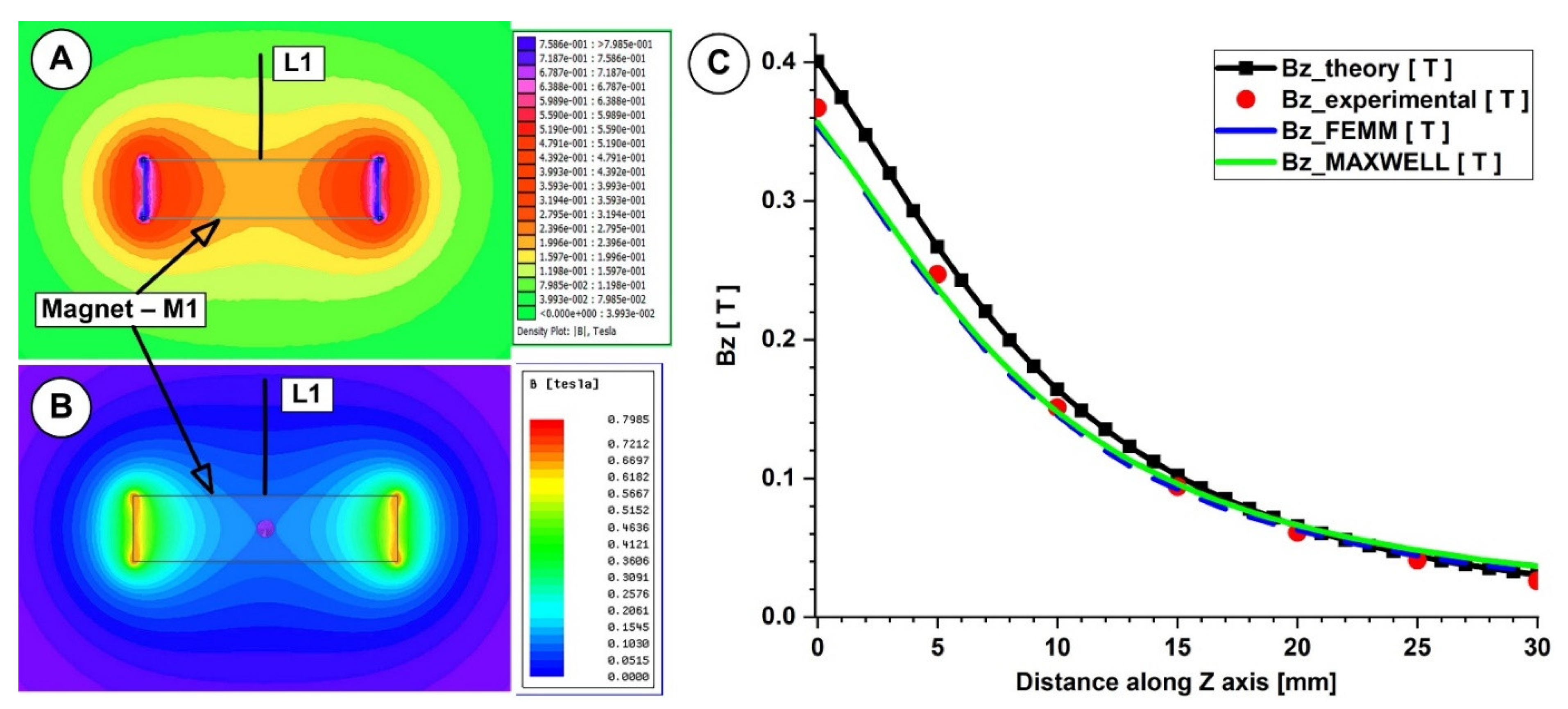
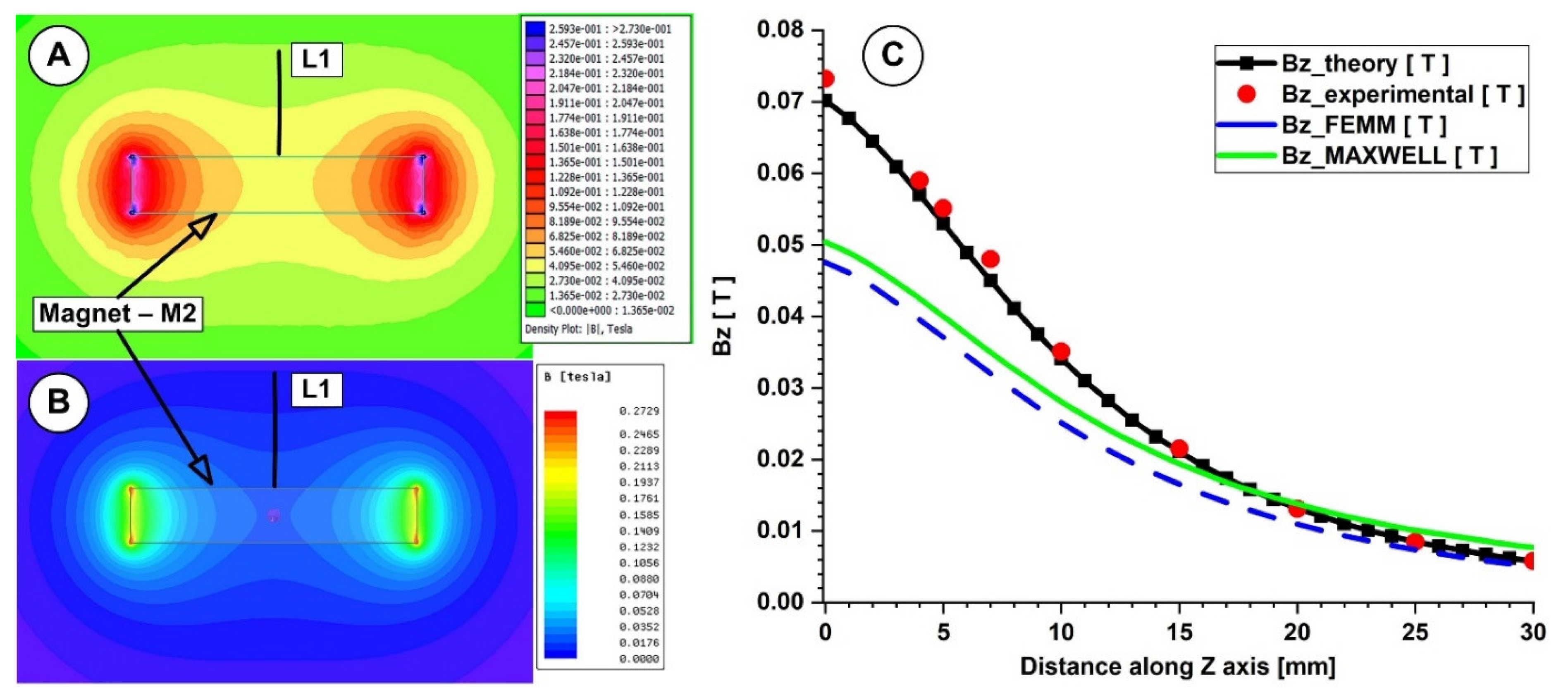
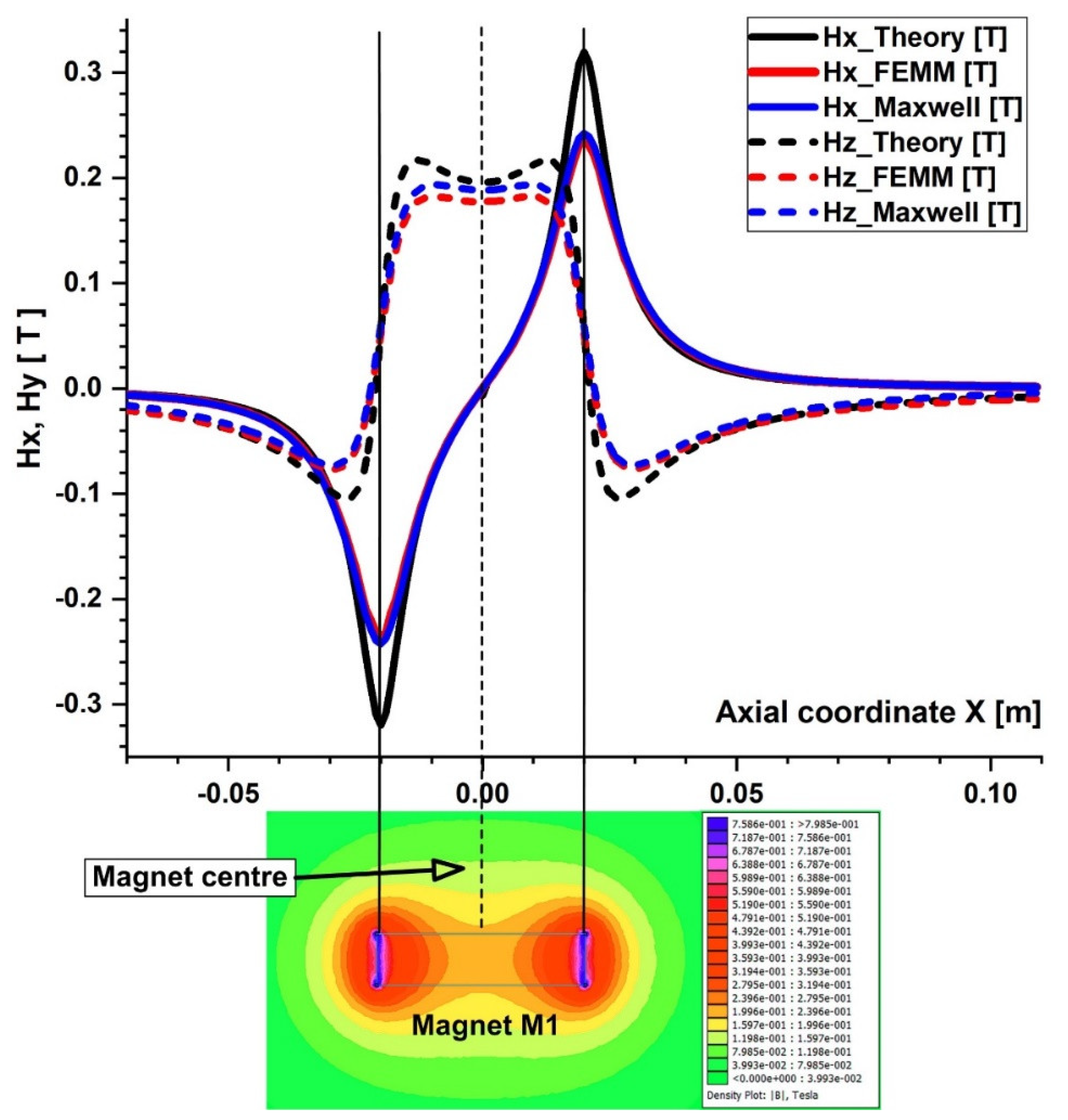
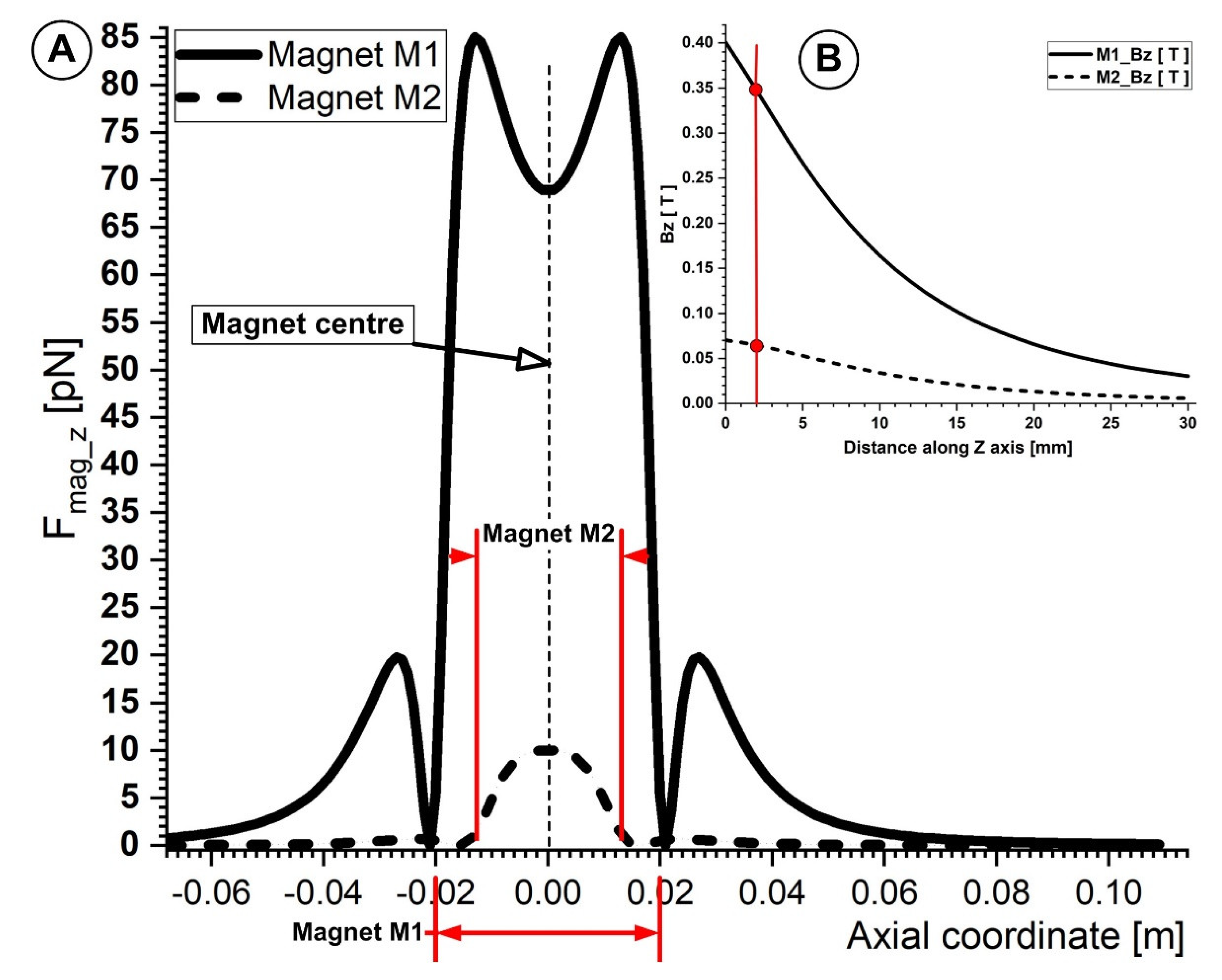
4.1. Calculation of the Permanent Magnets’ Magnetic Fields
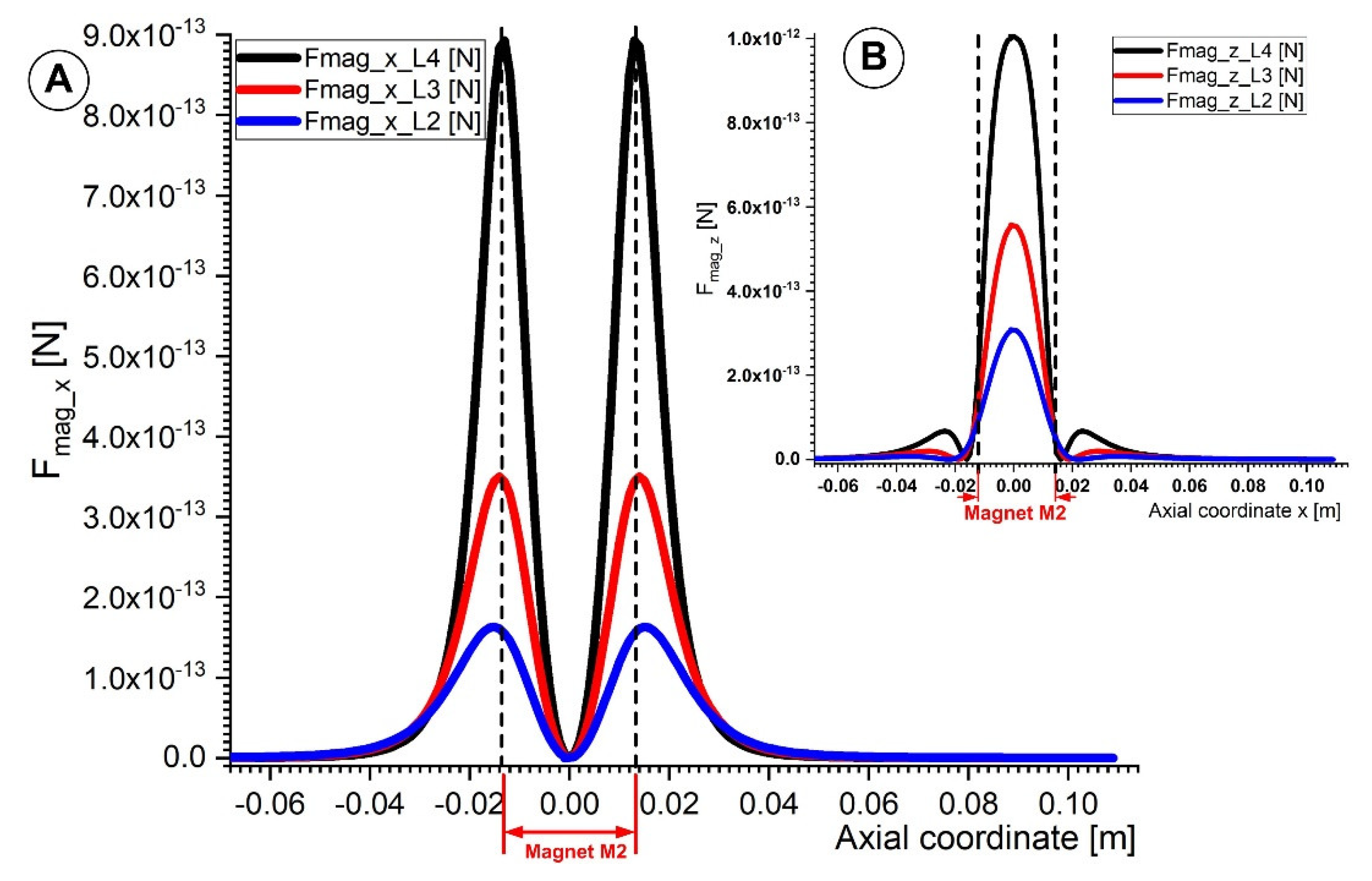
4.2. Calculation of the Magnetic Force Acting on the Micro-Sized Magnetoresposive Particle
5. Discussion
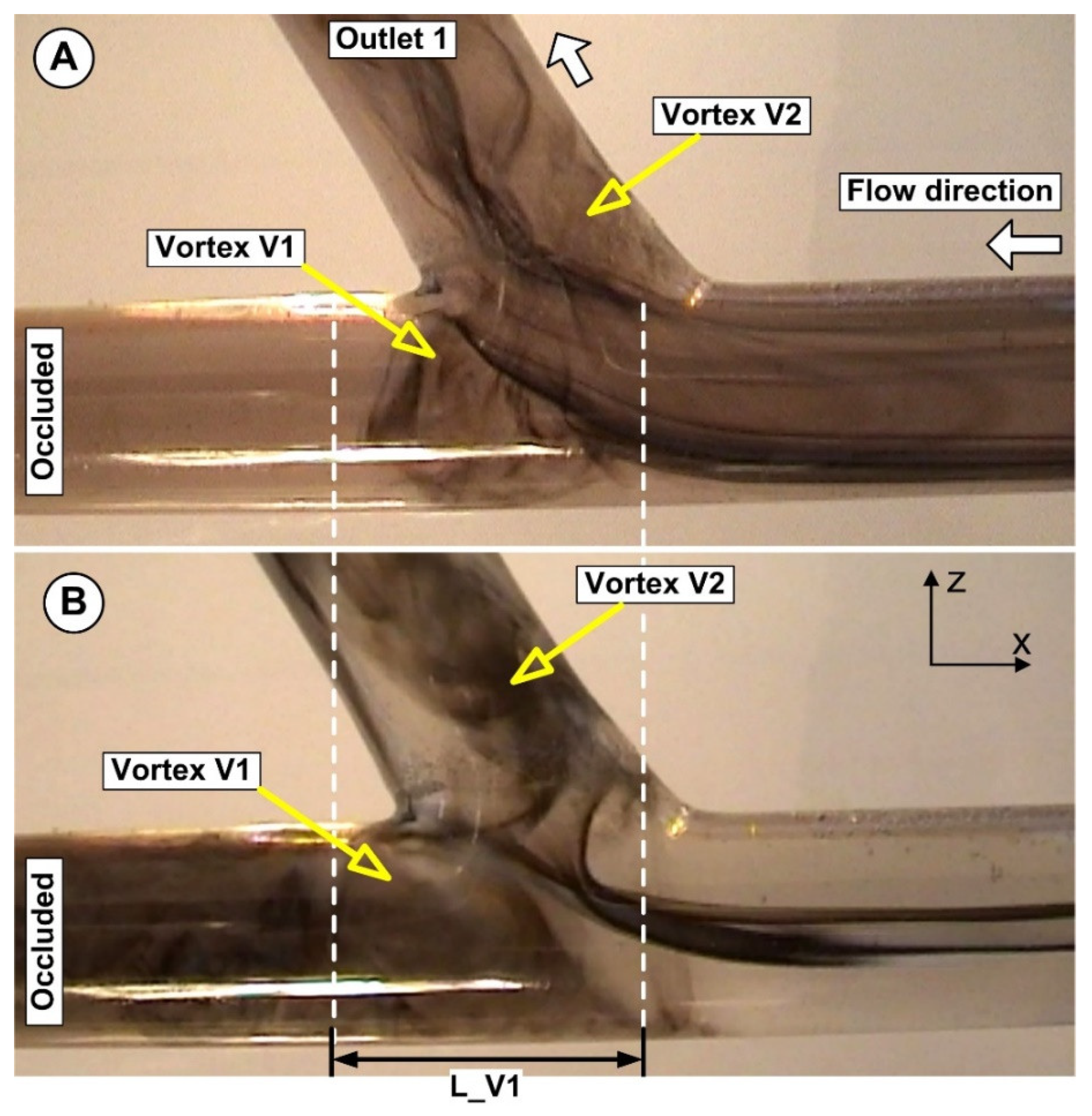
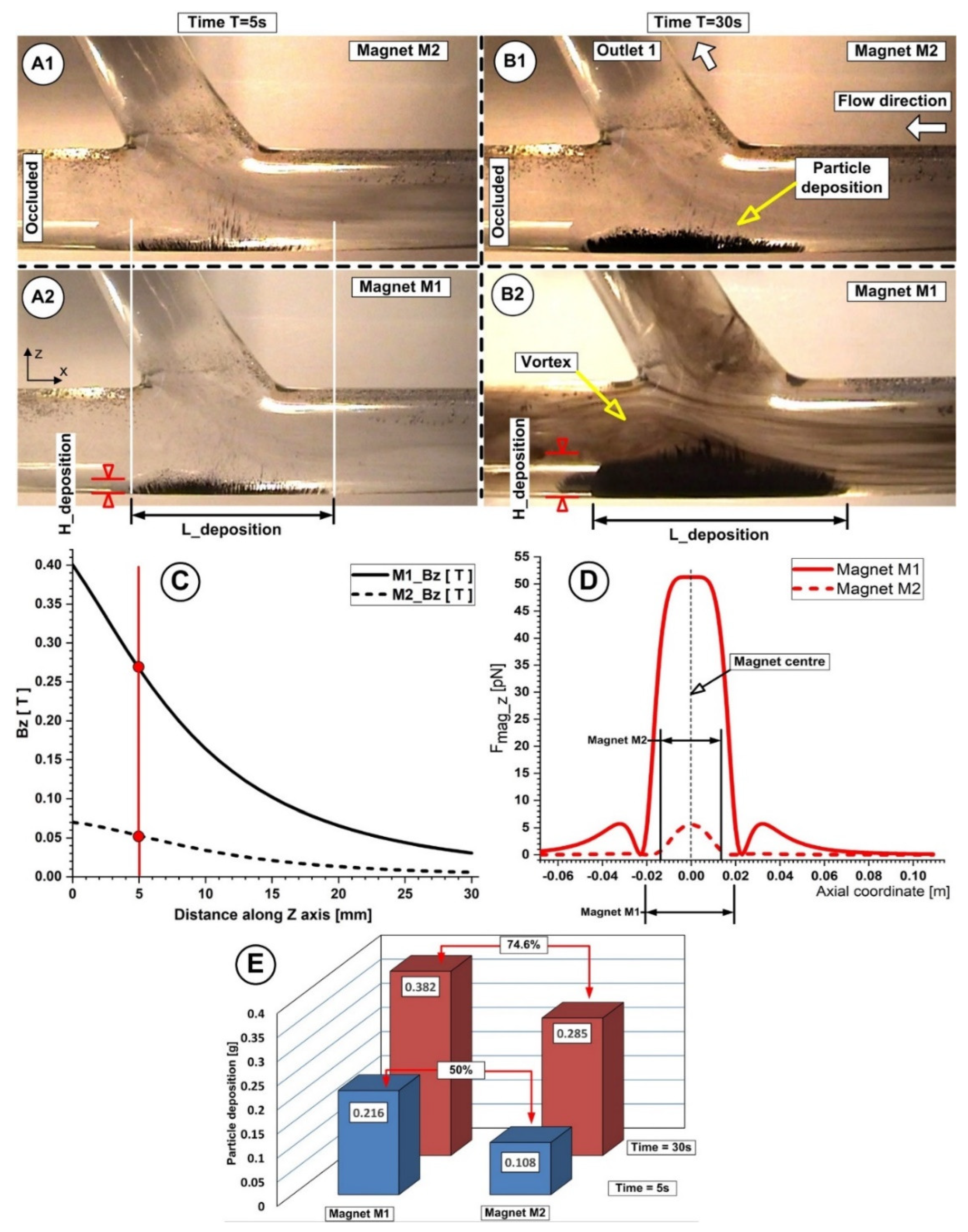
5.1. The Effect of Flow Rate on Particle Accumulation Evolution
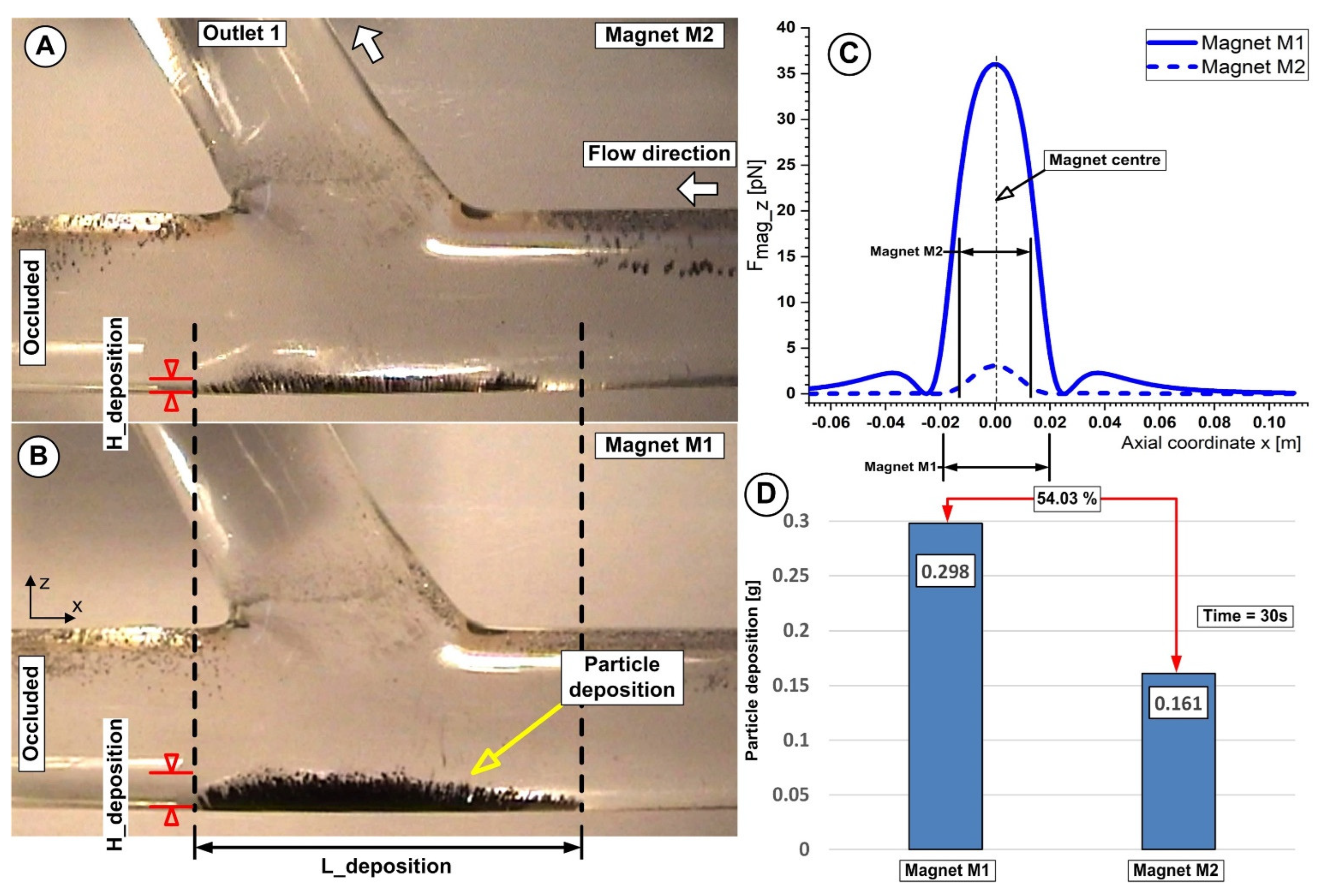
5.2. The Effect of Magnet Type on Particle Accumulation
5.3. Correlation between Magnet Distance and Particle Deposition
6. Conclusions
- (1)
- The deposition on the vessel wall is greatly influenced by the intensity of the magnetic field, the magnet type, the magnet size, and the magnetic characteristics of the ferromagnetic particles.
- (2)
- How well particles may be targeted depends on how the magnetic and drag forces are balanced. This is because the magnetic and the drag forces are proportional to the particle’s size in terms of its cube.
- (3)
- The results from the CFD models are qualitatively comparable with the measured magnetic field induction, magnetic field strength, and their fluctuation with the distance from the magnet surface.
- (1)
- The used magnets are clearly defined and, more importantly, investigated from the magnetic field point of view.
- (2)
- As described in Chapter 3.6, we used a glycerol-water solution during our experimental investigation to ensure that the rheological behavior of blood was replicated. There is a fundamental difference, to quantify the magnetic particle deposition in the in vitro test section, which tries to mimic the real working environment.
- (3)
- Our investigation consists of a comparison between two different software and analytical solutions, respectively, which is a novelty in terms of results validation.
- (4)
- In our research, we used ordinary commercially available magnets rather than special magnets or a magnet configuration designed for specific applications. Using ordinary magnets, we highlighted the possibility of creating an efficient magnetic system for an optimized drug-targeting technique.
7. Limitation
- (1)
- The use of relatively large Fe particles (4–6 μm).
- (2)
- The use of two different magnets (one neodymium and one ferrite) without being able to quantify the impact of each magnet’s geometry variation and level of magnetization on the effectiveness of capturing magnetizable particles.
- (3)
- The large diameter of the test section (8 mm tubes).
- (4)
- The vessel wall rugosity.
- (5)
- The use of a single-flow regime.
- (6)
- A single value for the artery bifurcation angle.
8. Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Voltairas, P.A.; Fotiadis, D.I.; Michalis, L.K. Hydrodynamics of magnetic drug targeting. J. Biomech. 2002, 35, 813–821. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Földesi, I.; Szabó, Á.; Iván, B.; Tombácz, E. PEGylation of Superparamagnetic Iron Oxide Nanoparticles with Self-Organizing Polyacrylate-PEG Brushes for Contrast Enhancement in MRI Diagnosis. Nanomaterials 2018, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.D.; Xu, W.; Wang, S.G.; Ke, Z.J. Hydrodynamic modeling of ferrofluid flow in magnetic targeting drug delivery. Appl. Math. Mech.-Engl. Ed. 2008, 29, 1341–1349. [Google Scholar] [CrossRef]
- Gleich, B.; Hellwig, N.; Bridell, H.; Jurgons, R.; Seliger, C.; Alexiou, C.; Wolf, B.; Weyh, T. Design and evaluation of magnetic fields for nanoparticle drug targeting in cancer. IEEE Trans. Nanotechnol. 2007, 6, 164–170. [Google Scholar] [CrossRef]
- Bernad, S.I.; Susan-Resiga, D.; Bernad, E. Hemodynamic Effects on Particle Targeting in the Arterial Bifurcation for Different Magnet Positions. Molecules 2019, 24, 2509. [Google Scholar] [CrossRef]
- Bernad, S.I.; Susan-Resiga, D.; Vekas, L.; Bernad, E.S. Drug targeting investigation in the critical region of the arterial bypass graft. J. Magn. Magn. Mater. 2019, 475, 14–23. [Google Scholar] [CrossRef]
- Uthamaraj, S.; Tefft, B.J.; Klabusay, M.; Hlinomaz, O.; Sandhu, G.; Dragomir-Daescu, D. Design and validation of a novel ferromagnetic bare metal stent capable of capturing and retaining endothelial cells. Ann. Biomed. Eng. 2014, 24, 2416. [Google Scholar] [CrossRef]
- Tefft, B.J.; Uthamaraj, S.; Harbuzariu, A.; Harburn, J.J.; Witt, T.A.; Newman, B.; Psaltis, P.J.; Hlinomaz, O.; Holmes, D.R., Jr.; Gulati, R.; et al. Nanoparticle-Mediated Cell Capture Enables Rapid Endothelialization of a Novel Bare Metal Stent. Tissue Eng Part A. 2018, 24, 1157–1166. [Google Scholar] [CrossRef]
- Garello, F.; Svenskaya, Y.; Parakhonskiy, B.; Filippi, M. Micro/Nanosystems for Magnetic Targeted Delivery of Bioagents. Pharmaceutics 2022, 14, 1132. [Google Scholar] [CrossRef]
- George, T.A.; Hsu, C.-C.; Meeson, A.; Lundy, D.J. Nanocarrier-Based Targeted Therapies for Myocardial Infarction. Pharmaceutics 2022, 14, 930. [Google Scholar] [CrossRef]
- Tombácz, E.; Turcu, R.; Socoliuc, V.; Vékás, L. Magnetic iron oxide nanoparticles: Recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem. Biophys. Res. Commun. 2015, 468, 442–453. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Kupcsik, E.; Tóth, I.Y.; Farkas, K.; Jedlovszky-Hajdú, A.; Tombácz, E. PEGylation of surfacted magnetite core-shell nanoparticles for biomedical application. Colloids Surf. A 2014, 460, 429–440. [Google Scholar] [CrossRef]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef]
- Salvador, M.; Marqués-Fernández, J.L.; Martínez-García, J.C.; Fiorani, D.; Arosio, P.; Avolio, M.; Brero, F.; Balanean, F.; Guerrini, A.; Sangregorio, C.; et al. Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy. Nanomaterials 2022, 12, 205. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Lamura, G.; Peddis, D.; Canepa, F. Optimization of a NdFeB permanent magnet configuration for in-vivo drug delivery experiments. J. Magn. Magn. Mater. 2021, 522, 167491. [Google Scholar] [CrossRef]
- Tharayil, J.J.; Goetz, S.M.; Bernabei, J.M.; Peterchev, A.V. Field Distribution of Transcranial Static Magnetic Stimulation in Realistic Human Head Model. Neuromodulation Technol. Neural Interface 2018, 21, 340–347. [Google Scholar] [CrossRef]
- Qiu, Y.; Tong, S.; Zhang, L.; Sakurai, Y.; Myers, D.R.; Hong, L.; Lam, W.A.; Bao, G. Magnetic forces enable controlled drug delivery by disrupting endothelial cell-cell junctions. Nat. Commun. 2017, 8, 15594. [Google Scholar] [CrossRef]
- Barnsley, L.C.; Carugo, D.; Stride, E. Optimized shapes of magnetic arrays for drug targeting applications. J. Phys. D Appl. Phys. 2016, 49, 225501. [Google Scholar] [CrossRef]
- Barnsley, L.C.; Carugo, D.; Aron, M.; Stride, E. Understanding the dynamics of superparamagnetic particles under the influence of high field gradient arrays. Phys Med Biol. 2017, 62, 2333–2360. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Sun, W.-F. Magnetic Force Probe Characterizations of Nanoscaled Ferromagnetic Domains: Finite-Element Magnetostatic Simulations. Nanomaterials 2022, 12, 2212. [Google Scholar] [CrossRef]
- Descamps, L.; Le Roy, D.; Tomba, C.; Deman, A.-l. Magnetic Polymers for Magnetophoretic Separation in Microfluidic Devices. Magnetochemistry 2021, 7, 100. [Google Scholar] [CrossRef]
- Boutopoulos, I.D.; Lampropoulos, D.S.; Bourantas, G.C.; Miller, K.; Loukopoulos, V.C. Two-Phase Biofluid Flow Model for Magnetic Drug Targeting. Symmetry 2020, 12, 1083. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Z.; Wang, Z.; Han, X. Rotational motion and lateral migration of an elliptical magnetic particle in a microchannel under a uniform magnetic field. Microfluid. Nanofluidics 2018, 22, 3. [Google Scholar] [CrossRef]
- Cao, Q.; Fan, Q.; Chen, Q.; Liu, C.; Han, X.; Li, L. Recent Advances in Manipulation of Micro- and Nano-objects with Magnetic Fields at Small Scales. Mater. Horiz. 2019, 7, 638–666. [Google Scholar] [CrossRef]
- Cao, Q.; Han, X.; Li, L. Configurations and control of magnetic fields for manipulating magnetic particles in microfluidic applications: Magnet systems and manipulation mechanisms. Lab Chip 2014, 14, 2762–2777. [Google Scholar] [CrossRef]
- Panina, L.V.; Gurevich, A.; Beklemisheva, A.; Omelyanchik, A.; Levada, K.; Rodionova, V. Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces. Cells 2022, 11, 950. [Google Scholar] [CrossRef]
- Cherry, E.M.; Maxim, P.G.; Eaton, J.K. Particle size, magnetic field, and blood velocity effects on particle retention in magnetic drug targeting. Med. Phys. 2010, 37, 175–182. [Google Scholar] [CrossRef]
- Schenck, J.F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2005, 87, 185–204. [Google Scholar] [CrossRef]
- Grief, A.D.; Richardson, G. Mathematical modelling of magnetically targeted drug delivery. J. Magn. Magn. Mater. 2005, 293, 455–463. [Google Scholar] [CrossRef]
- Liu, J.F.; Lan, Z.; Ferrari, C.; Stein, J.M.; Higbee-Dempsey, E.; Yan, L.; Amirshaghaghi, A.; Cheng, Z.; Issadore, D.; Tsourkas, A. Use of Oppositely Polarized External Magnets to Improve the Accumulation and Penetration of Magnetic Nanocarriers into Solid Tumors. ACS Nano. 2020, 14, 142–152. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Bai, J.; Wang, J.T.W.; Protti, A.; Southern, P.; Bogart, L.; Heidari, H.; Li, X.; Cakebread, A.; Asker, D.; et al. Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling, and Extrapolation to Humans. Nano Lett. 2016, 16, 5652–5660. [Google Scholar] [CrossRef]
- Mahmoodpour, M.; Goharkhah, M.; Ashjaee, M. Investigation on trajectories and capture of magnetic drug carrier nanoparticles after injection into a direct vessel. J. Magn. Magn. Mater. 2019, 497, 166065. [Google Scholar] [CrossRef]
- Manshadi, M.K.D.; Saadat, M.; Mohammadi, M.; Shamsi, M.; Dejam, M.; Kamali, R.; Sanati-Nezhad, A. Delivery of magnetic micro/nanoparticles and magnetic-based drug/cargo into arterial flow for targeted therapy. Drug Deliv. 2018, 25, 1963–1973. [Google Scholar] [CrossRef]
- Lunnoo, T.; Puangmali, T. Capture Efficiency of Biocompatible Magnetic Nanoparticles in Arterial Flow: A Computer Simulation for Magnetic Drug Targeting. Nanoscale Res. Lett. 2015, 10, 426. [Google Scholar] [CrossRef]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Pislaru, S.V.; Harbuzariu, A.; Agarwal, G.; Witt, T.; Gulati, R.; Sandhu, N.P.; Mueske, C.; Kalra, M.; Simari, R.D.; Sandhu, G.S. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation 2006, 114, I-314–I-318. [Google Scholar] [CrossRef]
- Camacho, J.M.; Sosa, V. Alternative method to calculate the magnetic field of permanent magnets with azimuthal symmetry. Rev. Mex. Fis. 2013, 59, 8–17. [Google Scholar]
- Bernad, S.I.; Crăciunescu, I.; Sandhu, G.S.; Dragomir-Daescu, D.; Tombácz, E.; Vékás, L.; Turcu, R. Fluid targeted delivery of functionalized magnetoresponsive nanocomposite particles to a ferromagnetic stent. J. Magn. Magn. Mater. 2021, 519, 167489. [Google Scholar] [CrossRef]
- Sharma, S.; Katiyar, V.K.; Singh, U. Mathematical modelling for trajectories of magnetic nanoparticles in a blood vessel under magnetic field. J. Magn. Magn. Mater. 2015, 379, 102–107. [Google Scholar] [CrossRef]
- Li, A.; Ahmadi, G. Dispersion and deposition of spherical particles from point sources in a turbulent channel flow. Aerosol Sci. Technol. 1992, 16, 209–226. [Google Scholar] [CrossRef]
- Khashan, S.; Furlani, E. Effects of particle-fluid coupling on particle transport and capture in a magnetophoretic microsystem. Microfluid. Nanofluidics 2012, 12, 565–580. [Google Scholar] [CrossRef]
- Li, X.L.; Yao, K.L.; Liu, Z.L. CFD study on the magnetic fluid delivering in the vessel in high-gradient magnetic field. J. Magn. Magn. Mater. 2008, 320, 1753. [Google Scholar] [CrossRef]
- Kayal, S.; Bandyopadhyay, D.; Mandal, T.K.; Ramanujan, R.V. The flow of magnetic nanoparticles in magnetic drug targeting. RSC Adv. 2011, 1, 238–246. [Google Scholar] [CrossRef]
- Lubbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dorken, B.; Herrmann, F.; Gurtler, R.; et al. Clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar]
- Goodwin, S.; Peterson, C.; Hoh, C.; Bittner, C. Targeting and retention of magnetic targeted carriers (MTCs) enhancing intra-arterial chemotherapy. J. Magn. Magn. Mater. 1999, 194, 132–139. [Google Scholar] [CrossRef]
- Ku, D.N. Blood Flow in Arteries. Annu. Rev. Fluid Mech. 1997, 29, 399–434. [Google Scholar] [CrossRef]

| Characteristics | Value |
|---|---|
| particle diameter | 4–6 μm |
| density | 7.86 g/cm3 |
| molar mass | 55.8 g/mol |
| Saturation Magnetization | Saturation Field | Coercive Field | Remanent Magnetization |
|---|---|---|---|
| Ms (A·m2/kg): 177 | Hs (kA/m): 600 | Hc (kA/m): 1.32 | Mr (A·m2/kg): 0.891 |
| Magnet | Shape | Material | Length—L (mm) | Width—W (mm) | Thickness—T (mm) |
|---|---|---|---|---|---|
| M1 | Rectangular | Neodymium—NdFeB | 40 | 20 | 10 |
| M2 | Rectangular | Ferrite | 26 | 29 | 5 |
| Magnet | Shape | Magnetization Direction | Material | Grade | Br (T) | Hcb (kA/m) | Hcj (kA/m) | BHmax (kJ/m3) |
|---|---|---|---|---|---|---|---|---|
| M1 | block | thickness | Neodymium | N52 | 1.42–1.47 | 860–995 | ≥955 | 380–422 |
| M2 | block | thickness | Ferrite | Y35 | 0.43–0.45 | 215–239 | 217–241 | 33.1–38.3 |
| Z-Position (mm) | Bz_Equation (T) | Bz_exp (T) | Bz_FEMM (T) | Bz_Maxwell (T) | (%) Diff. Equation—Exp | (%) Diff. Equation—FEMM | (%) Diff. Equation—Maxwell |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.4090 | 0.367 | 0.3532 | 0.3566 | 10.27 | 13.65 | 12.81 |
| 5.00 | 0.2727 | 0.247 | 0.2343 | 0.2371 | 9.42 | 14.08 | 13.05 |
| 10.00 | 0.1675 | 0.151 | 0.1456 | 0.148 | 9.85 | 13.09 | 11.64 |
| 15.00 | 0.1041 | 0.094 | 0.0923 | 0.0961 | 9.70 | 11.31 | 7.68 |
| 20.00 | 0.0671 | 0.061 | 0.0628 | 0.0663 | 9.09 | 6.43 | 1.19 |
| 25.00 | 0.0450 | 0.041 | 0.0443 | 0.0482 | 8.89 | 1.54 | 7.11 |
| 30.00 | 0.0312 | 0.026 | 0.0330 | 0.0367 | 16.67 | 5.80 | 17.63 |
| Z-Position (mm) | Bz_Equation (T) | Bz_Exp (T) | Bz_FEMM (T) | Bz_Maxwell (T) | (%) Diff. Equation—Exp | (%) Diff. Equation—FEMM | (%) Diff. Equation—Maxwell |
|---|---|---|---|---|---|---|---|
| 0.00 | 0.0780 | 0.0732 | 0.0476 | 0.0504 | 6.15 | 39.02 | 35.38 |
| 5.00 | 0.0589 | 0.0551 | 0.0371 | 0.04 | 6.45 | 37.00 | 32.09 |
| 10.00 | 0.0379 | 0.0351 | 0.0251 | 0.0281 | 7.39 | 33.71 | 25.86 |
| 15.00 | 0.0234 | 0.0215 | 0.0165 | 0.0194 | 8.12 | 29.40 | 17.09 |
| 20.00 | 0.0146 | 0.0131 | 0.0110 | 0.0138 | 10.27 | 24.98 | 5.48 |
| 25.00 | 0.0095 | 0.0085 | 0.0074 | 0.0101 | 10.53 | 22.32 | 6.32 |
| 30.00 | 0.0064 | 0.0058 | 0.0050 | 0.0077 | 9.38 | 21.64 | 20.31 |
| Materials | Properties | Value | Unit |
|---|---|---|---|
| Carrier fluid | ρf—fluid density ηf—fluid dynamic viscosity χf—fluid magnetic susceptibility | 1055 0.0036 −6.6 × 10−7 | kg/m3 kg/(m.s) [-] |
| Fe | ρFe—Fe particle density χFe—Fe particle magnetic susceptibility | 7860 4 | kg/m3 [-] |
| Symbol | Description | Default Value | Unit |
|---|---|---|---|
| meff | Mass of the Fe particle (one particle) | 8.88945 × 10−13 | (kg) |
| Magnetic particle total velocity | (m/s) | ||
| Fluid velocity (carrier fluid) | 0.12 | (m/s) | |
| Magnetic particle (Fe particle) velocity due to the magnetic force acting on the particle | (m/s) | ||
| Fluid mean velocity (carrier fluid) | (m/s) | ||
| The volume of magnetic particle | Vp = | (m3) | |
| Magnetic particle radius (Fe particle) | 4 ÷ 6 × 10−6 | (m) | |
| R | Artery (vessel) radius | 8 × 10−3 | (m) |
| z | Magnetic particle (Fe) coordinates along the z direction | (m) | |
| μ0 | The magnetic permeability of air | μ0 = 4π × 10−7 | (N/A2) |
| ρf | Fluid density (carrier fluid) | 1055 | (kg/m3) |
| Fluid flow rate (carrier fluid) | 6.0288 × 10−6 | (m3/s) | |
| g | Gravity acceleration | 9.81 | (m/s−2) |
| Item | Flow Rate Q (ml/min) | Flow Velocity vf (m/s) | Reynolds Number Re (-) | Main Vortex Length L_V1 (mm) | Particle Deposition * (g) | Magnet Type | Magnet Distance h (mm) |
|---|---|---|---|---|---|---|---|
| Q1 | 362 | 0.12 | 281 | 17 | 0.285 ± 0.00616 | M2 | 5 |
| Q2 | 754 | 0.25 | 586 | 22 | 0.214 ± 0.00734 | M2 | 5 |
| Magnet Distance h (mm) | Magnet Type | Magnetic Flux Density Bz (T) | Particle Deposition Quantity mFe (g) | Particle Deposition Length L_Deposition (mm) | Particle Deposition AVERAGE Thickness H_Deposition (mm) |
|---|---|---|---|---|---|
| 2 | M1 | 0.35 | 0.405 ± 0.00714 | 44 | 3 |
| M2 | 0.065 | 0.261 ± 0.00831 | 36 | 1.85 | |
| 5 | M1 | 0.27 | 0.382 ± 0.00817 | 41.5 | 2.2 |
| M2 | 0.051 | 0.285 ± 0.00616 | 34 | 1.11 | |
| 10 | M1 | 0.16 | 0.268 ± 0.00778 | 40 | 1.2 |
| M2 | 0.032 | 0.161 ± 0.00857 | 32 | 0.75 |
| Magnet Distance h (mm) | Magnet Type | Targeting Efficiency TE (%) | Targeting Efficiency Percentage Difference between M1 and M2 |
|---|---|---|---|
| 2 | M1 | 40.5% | 14.4% |
| M2 | 26.1% | ||
| 5 | M1 | 38.2% | 9.7% |
| M2 | 28.5% | ||
| 10 | M1 | 26.8% | 10.7% |
| M2 | 16.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernad, S.I.; Bernad, E. Magnetic Forces by Permanent Magnets to Manipulate Magnetoresponsive Particles in Drug-Targeting Applications. Micromachines 2022, 13, 1818. https://doi.org/10.3390/mi13111818
Bernad SI, Bernad E. Magnetic Forces by Permanent Magnets to Manipulate Magnetoresponsive Particles in Drug-Targeting Applications. Micromachines. 2022; 13(11):1818. https://doi.org/10.3390/mi13111818
Chicago/Turabian StyleBernad, Sandor I., and Elena Bernad. 2022. "Magnetic Forces by Permanent Magnets to Manipulate Magnetoresponsive Particles in Drug-Targeting Applications" Micromachines 13, no. 11: 1818. https://doi.org/10.3390/mi13111818
APA StyleBernad, S. I., & Bernad, E. (2022). Magnetic Forces by Permanent Magnets to Manipulate Magnetoresponsive Particles in Drug-Targeting Applications. Micromachines, 13(11), 1818. https://doi.org/10.3390/mi13111818








