Detection of Tomato Ringspot Virus Based on Microfluidic Impedance Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Principle of Microfluidic Impedance Detection
2.2. Preparation of Tomato Ringspot Virus
2.2.1. Materials
2.2.2. Method
- (1)
- We added 500 μL Plant Protein Extract A, 4 μL Protein Stabilizer, and 2 μL Protease Inhibitor (the above chemicals are from Bioengineering (Shanghai) Co., Ltd.’s plant protein extraction kit C500053) to a 1.5 mL centrifuge tube and mixed well with a constant temperature mixer (Model MSC-100, Hangzhou Ausheng Instrument Co., Ltd. (Block 9, No. 7, Turnang Science and Technology Economic Block, West Lake District, Hangzhou, Zhejiang Province, China));
- (2)
- Liquid nitrogen was poured into the mortar, the tomato ringspot virus-infected plant leaves were added, and everything was thoroughly crushed into the previously prepared 1.5 mL centrifuge tube;
- (3)
- The centrifuge tube was placed in a 4 °C mixed instrument at an oscillation speed of 800 rpm for one hour. The tube was then centrifuged for 15 min at 4 °C at a speed of 12,000 revolutions per minute in a frozen centrifuge (Model 5418R, Eppendorf Company, Hamburg, Germany);
- (4)
- In the last step, the supernatant was transferred from the centrifuge tube into a fresh 1.5 mL centrifuge tube, 1 μL of the separated supernatant was taken, and an ultra-micro spectrophotometer was used to measure the tomato ringspot virus concentration, which was 26.6 mg/mL (model Nanodrop 2000, Thermo Fisher Scientific, Waltham, MA, USA). Before being kept at −80 °C, the PBS was diluted to 10, 1, 0.1, 0.01, and 0.001 μg/mL.
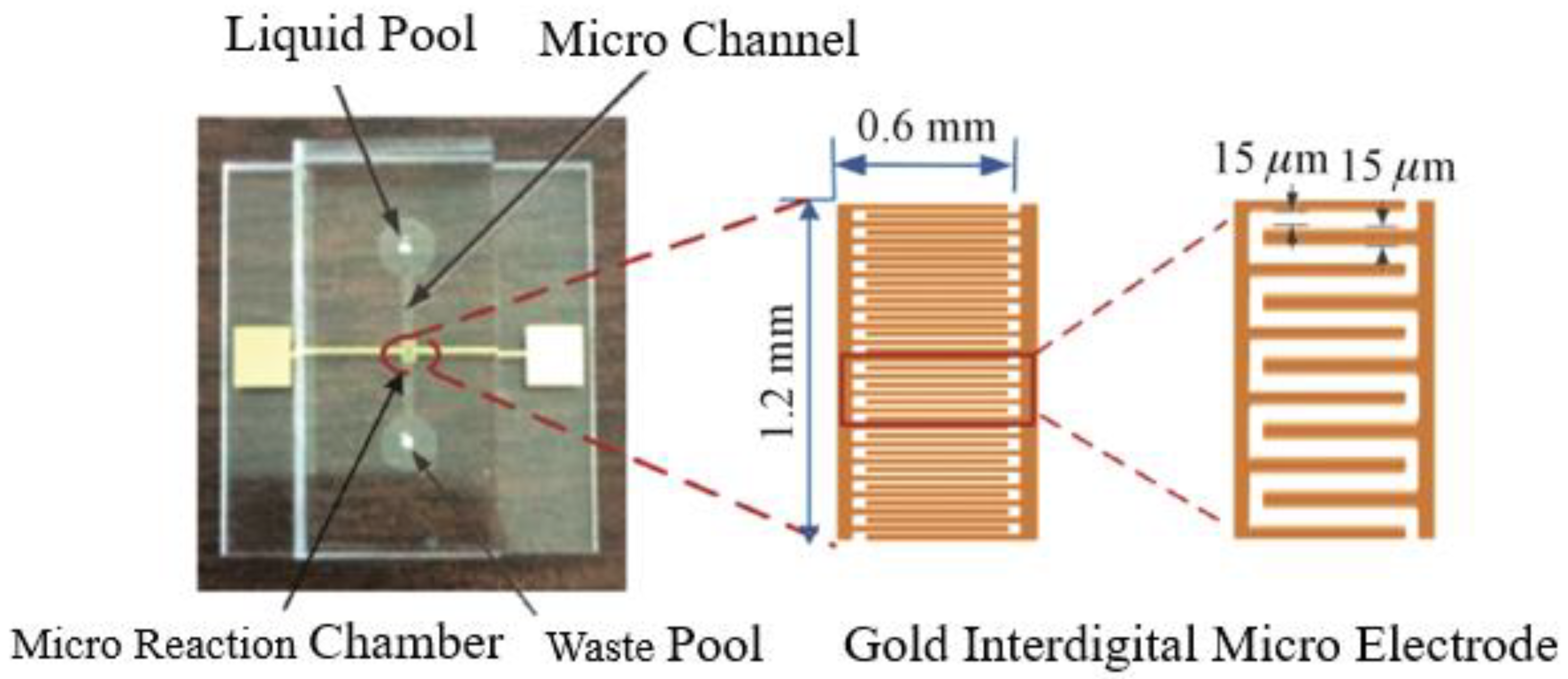
2.3. Microfluidic Impedance Detection Chips and Systems
2.4. Experimental Methods
- Measurement of the bare electrode: The impedance of the bare electrode was measured by injecting a PBS buffer. After the measurement was over, the microchannel and microelectrode were cleaned for 3 min with ultra-pure water;
- Antibody immobilization: the micro channel was filled with 15 μL of ToRSV monoclonal antibody (0.5 mg/mL) and set up for 2 h. The pH value of the PBS buffer solution at this moment was 7.4 and the ion concentration was 0.1 mol/L. Immobilization of antibodies by natural sedimentation adsorption, a physical adsorption technique that is relatively easy to use, had minimal impact on the activity of biomolecules compared to other techniques, and reduced the likelihood of non-specific immunity occurring. The unabsorbed antibody was rinsed with ultrapure water after 2 h, and the impedance was measured by injection of PBS buffer. The cleaning procedure was repeated after the measurement;
- (Bovine serum albumin)BSA sealing: 15 μL 1% BSA solution was injected and let to sit for 30 min. This shut the surface of the gold electrode that was not in contact with the antibody, preventing interference signals. BSA was washed away after 30 min, and PBS buffer was injected to determine the impedance value. Cleaning activities were repeated after the measurement was finished;
- Detection of ToRSV: 15 μL of ToRSV sample was injected and let to sit for 30 min, then the impedance was tested. The cleaning operation after each measurement was repeated until the impedance was similar to that of a bare electrode.
3. Results and Discussion
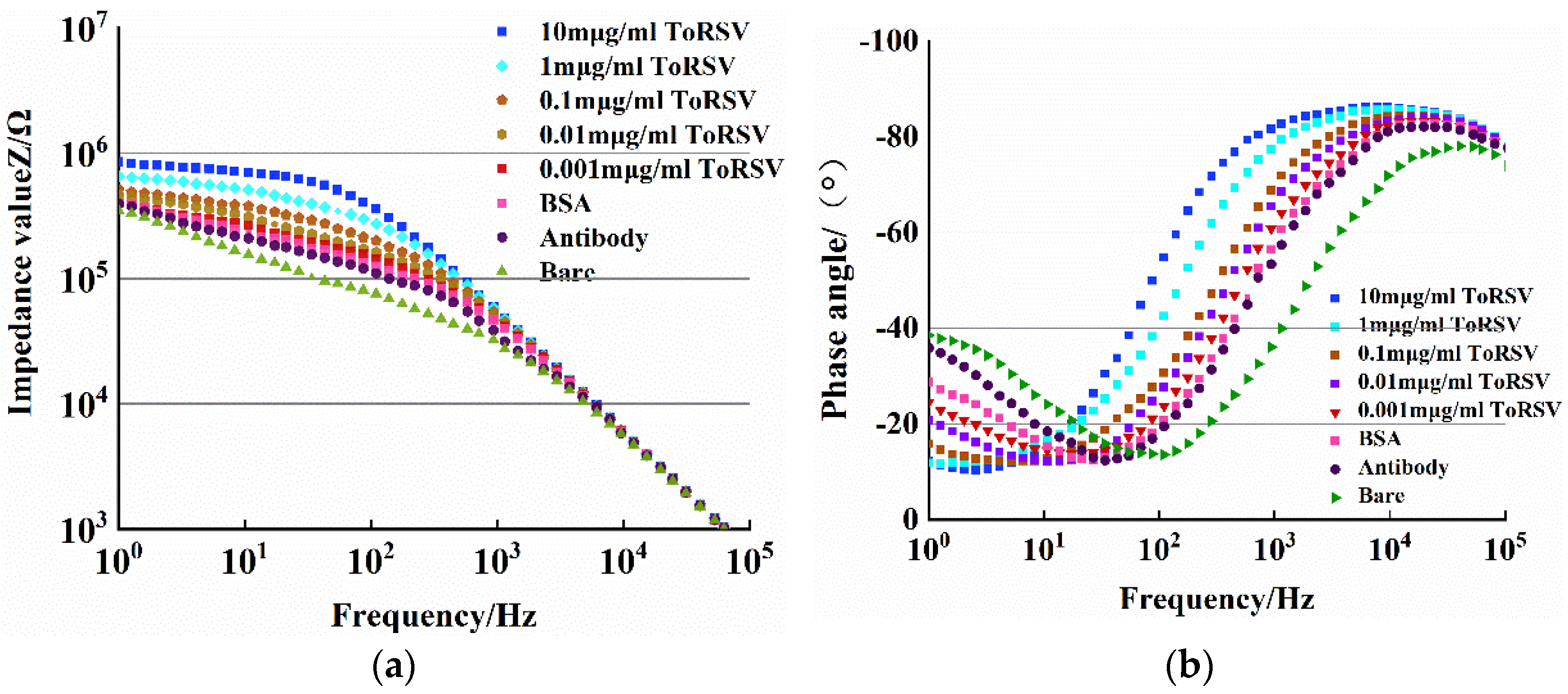
3.1. Impedance and Phase Angle Analysis
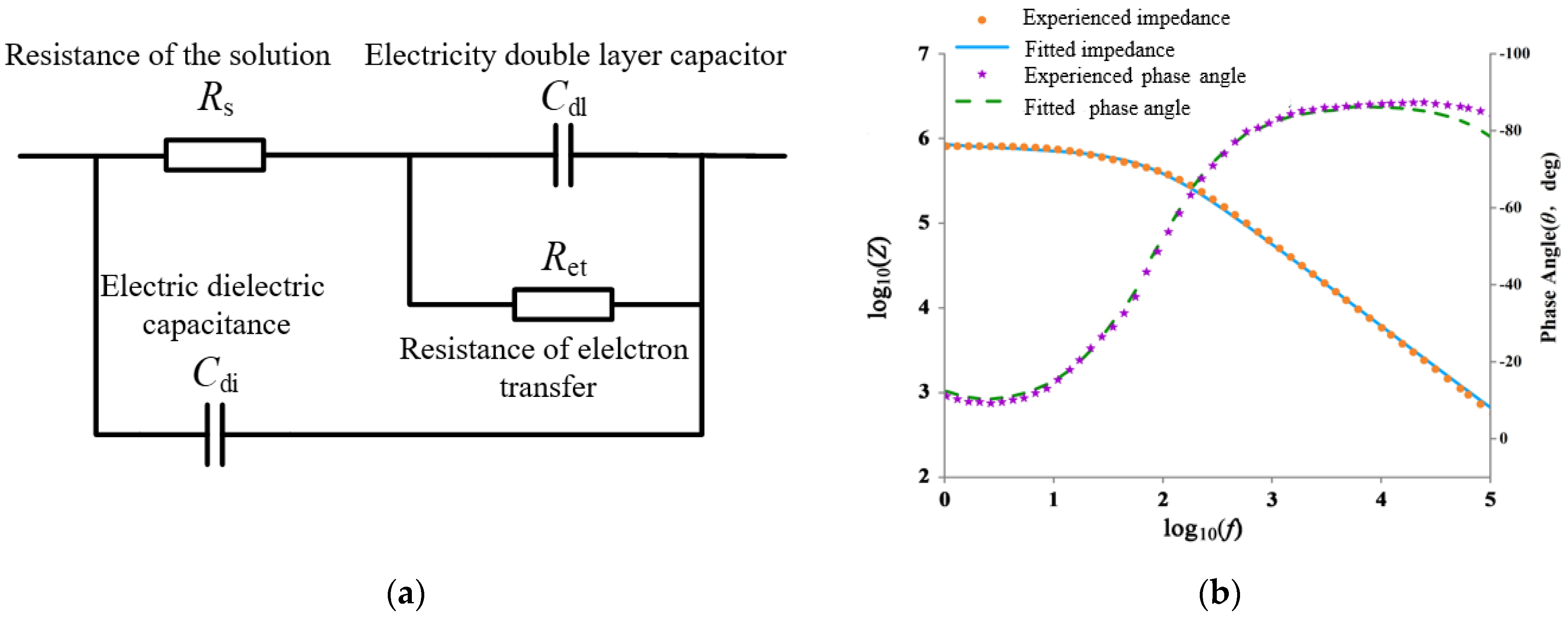
3.2. Equivalent Circuit Analysis
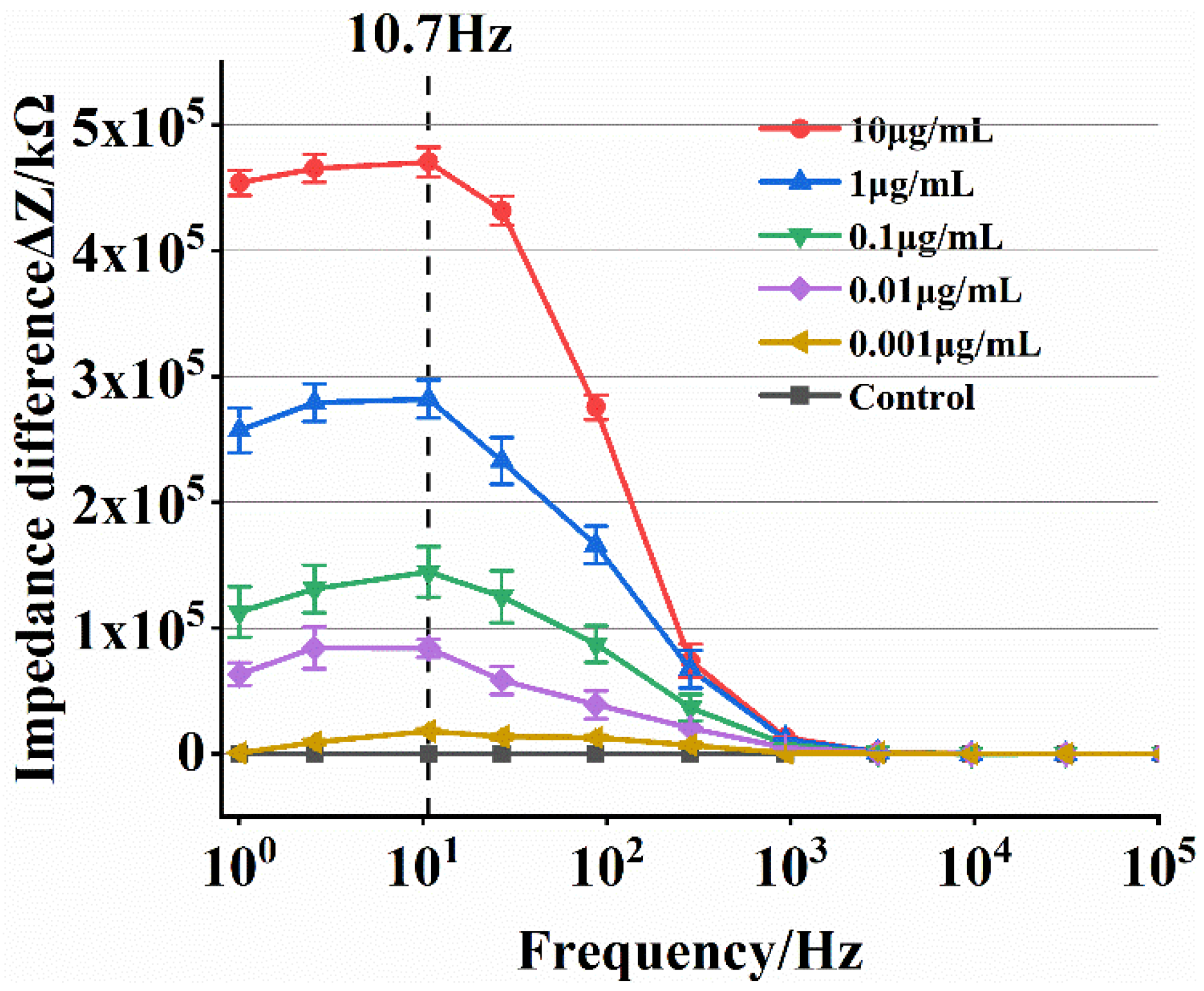
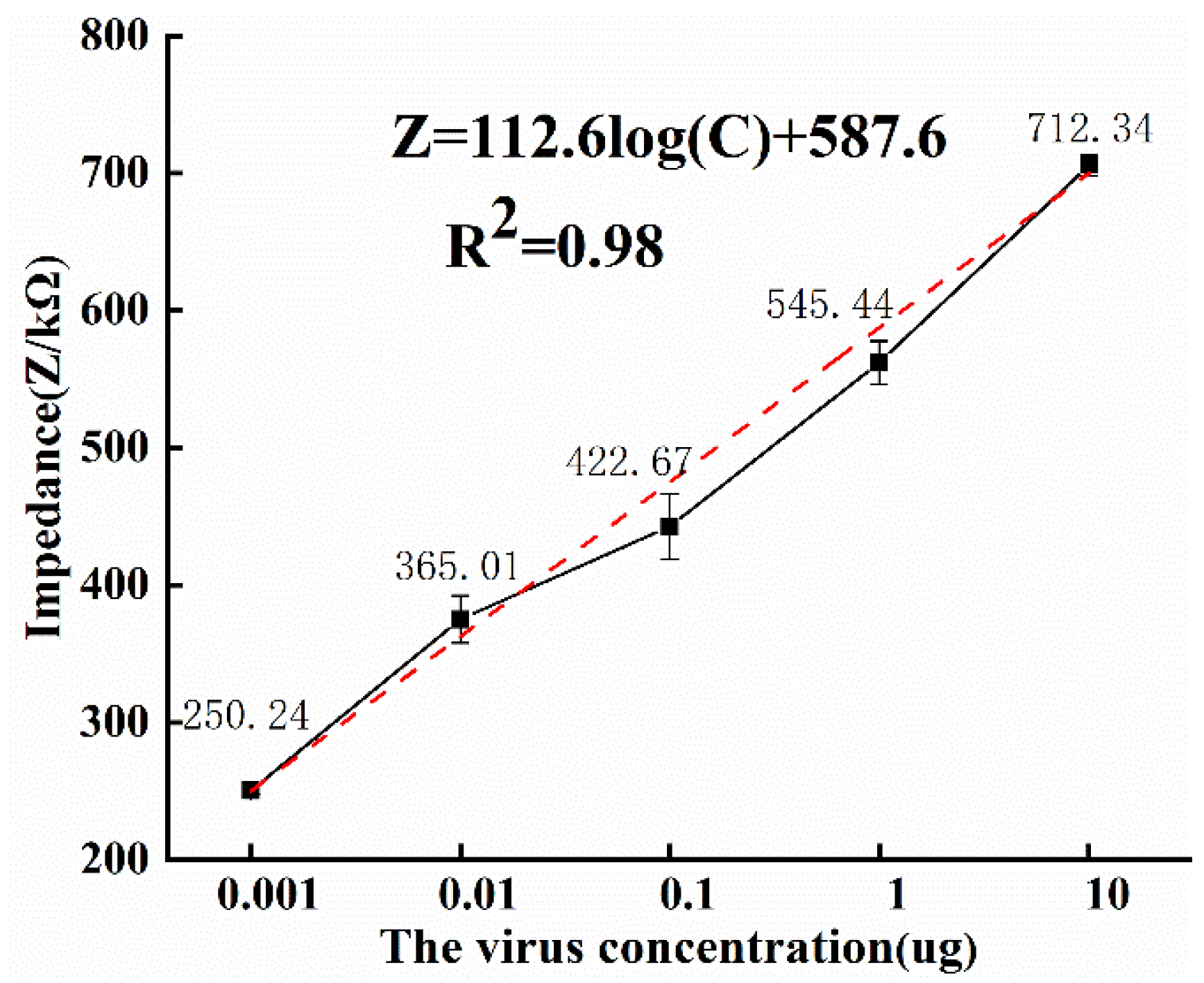
3.3. Detection of ToRSV Concentration
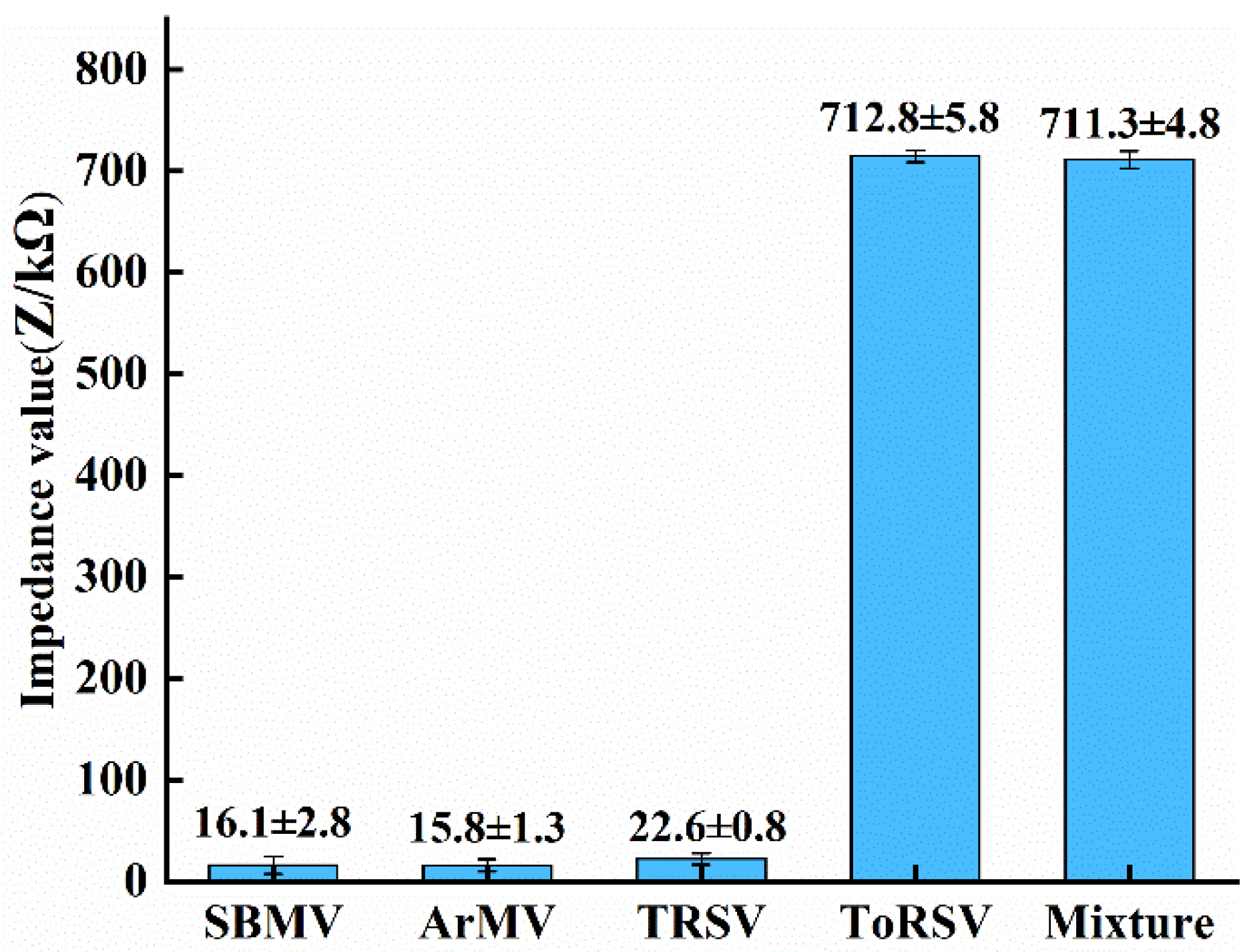
3.4. Specificity Test
4. Conclusions
- A microfluidic impedance sensor for ToRSV detection was designed. The ToRSV antibody was attached to the gold interdigital array microelectrode in the flow channel of the microfluidic chip. The combination of the antibody and ToRSV caused the impedance to change, and electrochemical impedance spectroscopy was made.
- For the study of the mechanism of ToRSV impedance creation and variation, a new equivalent circuit model is suggested. ToRSV at concentrations of 0.001~1 μg/mL was used for impedance detection, and it was shown that the impedance spectrum obtained from the microfluidic impedance sensor and the impedance spectrum produced by fitting the equivalent circuit model were in good agreement.
- The ideal detection frequency was determined using the impedance difference, and the results showed that the ideal detection frequency for this impedance biosensor to detect ToRSV concentration is 10.7 Hz. The ToRSV concentration and impedance value calculation model were built, and the detection specificity was evaluated. The experimental results reveal that the developed microfluidic impedance sensor has a detection limit of 0.0034 μg/mL, and good specificity and validity.
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, J.; Yuan, S.; Lu, N.; Ma, X.; Yang, Z.; Long, Y. Dual DPO-RT-PCR detection of peanut dwarf virus and tomato ringspot virus. J. Plant Prot. 2021, 48, 931–932. [Google Scholar] [CrossRef]
- Paudel, D.B.; Sanfacon, H. Mapping of sequences in the 5′ region and 3′ UTR of tomato ringspot virus RNA2 that facilitate cap-independent translation of reporter transcripts in vitro. PLoS ONE 2021, 16, e0249928. [Google Scholar] [CrossRef]
- Xu, T.; Zheng, X.; Zhang, X.L.; Chen, Y.; Mu, Y.; Chen, Y. Establishment of a real-time fluorescence quantitative PCR assay for tomato ringspot virus (TZSV). Shandong Agric. Sci. 2017, 49, 139–144. [Google Scholar] [CrossRef]
- Roberrt, R.M.; Jack, N.P.; Jennifer, K. The use of collagenase to improve the detection of plant viruses in vector nematodes by RT-PCR. J. Virol. Methods 2009, 155, 91–95. [Google Scholar] [CrossRef]
- Stewart, E.L.; Qu, X.; Overton, B.E.; Gildow, F.E.; Wenner, N.G.; Grove, D.S. Development of a Real-Time RT-PCR SYBR Green Assay for Tomato ring spot virus in Grape. Plant Dis. 2007, 91, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Zhao, X.-X.; Wang, D.; Zhang, P.-J.; Hu, X.-N.; Wei, S.; Liu, J.-Y.; Ye, Z.-H.; Yu, X.-P. A reverse transcription-cross-priming amplification method with lateral flow dipstick assay for the rapid detection of Bean pod mottle virus. Sci. Rep. 2022, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.G.; Cui, J.X.; Sheng, L. Detection of tobacco ringspot virus and tomato ringspot virus by semi-nested RT-PCR. J. Plant Prot. 2007, 01, 61–66. [Google Scholar]
- Yu, S.Q.; Zhang, J.H.; Zhang, H.L. Establishment of RT-LAMP assay for tomato ringspot virus. Plant Q. 2013, 27, 44–47. [Google Scholar]
- Kant, K.; Shahbazi, M.-A.; Dave, V.P.; Ngo, T.A.; Chidambara, V.A.; Than, L.Q.; Bang, D.D.; Wolff, A. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol. Adv. 2018, 36, 1003–1024. [Google Scholar] [CrossRef]
- Bruchmann, J.; Sachsenheimer, K.; Rapp, B.E.; Schwartz, T. Multi-channel microfluidic biosensor platform applied for online monitoring and screening of biofilm formation and activity. PLoS ONE 2015, 10, e0117300. [Google Scholar] [CrossRef]
- Yin, B.; Wan, X.; Qian, C.; Sohan, A.S.M.M.F.; Zhou, T.; Yue, W. Enzyme Method-Based Microfluidic Chip for the Rapid Detection of Copper Ions. Micromachines 2021, 12, 1380. [Google Scholar] [CrossRef]
- Ahmed, I.; Akram, Z.; Bule, M.H.; Iqbal, H.M.N. Advancements and Potential Applications of Microfluidic Approaches—A Review. Chemosensors 2018, 6, 46. [Google Scholar] [CrossRef]
- Pratikkumar, S.; Tharangattu, N.N.; Chen-zhong, L.; Alwarappan, S. Probing the biocompatibility of MoS2 nanosheets by cytotoxicity assay and electrical impedance spectroscopy. Nanotechnology 2015, 26, 315102. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Cai, G.; Xiong, Y.; Li, Y.; Wang, M.; Huo, H.; Lin, J. Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens. Bioelectron. 2016, 8, 770–776. [Google Scholar] [CrossRef]
- Wang, C.H.; Cui, C.J.; Gu, X.J. Study on microfork finger electrode impedance biosensor for the detection of Staphylococcus aureus. Food Res. Dev. 2020, 10, 184–192. [Google Scholar]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157:H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2018, 15, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Dou, W.; Zhao, G. Amperometric immunoassay for the detection of Salmonella pullorum using a screen—Printed carbon electrode modified with gold nanoparticle-coated reduced graphene oxide and immunomagnetic beads. Microchim. Acta 2016, 183, 757–764. [Google Scholar] [CrossRef]
- Tang, M.; Wang, G.; Kong, S.-K.; Ho, H.-P. A Review of Biomedical Centrifugal Microfluidic Platforms. Micromachines 2016, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, L.; Huang, F.; Cai, G.; Xi, X.; Lin, J. A microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E. coli O157:H7. Sens. Actuators B Chem. 2018, 259, 1013–1021. [Google Scholar] [CrossRef]
- Thomas, S.H.; David, S.; Niels, B.L. Fast prototyping of injection molded polymer microfluidic chips. J. Micromech. Microeng. 2010, 20, 015020. [Google Scholar] [CrossRef]
- Liu, J.; Jasim, I.; Shen, Z.; Zhao, L.; Dweik, M.; Zhang, S.; Almasri, M. A microfluidic based biosensor for rapid detection of Salmonella in food products. PLoS ONE 2019, 14, e0216873. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sjoberg, R.; Morisette, D.T.; Bashir, R. Impedance Microbiology-on-a-Chip: Microfluidic Bioprocessor for Rapid Detection of Bacterial Metabolism. J. Microelectromech. Syst. 2015, 14, 829–838. [Google Scholar] [CrossRef]
- Madhukar, V.; Li, Y. Interdigitated array microelectrode based impedance biosensor coupled with magnetic nanoparticle–antibody conjugates for detection of Escherichia coli O157:H7 in food samples. Biosens. Bioelectron. 2006, 22, 2408–2414. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Z.Z.; Tan, J.; Ji, S.M.; Sun, J.W. Two new quinoline-based regenerable fluorescent probes with AIE characteristics for selective recognition of Cu2+ in aqueous solution and test strips. Analyst 2018, 20, 4870–4886. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Ye, B.; Xi, Y.; Yuan, M. Detection of Tomato Ringspot Virus Based on Microfluidic Impedance Sensor. Micromachines 2022, 13, 1764. https://doi.org/10.3390/mi13101764
Li C, Ye B, Xi Y, Yuan M. Detection of Tomato Ringspot Virus Based on Microfluidic Impedance Sensor. Micromachines. 2022; 13(10):1764. https://doi.org/10.3390/mi13101764
Chicago/Turabian StyleLi, Chen, Bo Ye, Yongxin Xi, and Mu Yuan. 2022. "Detection of Tomato Ringspot Virus Based on Microfluidic Impedance Sensor" Micromachines 13, no. 10: 1764. https://doi.org/10.3390/mi13101764
APA StyleLi, C., Ye, B., Xi, Y., & Yuan, M. (2022). Detection of Tomato Ringspot Virus Based on Microfluidic Impedance Sensor. Micromachines, 13(10), 1764. https://doi.org/10.3390/mi13101764




