Electrochemical Testing of a New Polyimide Thin Film Electrode for Stimulation, Recording, and Monitoring of Brain Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodes
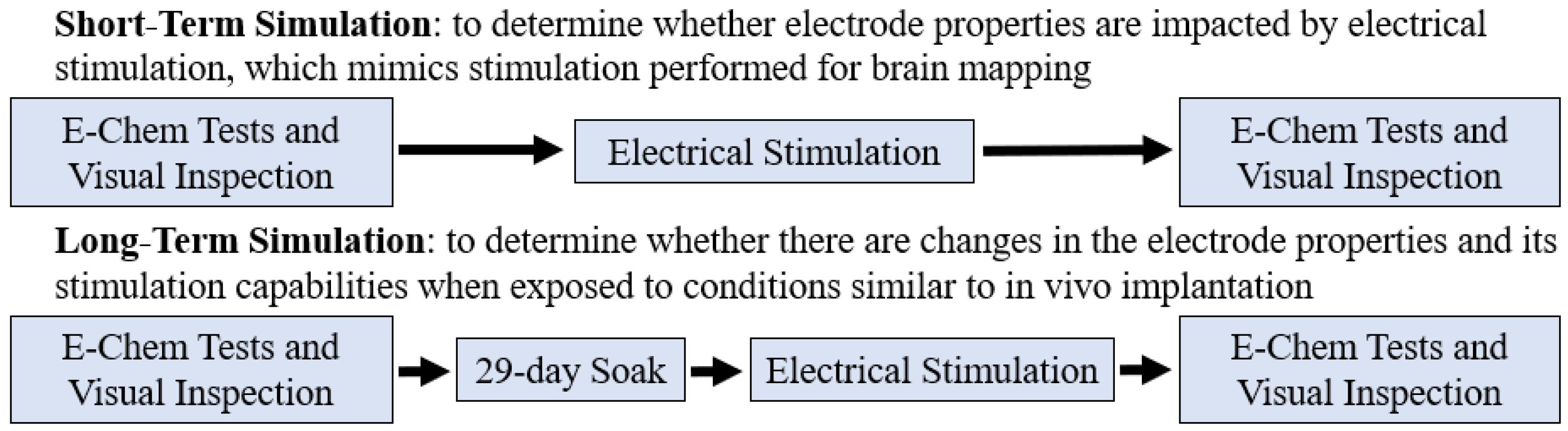
2.2. Testing Paradigms
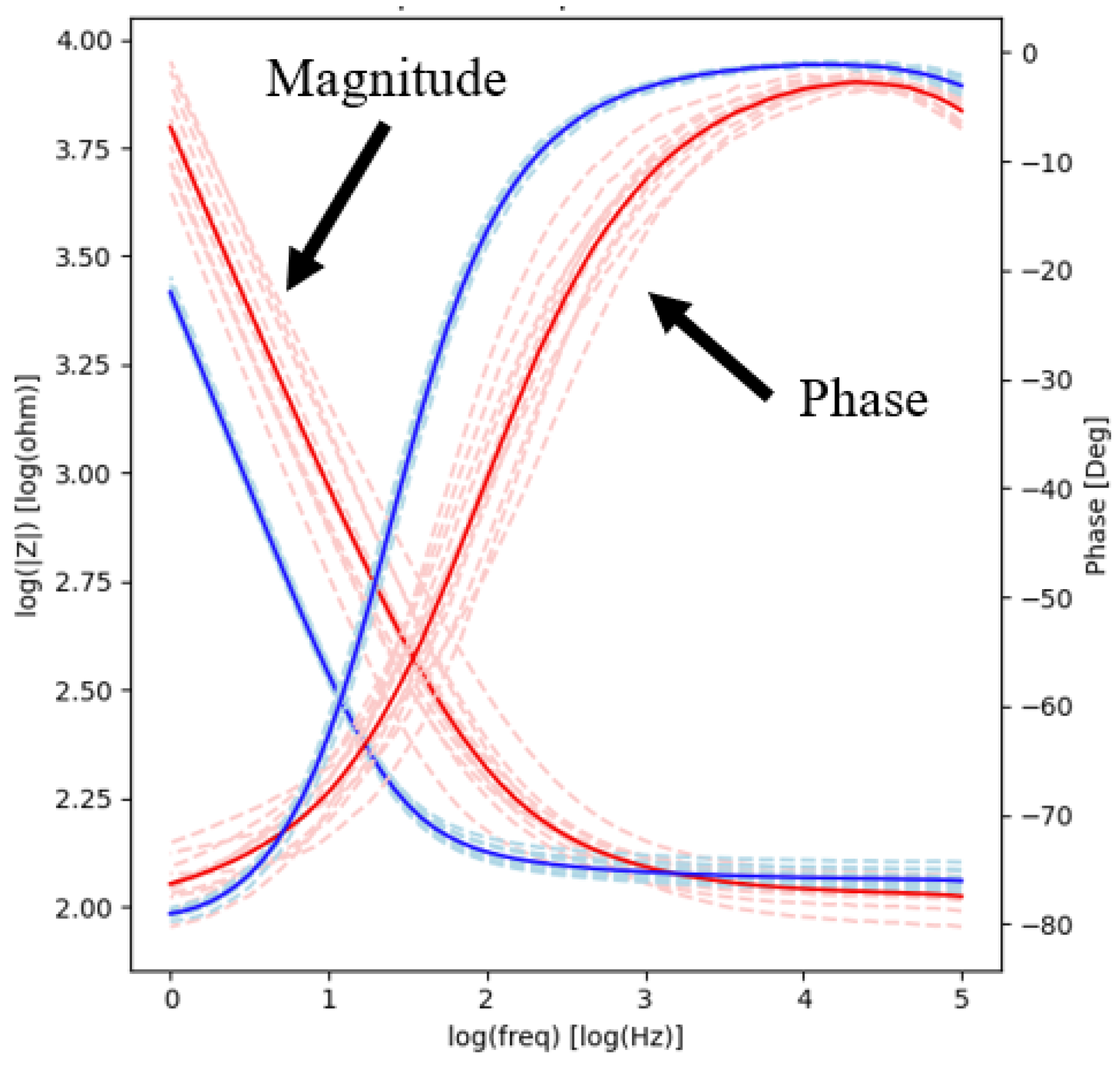
2.3. Electrochemical Impedance Spectroscopy
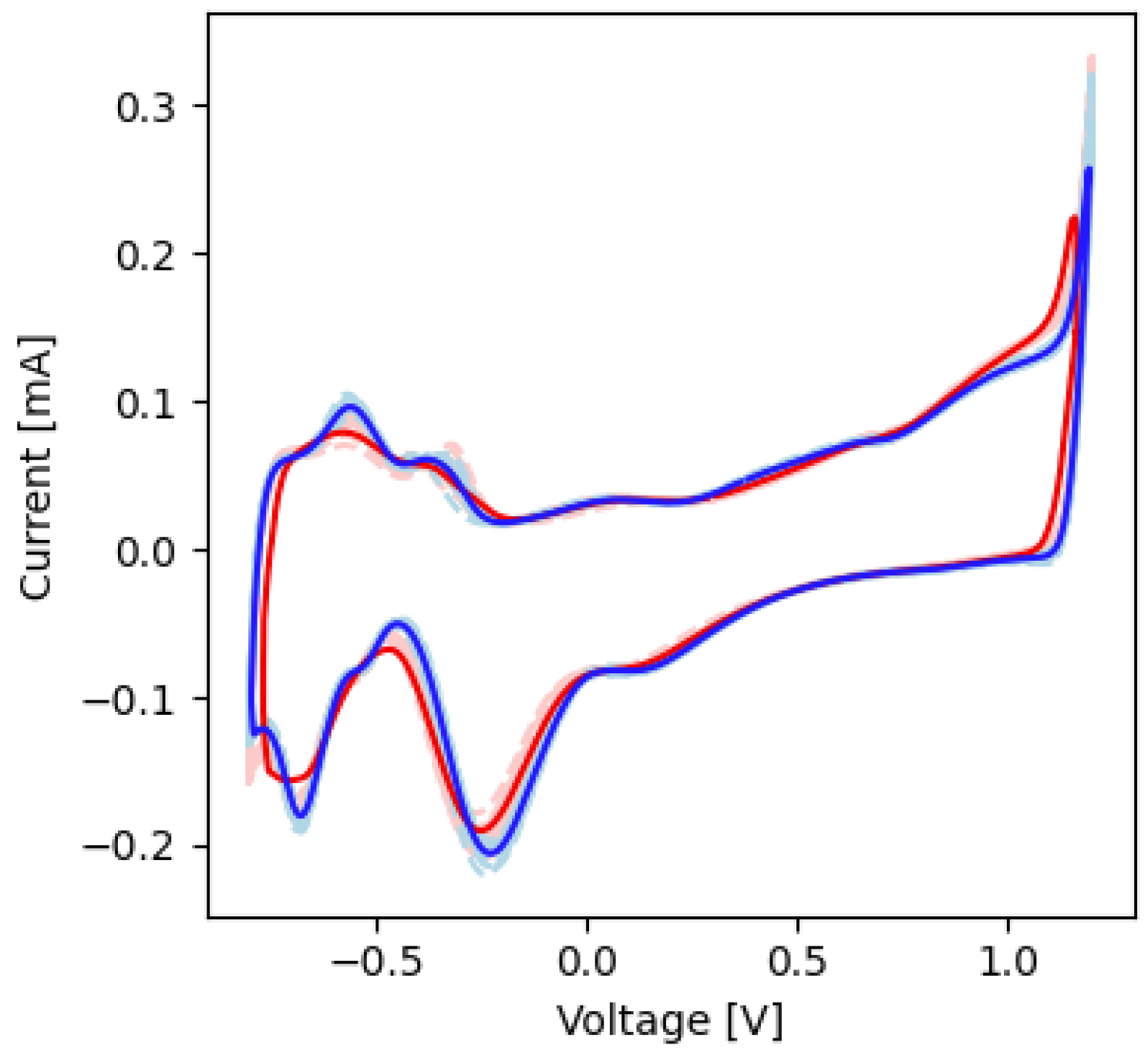
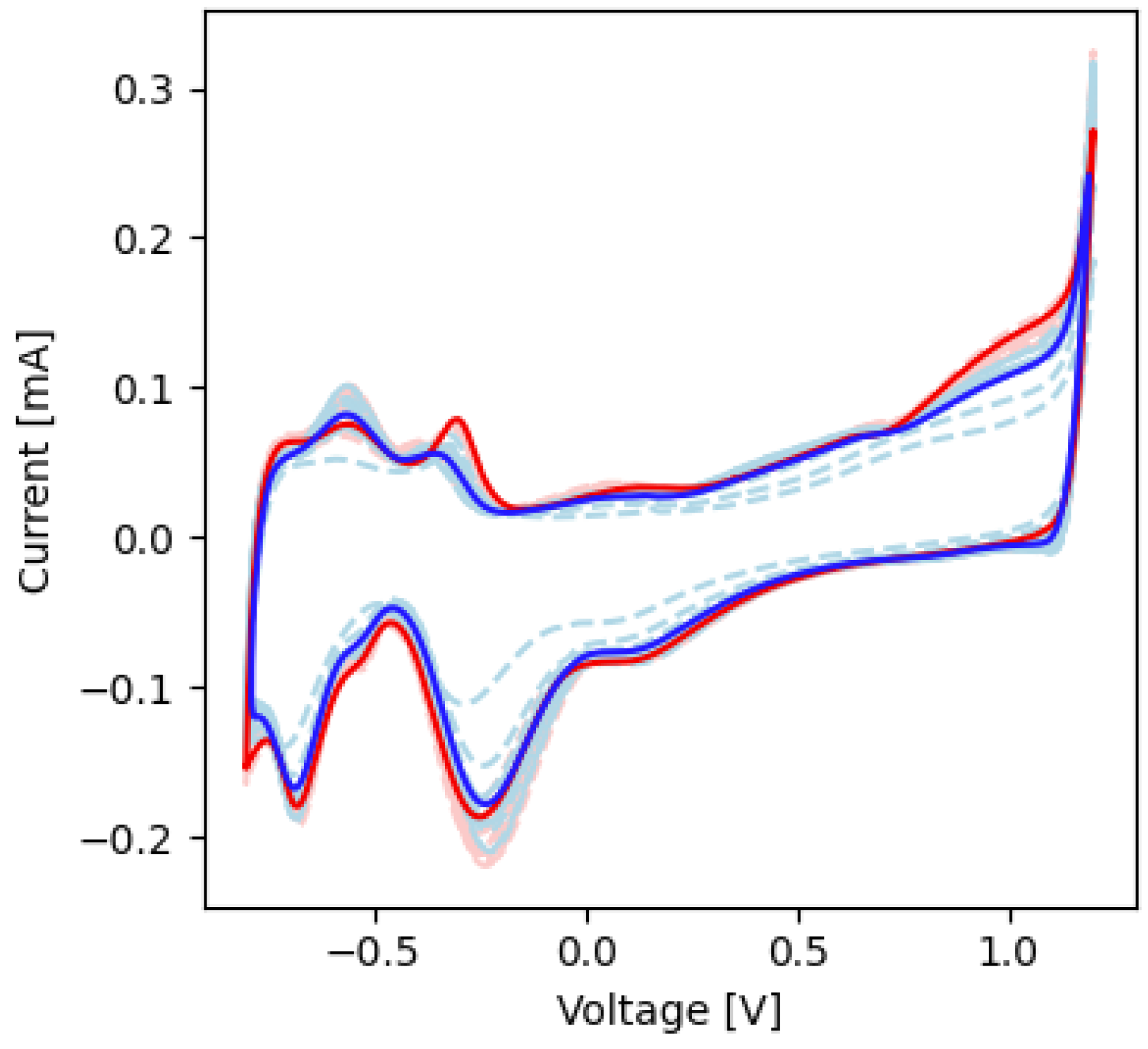
2.4. Cyclic Voltammetry
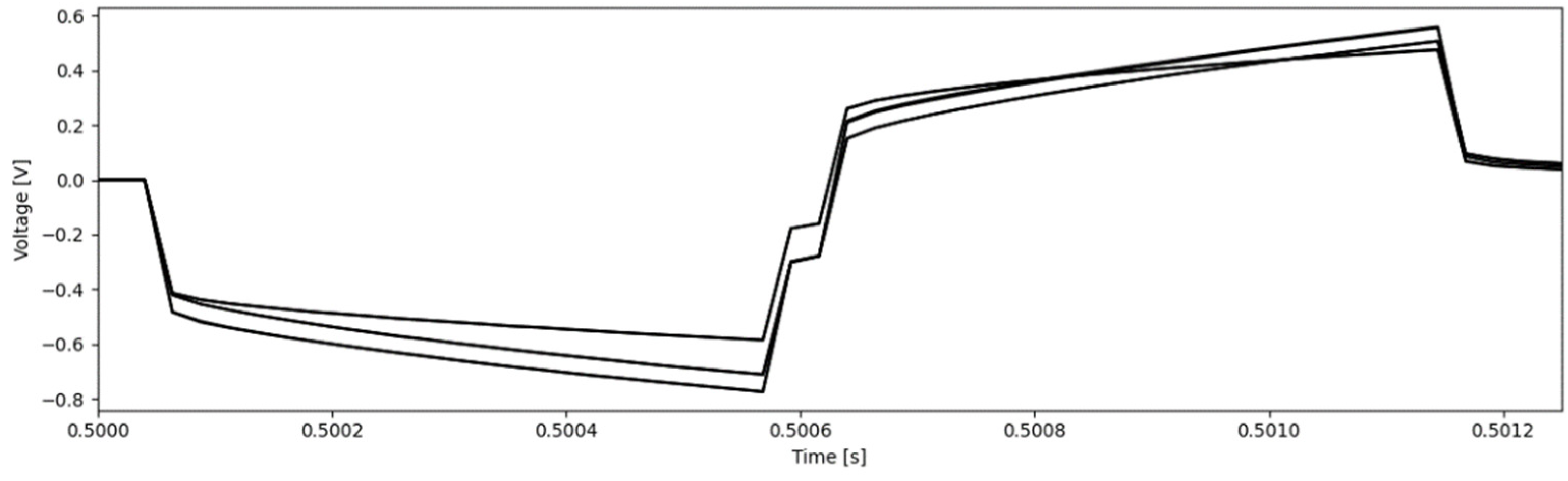
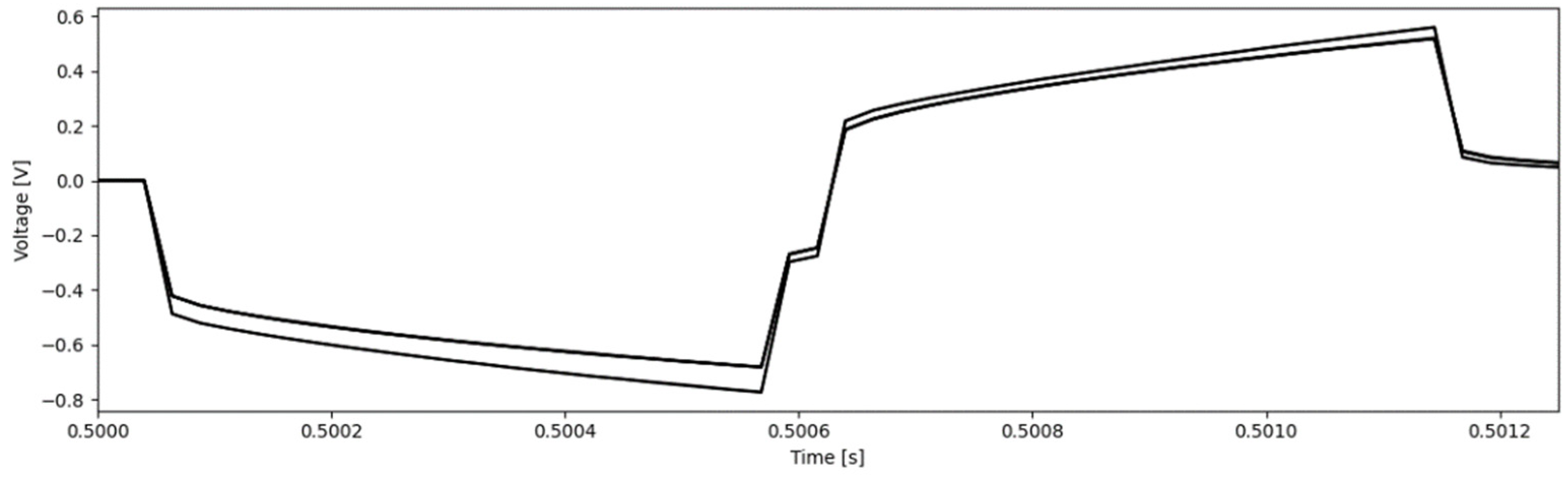
2.5. Voltage Transients
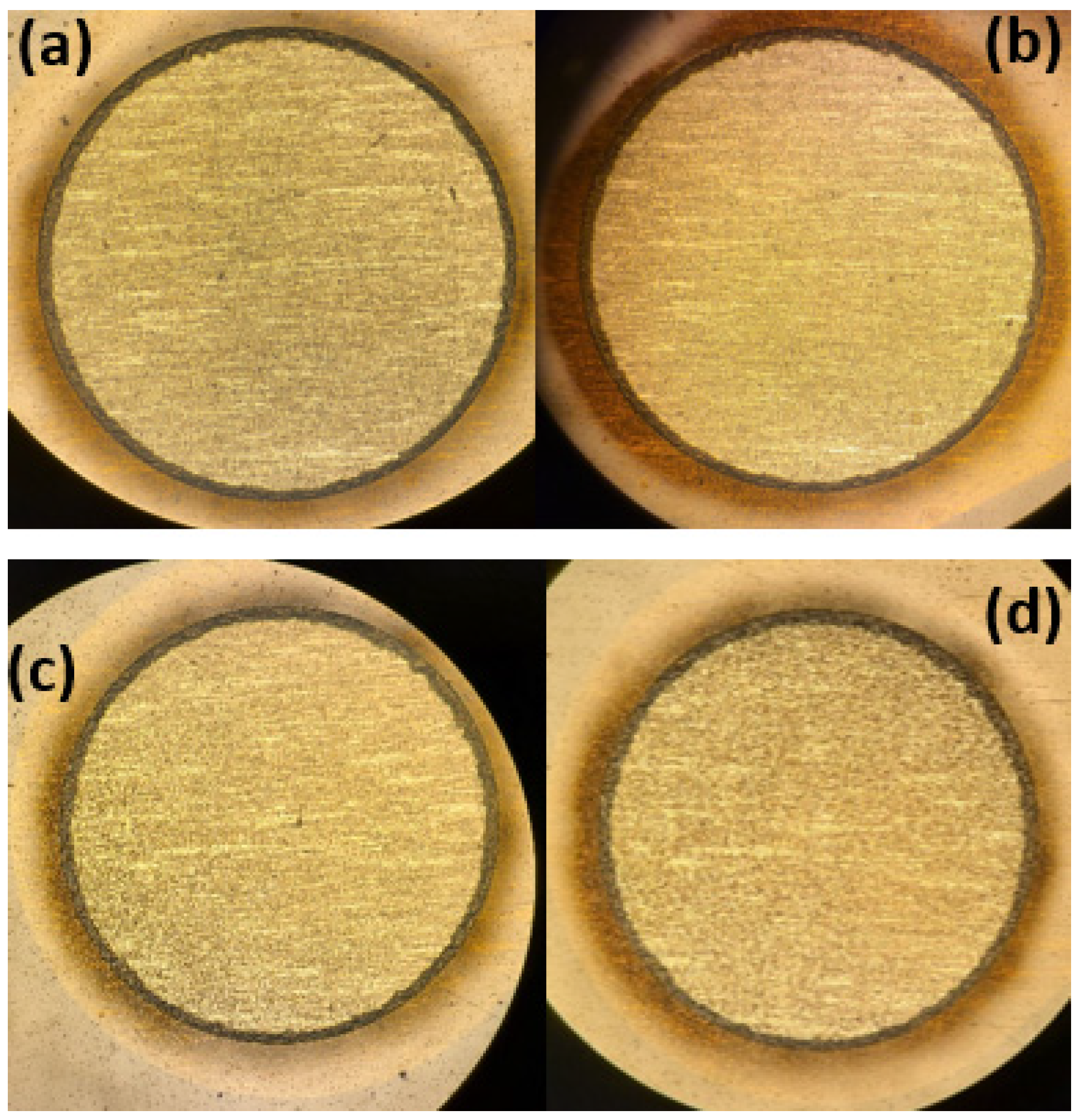
2.6. Visual Inspection
2.7. Statistical Analysis
3. Results
3.1. Short-Term Electrochemical Testing
3.2. Long-Term Electrochemical Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, L.H.; Parker, J.J.; Ho, A.L.; Feng, A.Y.; Kumar, K.K.; Chen, K.S.; Ojukwu, D.I.; Shuer, L.M.; Grant, G.A.; Graber, K.D.; et al. Contemporaneous evaluation of patient experience, surgical strategy, and seizure outcomes in patients undergoing stereoelectroencephalography or subdural electrode monitoring. Epilepsia 2021, 62, 74–84. [Google Scholar] [CrossRef]
- Kim, L.H.; Parker, J.J.; Ho, A.L.; Pendharkar, A.V.; Sussman, E.S.; Halpern, C.H.; Porter, B.; Grant, G.A. Postoperative outcomes following pediatric intracranial electrode monitoring: A case for stereoelectroencephalography (SEEG). Epilepsy Behav. 2020, 104, 106905. [Google Scholar] [CrossRef] [PubMed]
- Mullin, J.P.; Shriver, M.; Alomar, S.; Najm, I.; Bulacio, J.; Chauvel, P.; Gonzalez-Martinez, J. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia 2016, 57, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Punia, V.; Bulacio, J.; Gonzalez-Martinez, J.; Abdelkader, A.; Bingaman, W.; Najm, I.; Stojic, A. Extra operative intracranial EEG monitoring for epilepsy surgery in elderly patients. Epilepsy Behav. Case Rep. 2018, 10, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Sacino, M.F.; Huang, S.S.; Schreiber, J.; Gaillard, W.D.; Oluigbo, C.O. Is the use of Stereotactic Electroencephalography Safe and Effective in Children? A Meta-Analysis of the use of Stereotactic Electroencephalography in Comparison to Subdural Grids for Invasive Epilepsy Monitoring in Pediatric Subjects. Neurosurgery 2019, 84, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Gupta, D.; Brunner, P.; Gunduz, A.; Adamo, M.A.; Ritaccio, A.; Schalk, G. Recording human electrocorticographic (ECoG) signals for neuroscientific research and real-time functional cortical mapping. J. Vis. Exp. 2012, 64, e3993. [Google Scholar] [CrossRef]
- Jayakar, P.; Gotman, J.; Harvey, A.S.; Palmini, A.; Tassi, L.; Schomer, D.; Dubeau, F.; Bartolomei, F.; Yu, A.; Krsek, P.; et al. Diagnostic utility of invasive EEG for epilepsy surgery: Indications, modalities, and techniques. Epilepsia 2016, 57, 1735–1747. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Axmacher, N.; Mormann, F.; Halgren, E.; Crone, N.E. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog. Neurobiol. 2012, 98, 279–301. [Google Scholar] [CrossRef]
- Miller, K.J.; Honey, C.J.; Hermes, D.; Rao, R.P.; denNijs, M.; Ojemann, J.G. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. Neuroimage 2014, 85 Pt 2, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Roland, J.; Brunner, P.; Johnston, J.; Schalk, G.; Leuthardt, E.C. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010, 18, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Taplin, A.M.; de Pesters, A.; Brunner, P.; Hermes, D.; Dalfino, J.C.; Adamo, M.A.; Ritaccio, A.L.; Schalk, G. Intraoperative mapping of expressive language cortex using passive real-time electrocorticography. Epilepsy Behav. Case Rep. 2016, 5, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Worrell, G.; Gotman, J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: Clinical studies. Biomark. Med. 2011, 5, 557–566. [Google Scholar] [CrossRef]
- Kuncel, A.M.; Grill, W.M. Selection of stimulus parameters for deep brain stimulation. Clin. Neurophysiol. 2004, 115, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Allitt, B.J.; Paolini, A.G. Predicting neural recording performance of implantable electrodes. Analyst 2019, 144, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Lempka, S.F.; Johnson, M.D.; Barnett, D.W.; Moffitt, M.A.; Otto, K.J.; Kipke, D.R.; McIntyre, C.C. Optimization of microelectrode design for cortical recording based on thermal noise considerations. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 3361–3364. [Google Scholar] [CrossRef]
- McCreery, D.B.; Agnew, W.F.; Yuen, T.G.; Bullara, L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans. Biomed. Eng. 1990, 37, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.V. A model of safe levels for electrical stimulation. IEEE Trans. Biomed. Eng. 1992, 39, 424–426. [Google Scholar] [CrossRef]
- Szabo, B.; Gueli, C.; Eickenscheidt, M.; Stieglitz, T. Polyimide-based Thin Film Conductors for High Frequency Data Transmission in Ultra-Conformable Implants. Curr. Dir. Biomed. Eng. 2020, 6, 481–485. [Google Scholar] [CrossRef]
- Araki, T.; Otsubo, H.; Makino, Y.; Elliott, I.; Iida, K.; Ochi, A.; Weiss, S.K.; Chuang, S.H.; Rutka, J.T.; Snead, O.C. Efficacy of dexamathasone on cerebral swelling and seizures during subdural grid EEG recording in children. Epilepsia 2006, 47, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.S.; Alexopoulos, A.V.; Bingaman, W.E.; Gonzalez-Martinez, J.; Prayson, R.A. Pathologic findings associated with invasive EEG monitoring for medically intractable epilepsy. Am. J. Clin. Pathol. 2012, 138, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Mocco, J.; Komotar, R.J.; Ladouceur, A.K.; Zacharia, B.E.; Goodman, R.R.; McKhann, G.M. Radiographic characteristics fail to predict clinical course after subdural electrode placement. Neurosurgery 2006, 58, 120–125; discussion 120–125. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Dlouhy, B.J.; Nakagawa, D.; Kamm, J.; Hasan, D.; Howard, M.A.; Kawasaki, H. Bone flap elevation for intracranial EEG monitoring: Technical note. J. Neurosurg. 2018, 129, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.A.; Esquenazi, Y.; Johnson, J.; Zhu, P.; Tandon, N. The Brain is Not Flat: Conformal Electrode Arrays Diminish Complications of Subdural Electrode Implantation, A Series of 117 Cases. World Neurosurg. 2020, 144, e734–e742. [Google Scholar] [CrossRef] [PubMed]
- Van Gompel, J.J.; Worrell, G.A.; Bell, M.L.; Patrick, T.A.; Cascino, G.D.; Raffel, C.; Marsh, W.R.; Meyer, F.B. Intracranial electroencephalography with subdural grid electrodes: Techniques, complications, and outcomes. Neurosurgery 2008, 63, 498–505; discussion 505–506. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, A.; Kridner, D.; Mertens, S.; Christianson, M.; Rosa, D.; Diaz-Botia, C.A. First Food and Drug Administration Cleared Thin-Film Electrode for Intracranial Stimulation, Recording, and Monitoring of Brain Activity-Part 1: Biocompatibility Testing. Front. Neurosci. 2022, 16, 876877. [Google Scholar] [CrossRef]
- Boehler, C.; Carli, S.; Fadiga, L.; Stieglitz, T.; Asplund, M. Tutorial: Guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 2020, 15, 3557–3578. [Google Scholar] [CrossRef]
- Gardner, A.T.; Strathman, H.J.; Walker, R.M. A multiplexed electrochemical measurement system for characterization of implanted electrodes. In Proceedings of the 2020 IEEE International Symposium on Circuits and Systems (ISCAS), Seville, Spain, 12–14 October 2020. [Google Scholar] [CrossRef]
- Lempka, S.F.; Miocinovic, S.; Johnson, M.D.; Vitek, J.L.; McIntyre, C.C. In vivo impedance spectroscopy of deep brain stimulation electrodes. J. Neural Eng. 2009, 6, 046001. [Google Scholar] [CrossRef]
- Wilks, S.J.; Richner, T.J.; Brodnick, S.K.; Kipke, D.R.; Williams, J.C.; Otto, K.J. Voltage biasing, cyclic voltammetry, & electrical impedance spectroscopy for neural interfaces. J. Vis. Exp. 2012, 60, e3566. [Google Scholar] [CrossRef]
- Doering, M.; Kieninger, J.; Urban, G.A.; Weltin, A. Electrochemical microelectrode degradation monitoring: In situ investigation of platinum corrosion at neutral pH. J. Neural Eng. 2022, 19, 016005. [Google Scholar] [CrossRef]
- Jiang, X.; Sui, X.; Lu, Y.; Yan, Y.; Zhou, C.; Li, L.; Ren, Q.; Chai, X. In vitro and in vivo evaluation of a photosensitive polyimide thin-film microelectrode array suitable for epiretinal stimulation. J. Neuroeng. Rehabil. 2013, 10, 48. [Google Scholar] [CrossRef]
- Sun, A.; Franc, J.; Macdonald, D.D. Growth and Properties of Oxide Films on Platinum: I. EIS and X-Ray Photoelectron Spectroscopy Studies. J. Electrochem. Soc. 2006, 153, B260. [Google Scholar] [CrossRef]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Cogan, S.F. Electrochemical characterization of high frequency stimulation electrodes: Role of electrode material and stimulation parameters on electrode polarization. J. Neural Eng. 2018, 15, 036023. [Google Scholar] [CrossRef] [PubMed]
- Hudak, E.M.; Kumsa, D.W.; Martin, H.B.; Mortimer, J.T. Electron transfer processes occurring on platinum neural stimulating electrodes: Calculated charge-storage capacities are inaccessible during applied stimulation. J. Neural Eng. 2017, 14, 046012. [Google Scholar] [CrossRef] [PubMed]
- Weiland, J.D.; Anderson, D.J. Chronic neural stimulation with thin-film, iridium oxide electrodes. IEEE Trans. Biomed. Eng. 2000, 47, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Perez, A.; Gabriel, G.; Rebollo, B.; Illa, X.; Guimera-Brunet, A.; Hernandez-Ferrer, J.; Martinez, M.T.; Villa, R.; Sanchez-Vives, M.V. Quantification of Signal-to-Noise Ratio in Cerebral Cortex Recordings Using Flexible MEAs With Co-localized Platinum Black, Carbon Nanotubes, and Gold Electrodes. Front. Neurosci. 2018, 12, 862. [Google Scholar] [CrossRef]
- Fontes, M.B.A. Electrodes for bio-application: Recording and stimulation. J. Phys. Conf. Ser. 2013, 421, 012019. [Google Scholar] [CrossRef]
- Kyaw, T.T.; Hanawa, T.; Kasugai, S. Investigation of different electrochemical cleaning methods on contaminated healing abutments in vitro: An approach for metal surface decontamination. Int. J. Implant. Dent. 2020, 6, 64. [Google Scholar] [CrossRef]
- Harris, A.T. Current perspectives on the safe electrical stimulation of peripheral nerves with platinum electrodes. Bioelectron. Med. 2020, 3, 199–211. [Google Scholar] [CrossRef]
- Fan, B.; Rodriguez, A.V.; Vercosa, D.G.; Kemere, C.; Robinson, J.T. Sputtered porous Pt for wafer-scale manufacture of low-impedance flexible microelectrodes. J. Neural Eng. 2020, 17, 036029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, H.; Bhonsle, S.P.; Wang, Y.; Davalos, R.V.; Yao, C. Ablation outcome of irreversible electroporation on potato monitored by impedance spectrum under multi-electrode system. Biomed. Eng. Online 2018, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Matteucci, P.B.; Dodds, C.W.; Palmer, J.; Dueck, W.F.; Hassarati, R.T.; Byrnes-Preston, P.J.; Lovell, N.H.; Suaning, G.J. Laser patterning of platinum electrodes for safe neurostimulation. J. Neural Eng. 2014, 11, 056017. [Google Scholar] [CrossRef] [PubMed]


| 100 Hz | 1 kHz | |||
|---|---|---|---|---|
| Magnitude (Ω) | Phase (°) | Magnitude (Ω) | Phase (°) | |
| Before | 218.72 ± 39.29 | −37.22 ± 5.72 | 133.02 ± 8.33 | −10.88 ± 2.78 |
| After | 146.83 ± 5.43 * | −20.46 ± 0.74 * | 127.32 ± 5.48 | −4.29 ± 0.25 * |
| Before Stimulation | After Stimulation | Relevant Reaction | ||
|---|---|---|---|---|
| Peak Voltage (V) | Peak Current (mA) | Peak Voltage (V) | Peak Current (mA) | |
| −0.25 | −0.18 | −0.22 | −0.21 | Pt-O reduction |
| −0.70 | −0.14 | −0.70 | −0.17 | Pt-H reduction |
| −0.52 | 0.08 | −0.51 | 0.10 | Pt-H oxidation |
| −0.45 | 0.07 | −0.45 | 0.08 | Pt-H oxidation |
| 100 Hz | 1 kHz | |||
|---|---|---|---|---|
| Magnitude (Ω) | Phase (°) | Magnitude (Ω) | Phase (°) | |
| Before | 211.23 ± 39.29 | −39.20 ± 5.72 | 124.01 ± 8.33 | −11.75 ± 2.78 |
| After | 133.81 ± 5.43 * | −16.39 ± 0.74 * | 120.39 ± 5.48 * | −3.28 ± 0.25 * |
| Before Stimulation | After Stimulation | Relevant Reaction | ||
|---|---|---|---|---|
| Peak Voltage (V) | Peak Current (mA) | Peak Voltage (V) | Peak Current (mA) | |
| −0.25 | −0.18 | −0.23 | −0.17 | Pt-O reduction |
| −0.75 | −0.17 | −0.76 | −0.16 | Pt-H reduction |
| −0.55 | 0.07 | −0.55 | 0.08 | Pt-H oxidation |
| −0.42 | 0.08 | −0.40 | 0.06 | Pt-H oxidation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, S.; Kullmann, A.; Mertens, S.; Rosa, D.; Diaz-Botia, C.A. Electrochemical Testing of a New Polyimide Thin Film Electrode for Stimulation, Recording, and Monitoring of Brain Activity. Micromachines 2022, 13, 1798. https://doi.org/10.3390/mi13101798
Ong S, Kullmann A, Mertens S, Rosa D, Diaz-Botia CA. Electrochemical Testing of a New Polyimide Thin Film Electrode for Stimulation, Recording, and Monitoring of Brain Activity. Micromachines. 2022; 13(10):1798. https://doi.org/10.3390/mi13101798
Chicago/Turabian StyleOng, Samuel, Aura Kullmann, Steve Mertens, Dave Rosa, and Camilo A Diaz-Botia. 2022. "Electrochemical Testing of a New Polyimide Thin Film Electrode for Stimulation, Recording, and Monitoring of Brain Activity" Micromachines 13, no. 10: 1798. https://doi.org/10.3390/mi13101798
APA StyleOng, S., Kullmann, A., Mertens, S., Rosa, D., & Diaz-Botia, C. A. (2022). Electrochemical Testing of a New Polyimide Thin Film Electrode for Stimulation, Recording, and Monitoring of Brain Activity. Micromachines, 13(10), 1798. https://doi.org/10.3390/mi13101798






