Design and Implementation of a Prosthesis System Controlled by Electromyographic Signals Means, Characterized with Artificial Neural Networks
Abstract
1. Introduction
2. Preliminaries
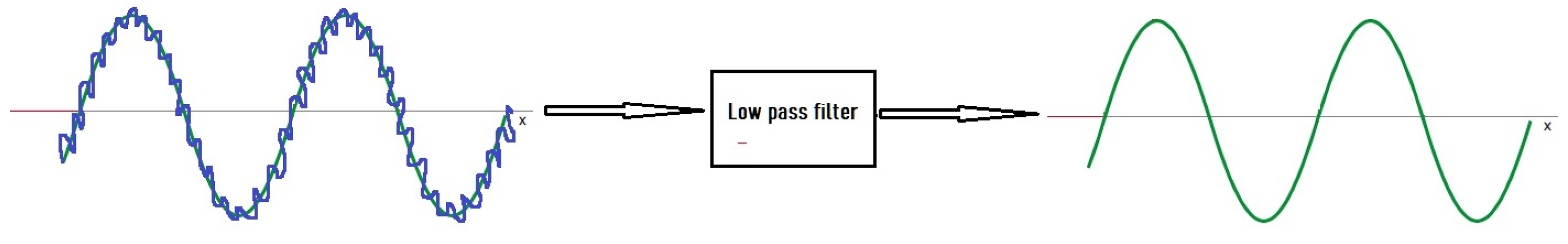
2.1. Filters
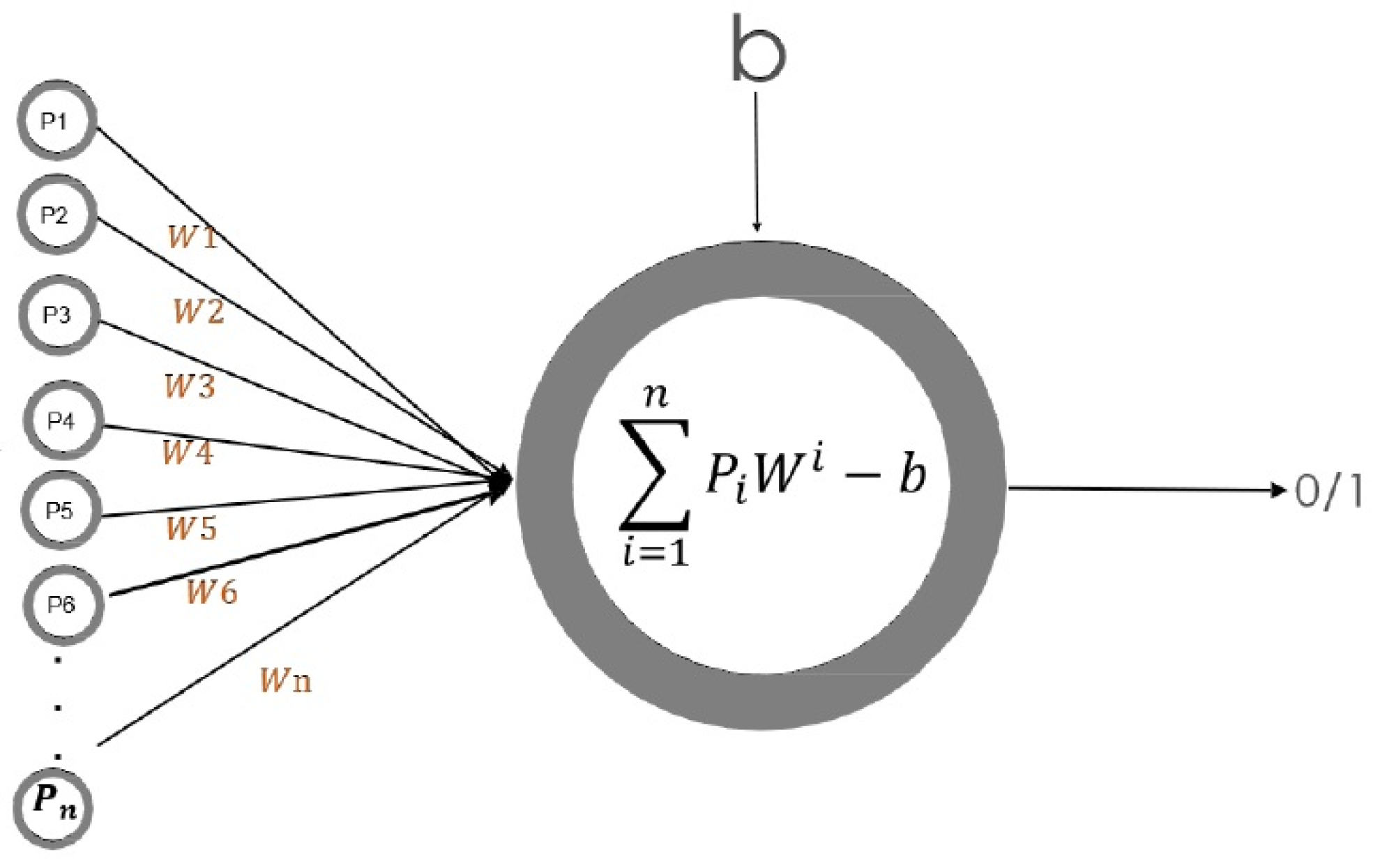
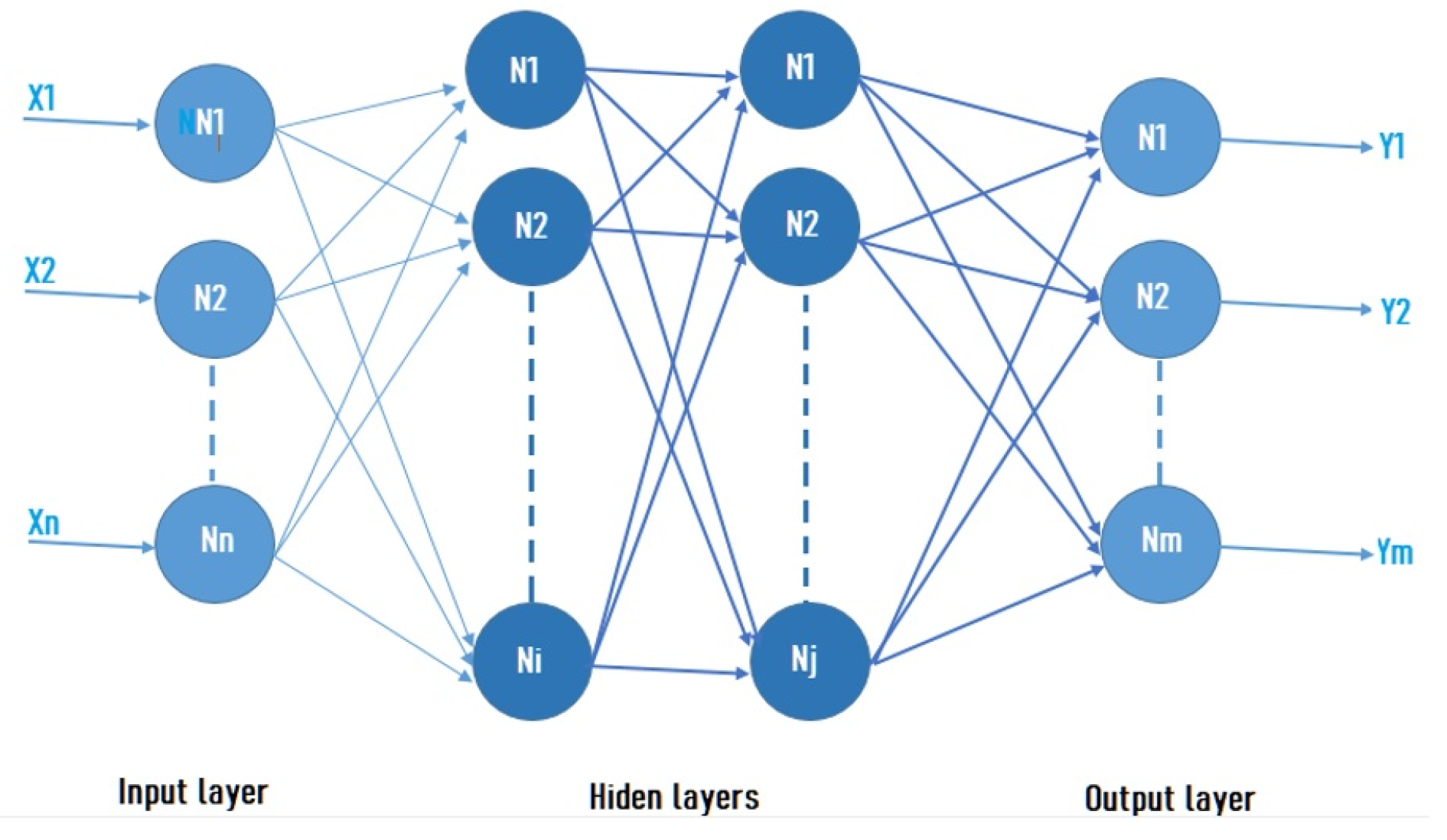
2.2. Artificial Neural Networks
3. Materials and Methods
3.1. Electromyographic Sensor Design
3.2. Signals Conditioning
3.2.1. Pre-Amplification
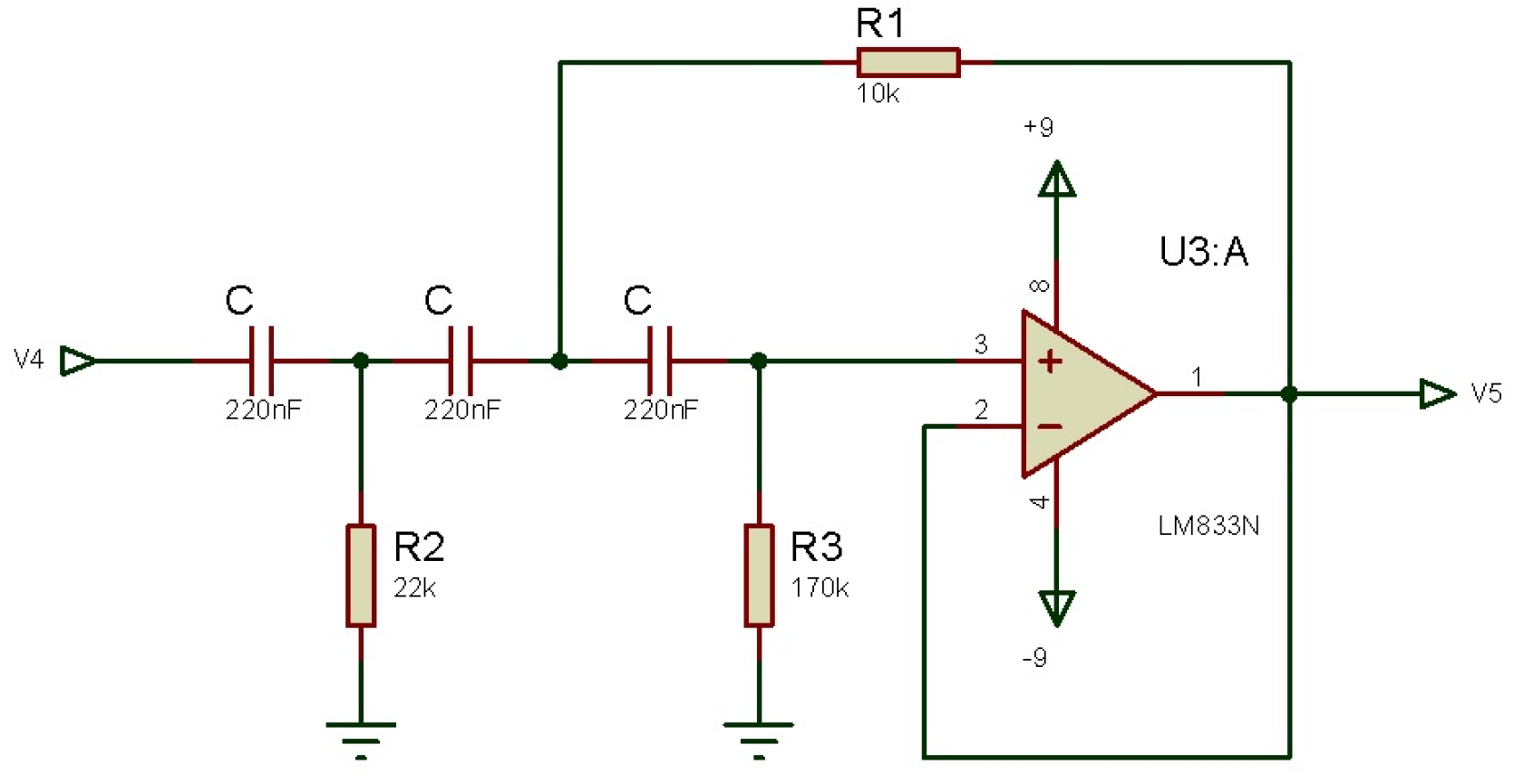
3.2.2. Third Order Low-Pass Butterworth Filter
3.2.3. Third Order High Pass Filter
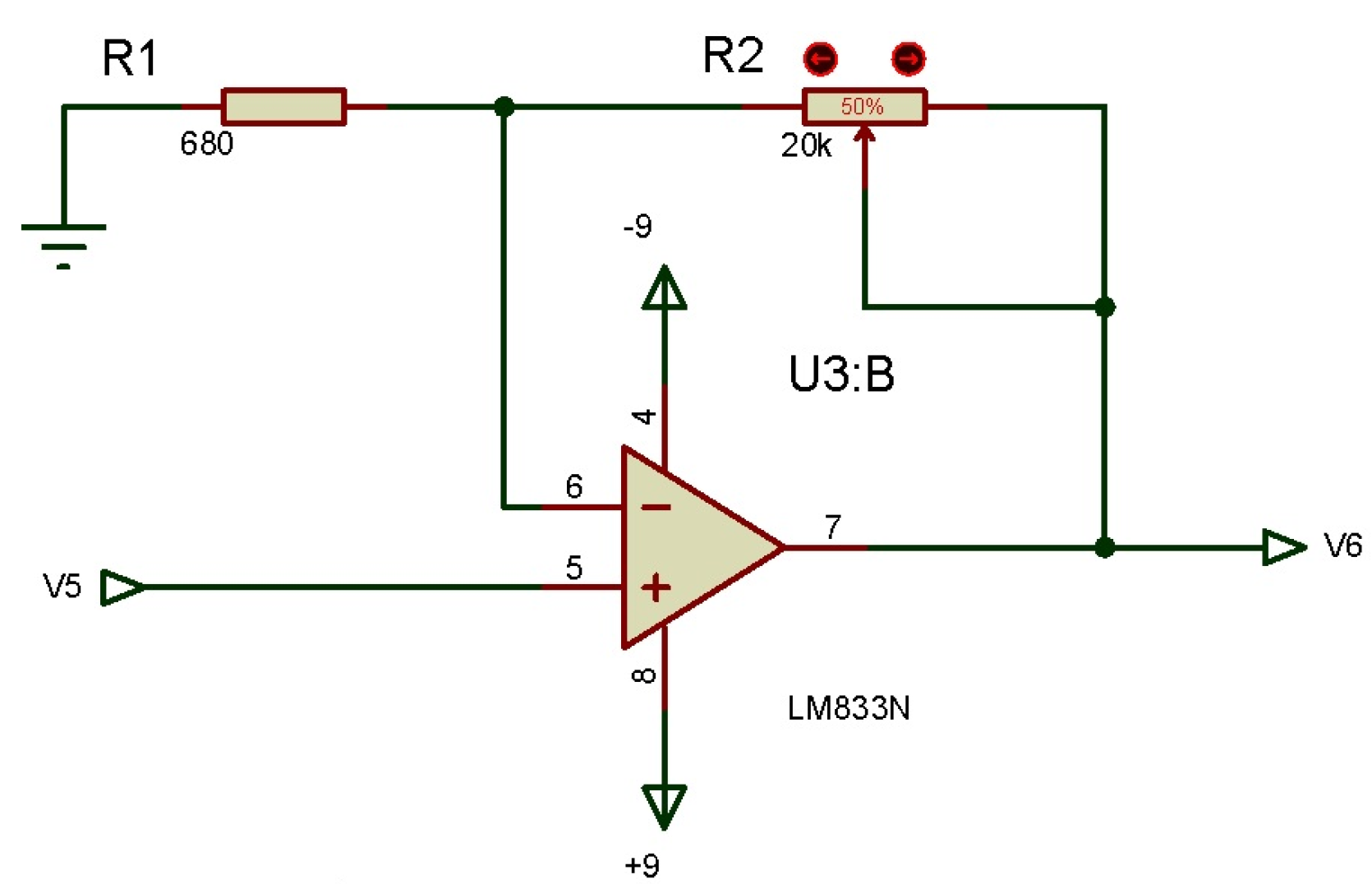
3.2.4. Non-Inverting Amplifier
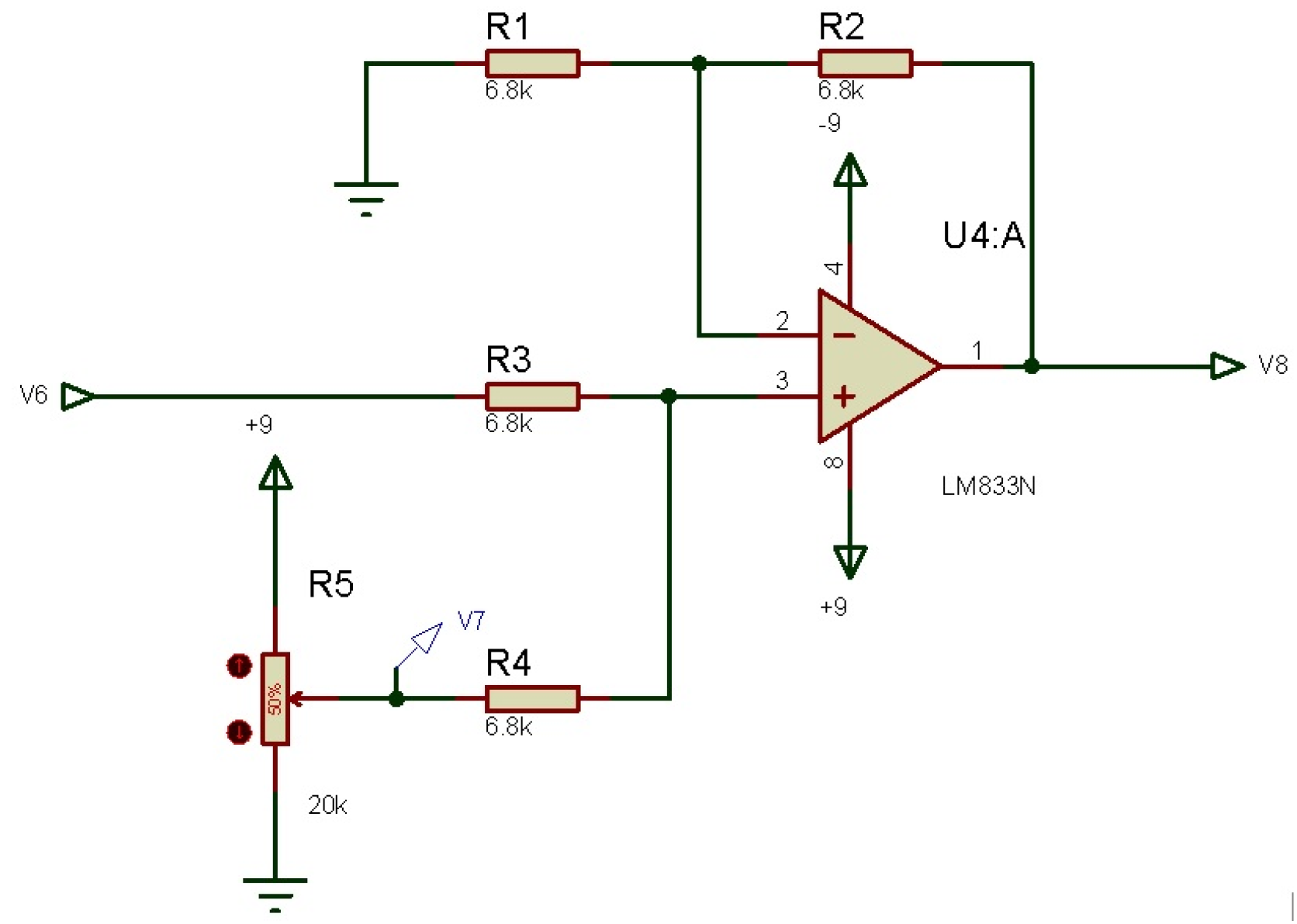
3.2.5. Offset (Non-Inverting Summing Amplifier)
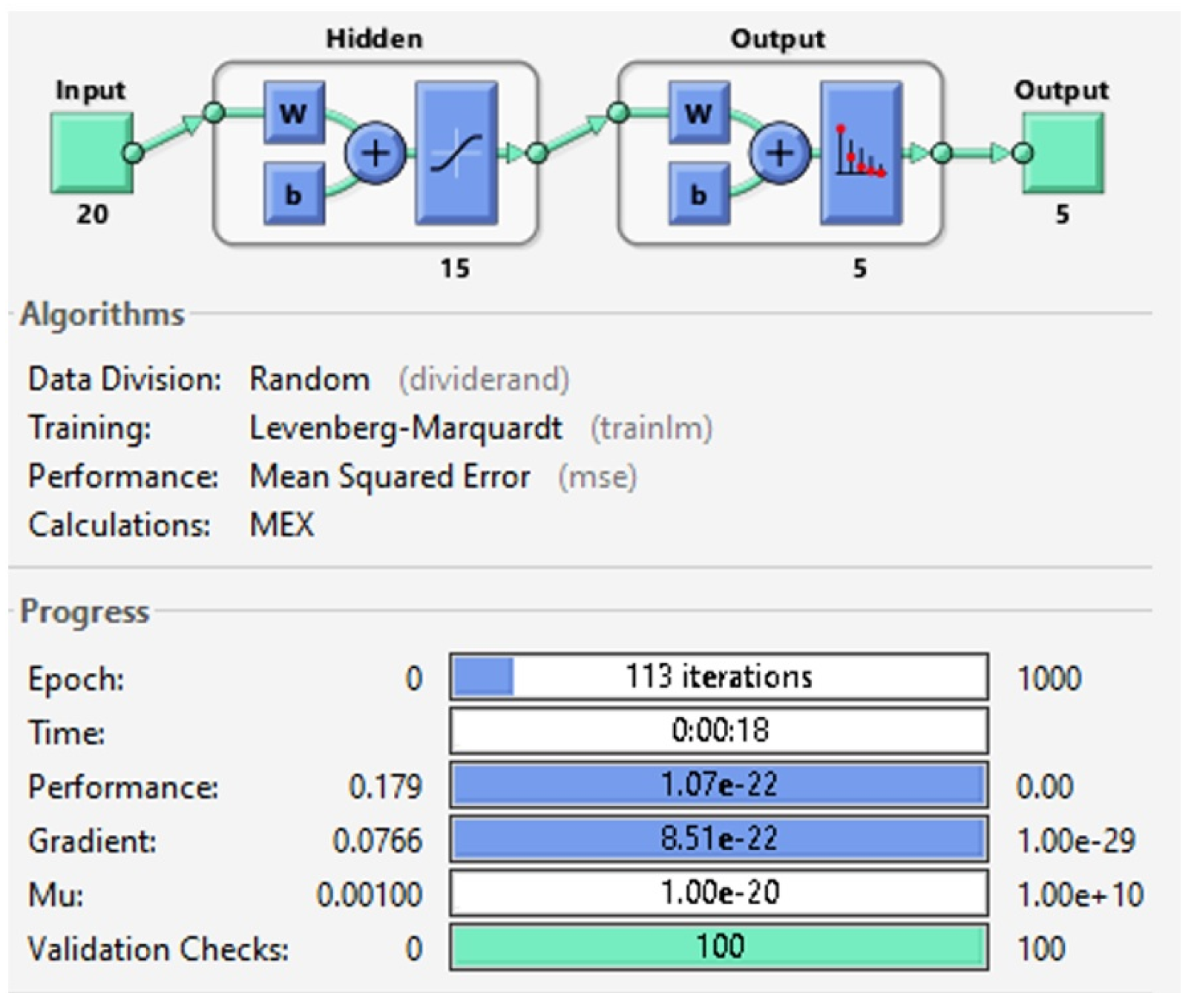
3.3. Neural Network Design
Extraction of Features
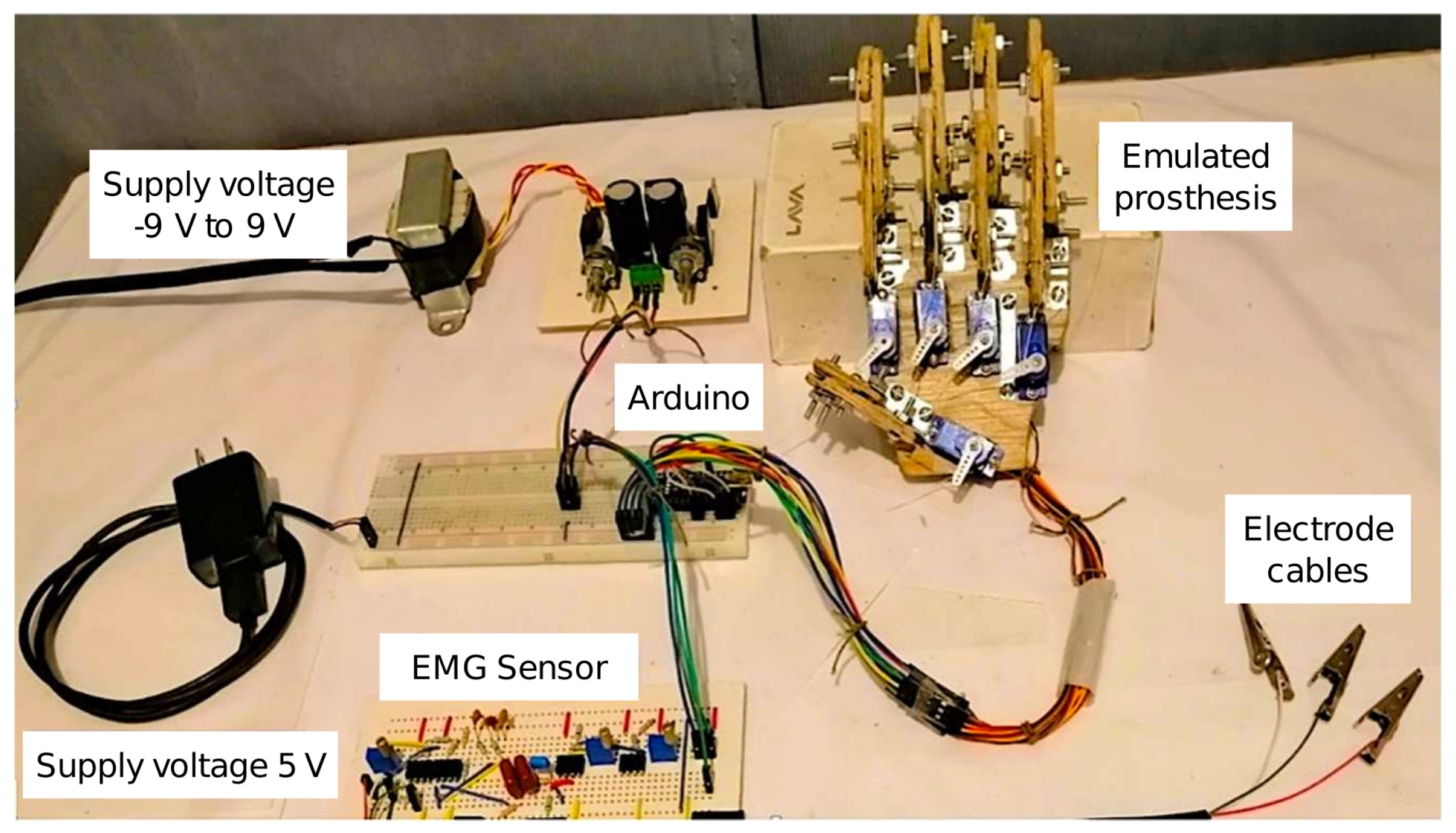
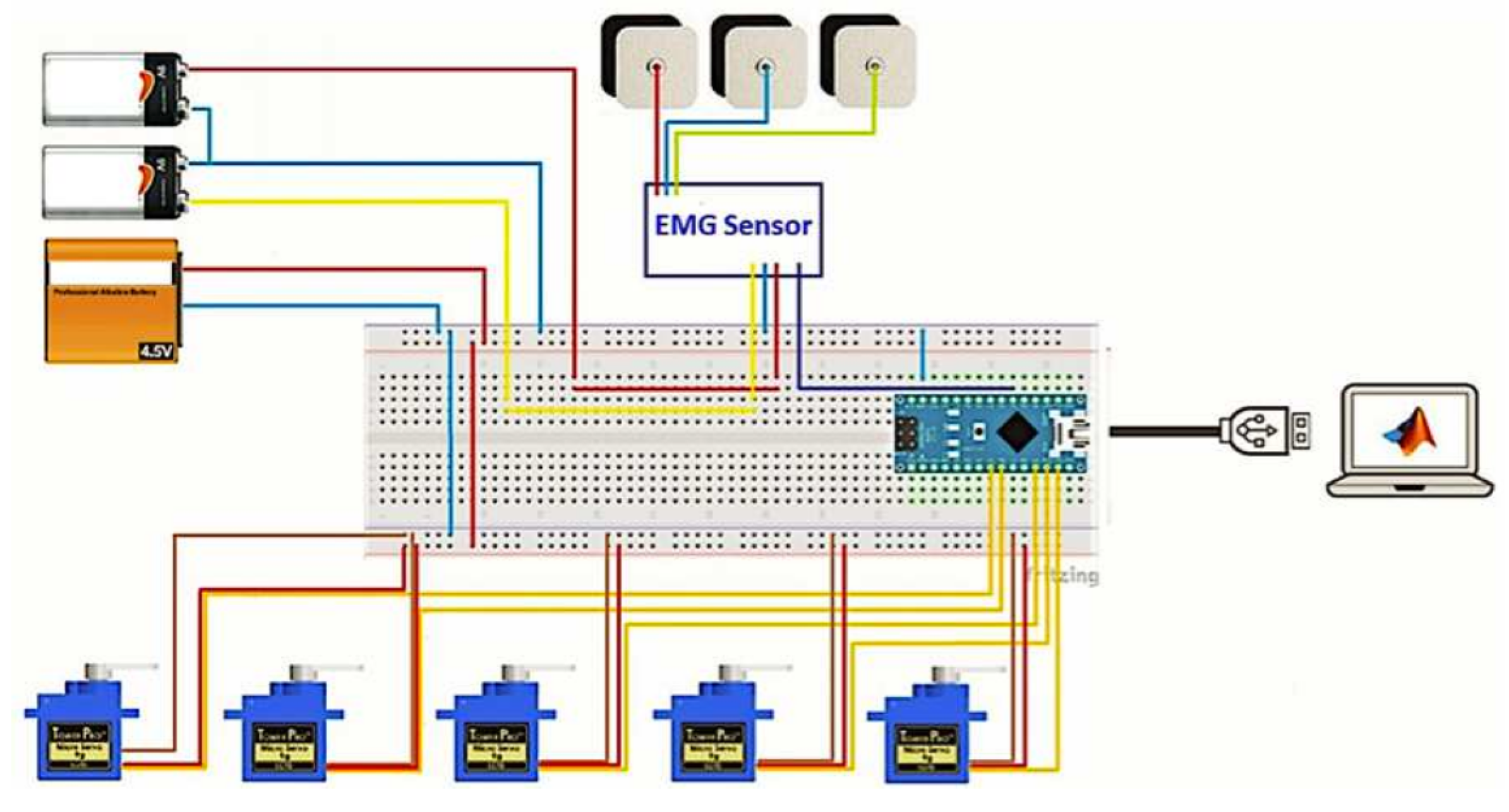
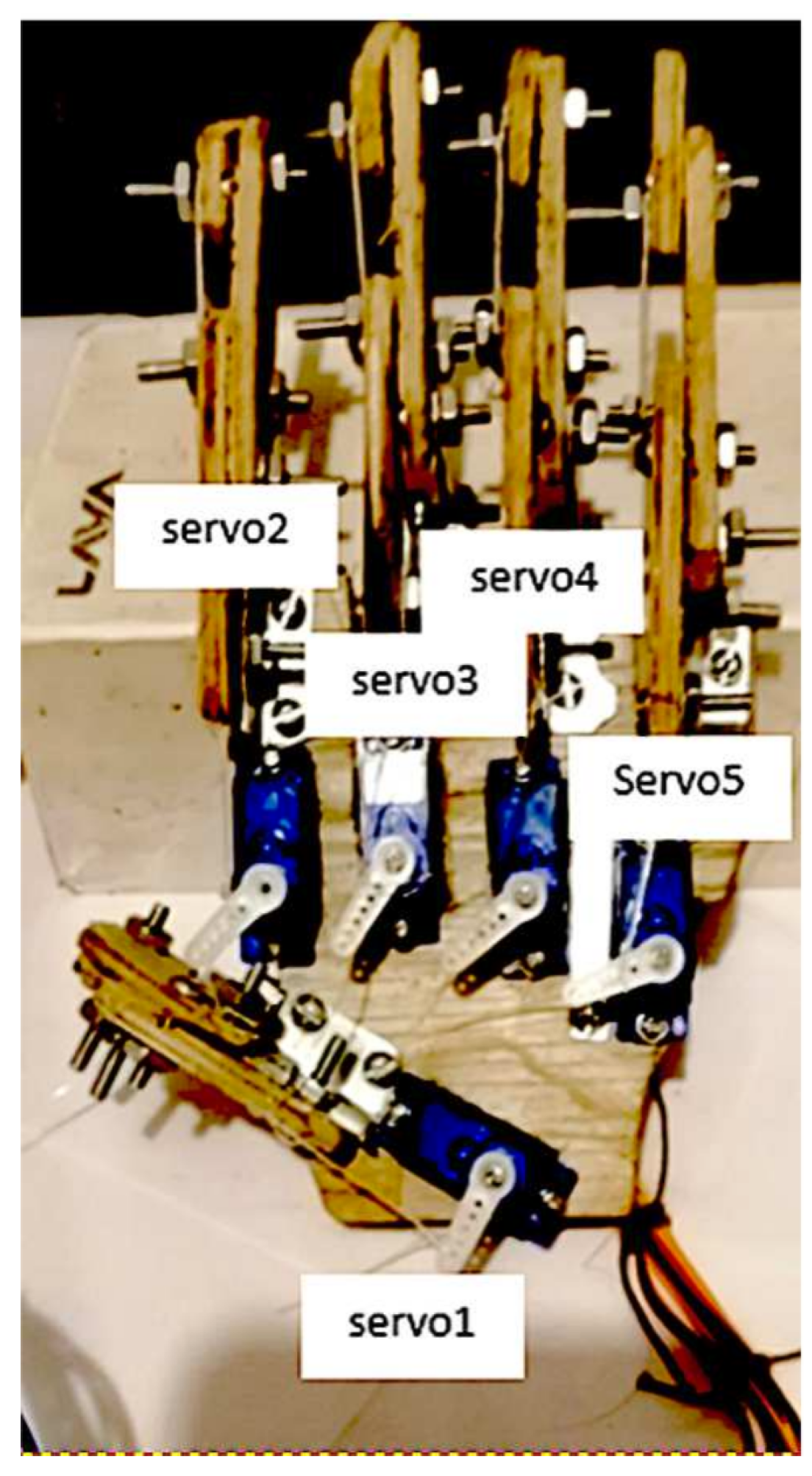
3.4. Experimental Platform
4. Results
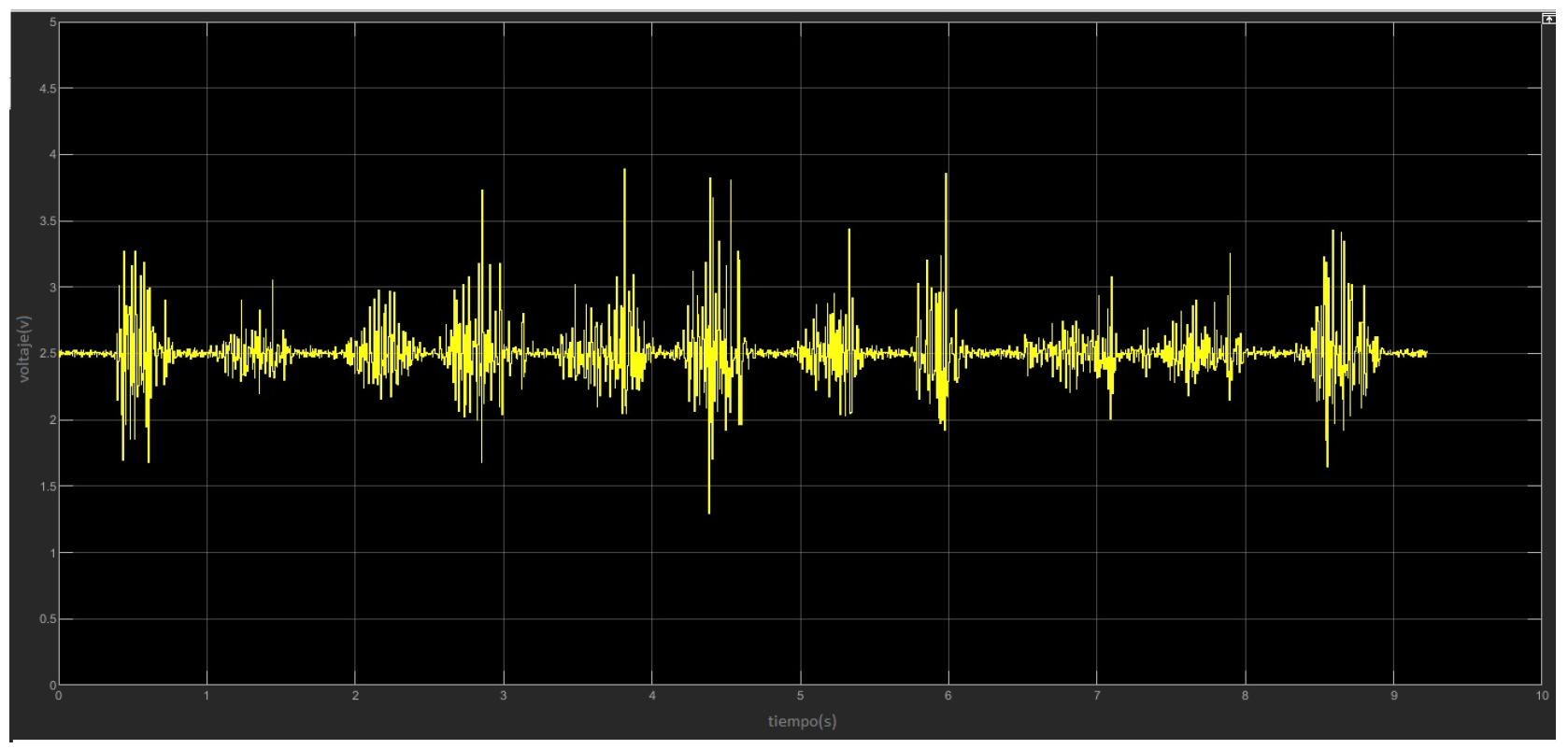
4.1. Implementation and Testing of EMG Sensor with MATLAB
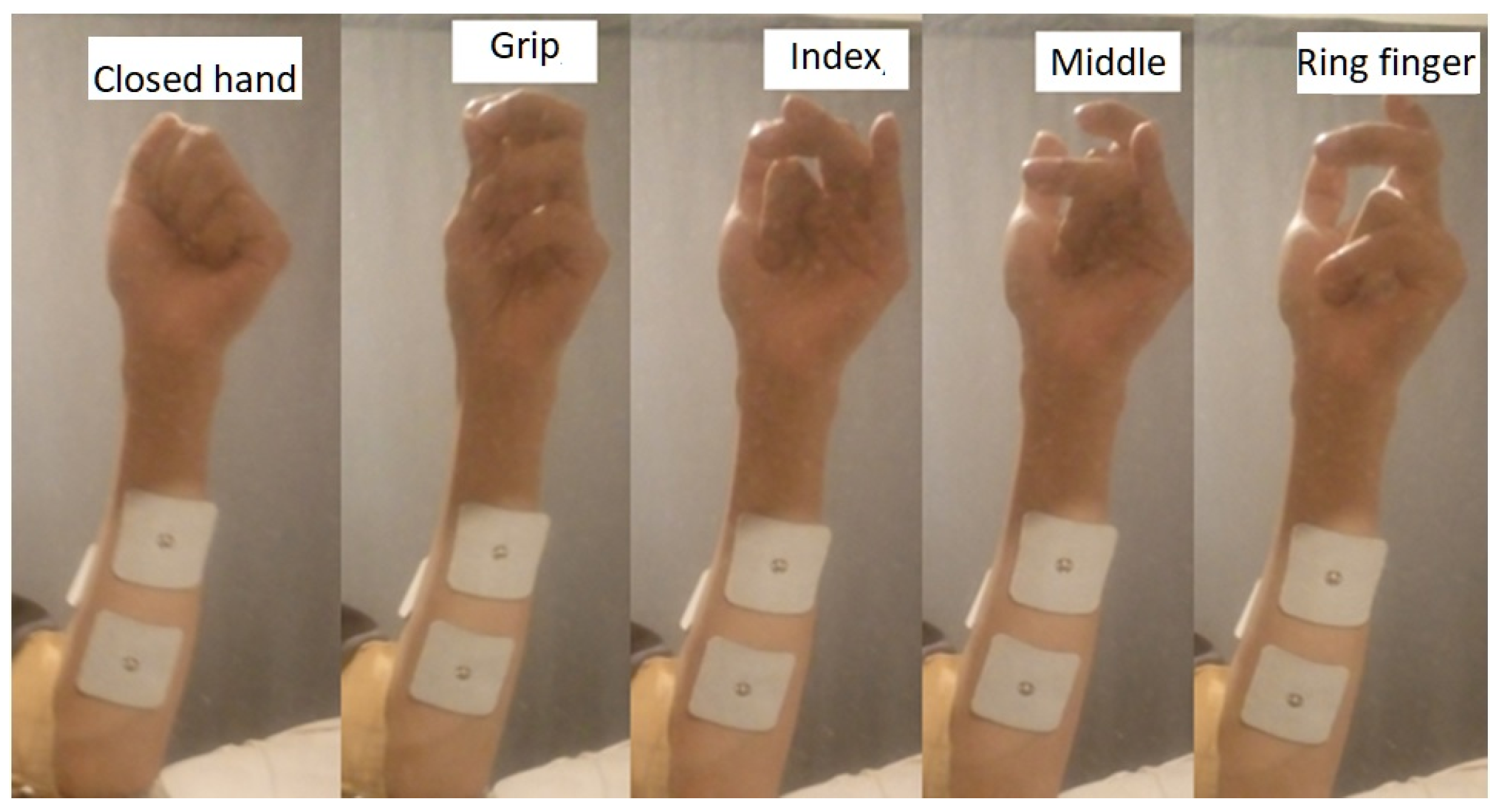
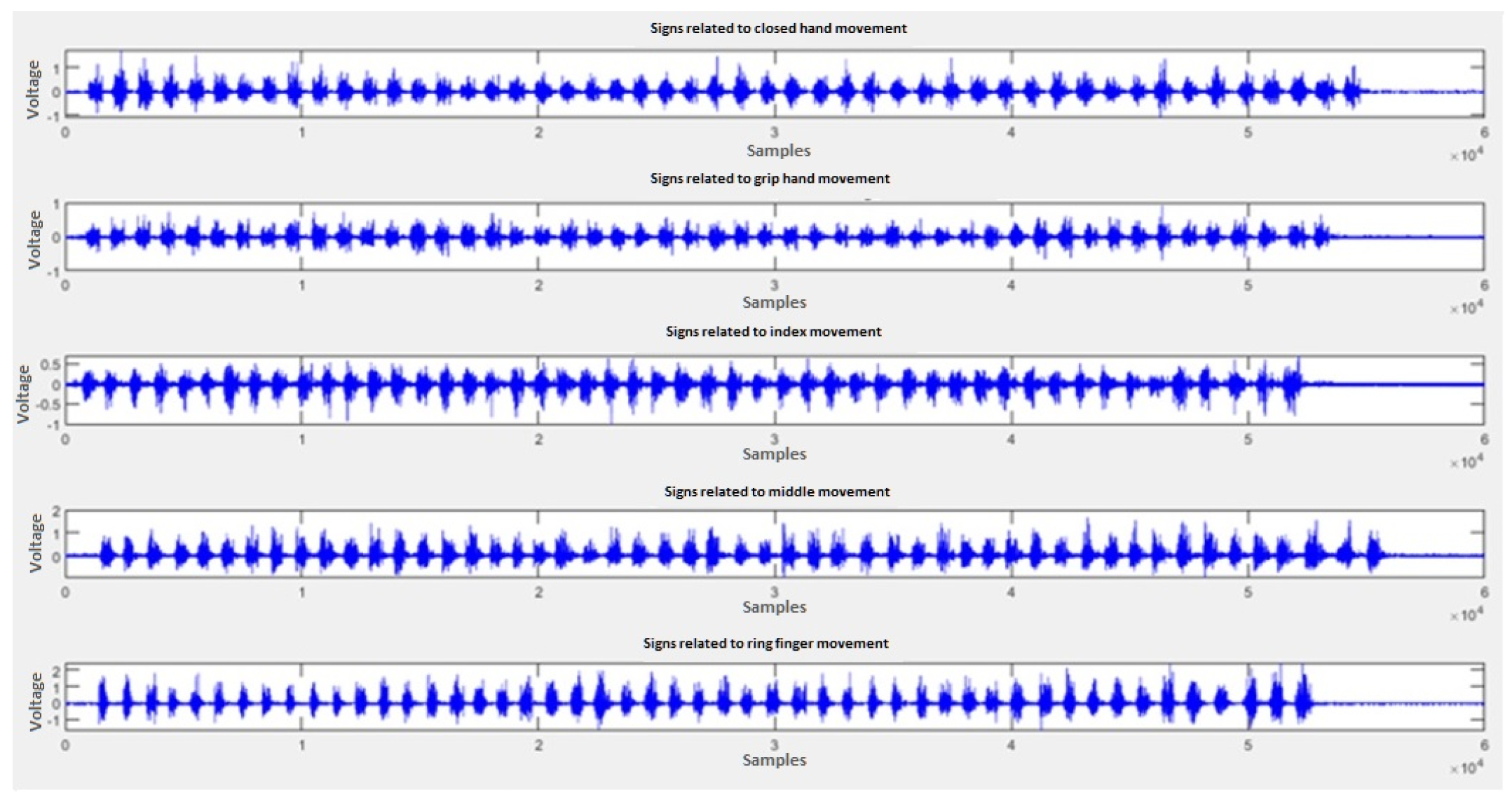
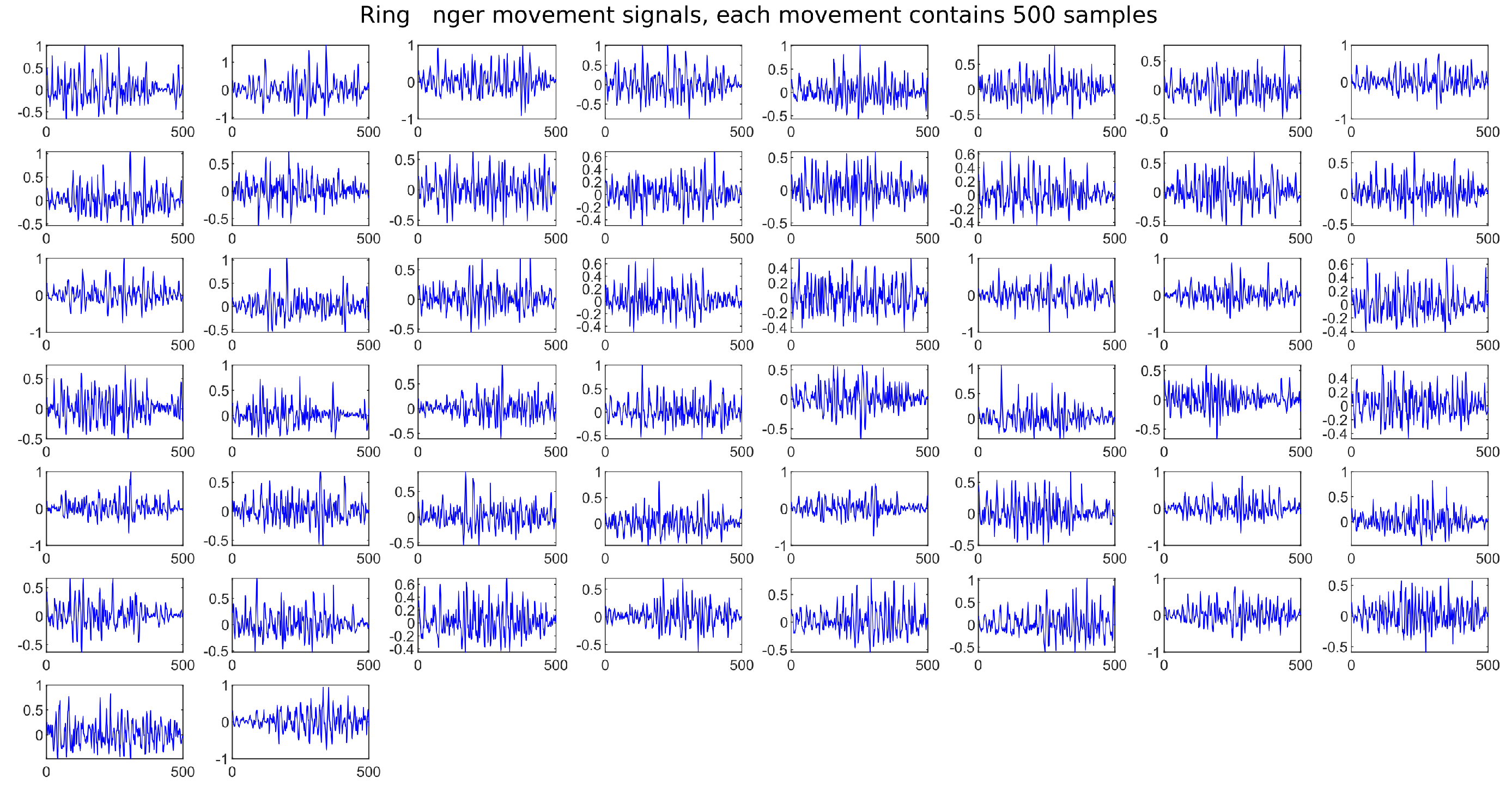
4.2. Data Acquisition
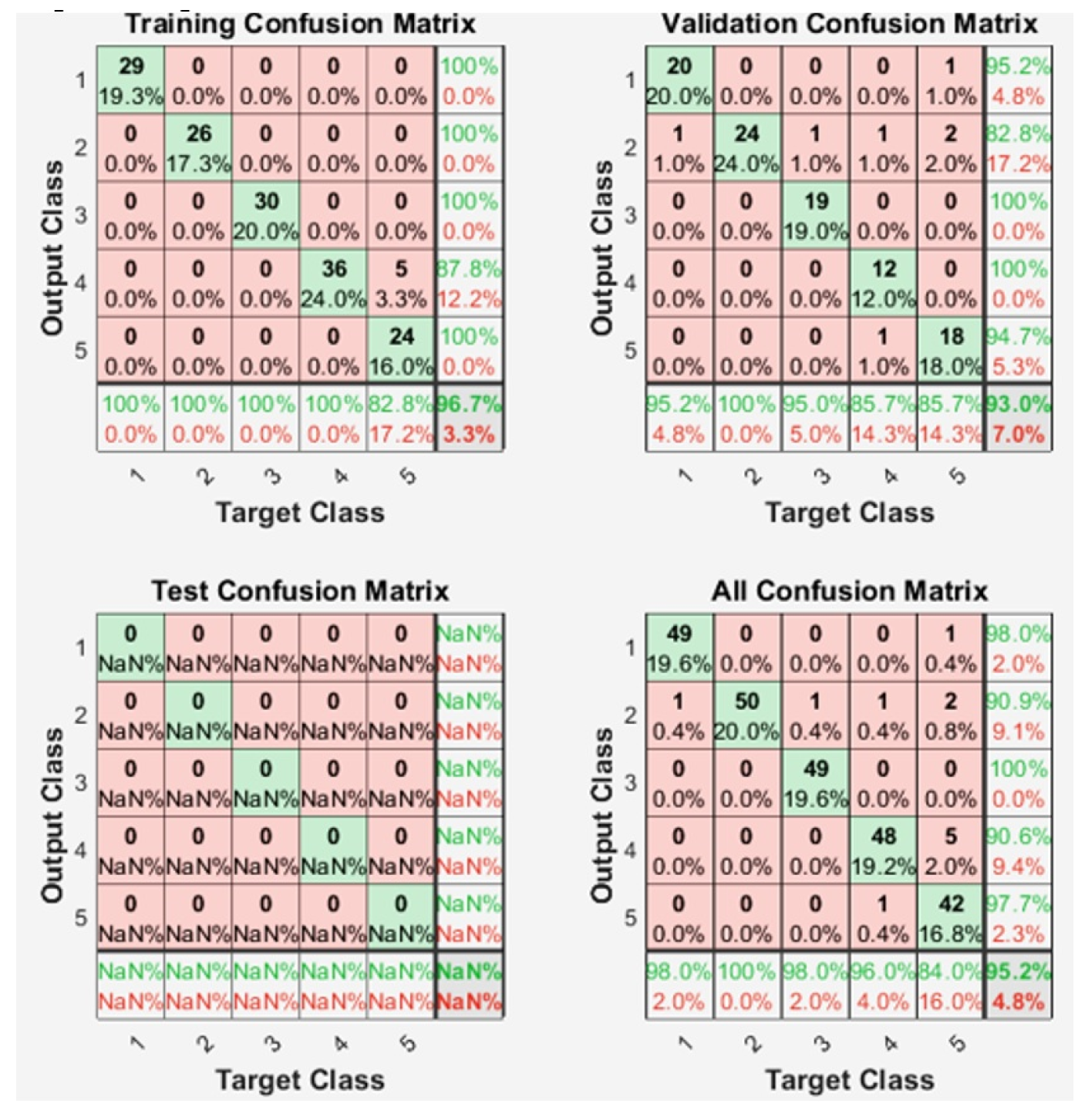
4.3. Training of the Neural Network
4.4. Emulation of a Human Hand
4.5. Online Execution
5. Conclusions
Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, C.L.; Westcott-McCoy, S.; Weaver, M.R.; Haagsma, J.; Kartin, D. Global prevalence of traumatic non-fatal limb amputation. Prosthetics Orthot. Int. 2021, 45, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Kim, J.; André, E. From physiological signals to emotions: Implementing and comparing selected methods for feature extraction and classification. In Proceedings of the 2005 IEEE International Conference on Multimedia and Expo, Amsterdam, The Netherlands, 6 July 2005; IEEE: Piscataway, NJ, USA, 2005; pp. 940–943. [Google Scholar]
- Dubost, G.; Tanaka, A. A wireless, network-based biosensor interface for music. In Proceedings of the 2002 International Computer Music Conference (ICMC 2002), Gothenburg, Sweden, 16–21 September 2002. [Google Scholar]
- Tröster, G. The agenda of wearable healthcare. Yearb. Med. Inform. 2005, 14, 125–138. [Google Scholar] [CrossRef]
- Crawford, B.; Miller, K.; Shenoy, P.; Rao, R. Real-time classification of electromyographic signals for robotic control. In Proceedings of the 20th National Conference on Artificial Intelligenc, Online, 9 July 2005; Volume 2, pp. 523–528. [Google Scholar]
- Fistre, J.; Tanaka, A. RealTime EMG Gesture Recognition for Consumer Electronics Device Control; Sony CSL Paris Open House 10/2002: Paris, France, 2002. [Google Scholar]
- Costanza, E.; Perdomo, A.; Inverso, S.A.; Allen, R. EMG as a subtle input interface for mobile computing. In Proceedings of the International Conference on Mobile Human-Computer Interaction, Online, 26 June–1 July 2004; Springer: Berlin/Heidelberg, Germany, 2004; pp. 426–430. [Google Scholar]
- Khanna, A.; Muthukumaraswamy, S.A. Cost-effective system for the classification of muscular intent using surface electromyography and artificial neural networks. In Proceedings of the 2017 International Conference of Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 20–22 April 2017; IEEE: Piscataway, NJ, USA, 2017; Volume 2, pp. 366–371. [Google Scholar]
- Song, M.S.; Kang, S.G.; Lee, K.T.; Kim, J. Wireless, skin-mountable EMG sensor for human–machine interface application. Micromachines 2019, 10, 879. [Google Scholar] [CrossRef]
- Toledo-Pérez, D.C.; Rodríguez-Reséndiz, J.; Gómez-Loenzo, R.A.; Jauregui-Correa, J. Support vector machine-based EMG signal classification techniques: A review. Appl. Sci. 2019, 9, 4402. [Google Scholar] [CrossRef]
- Sangjun, O.; Mallipeddi, R.; Lee, M. Real Time Hand Gesture Recognition Using Random Forest and Linear Discriminant Analysis. In Proceedings of the 3rd International Conference on Human-Agent Interaction, Daegu, Korea, 21–24 October 2015; pp. 279–282. [Google Scholar]
- Benalcázar, M.E.; Jaramillo, A.G.; Zea, A.; Páez, A.; Andaluz, V.H. Hand gesture recognition using machine learning and the Myo armband. In Proceedings of the 2017 25th European Signal Processing Conference (EUSIPCO), Kos Island, Greece, 28 August–2 September 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1040–1044. [Google Scholar]
- Haider, I.; Shahbaz, M.; Abdullah, M.; Nazim, M. Feature extraction for identification of extension and flexion movement of wrist using EMG signals. In Proceedings of the 2015 IEEE 28th Canadian Conference on Electrical and Computer Engineering (CCECE), Halifax, NS, Canada, 3–6 May 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 792–795. [Google Scholar]
- Joshi, A.; Monnier, C.; Betke, M.; Sclaroff, S. Comparing random forest approaches to segmenting and classifying gestures. Image Vis. Comput. 2017, 58, 86–95. [Google Scholar] [CrossRef]
- Khezri, M.; Jahed, M.; Sadati, N. Neuro-fuzzy surface EMG pattern recognition for multifunctional hand prosthesis control. In Proceedings of the 2007 IEEE International Symposium on Industrial Electronics, Vigo, Spain, 4–7 June 2007; IEEE: Pisactaway, NJ, USA, 2007; pp. 269–274. [Google Scholar]
- Chan, F.H.; Yang, Y.S.; Lam, F.; Zhang, Y.T.; Parker, P.A. Fuzzy EMG classification for prosthesis control. IEEE Trans. Rehabil. Eng. 2000, 8, 305–311. [Google Scholar] [CrossRef]
- Gini, G.; Arvetti, M.; Somlai, I.; Folgheraiter, M. Acquisition and analysis of EMG signals to recognize multiple hand movements for prosthetic applications. Appl. Bionics Biomech. 2012, 9, 145–155. [Google Scholar] [CrossRef]
- Raurale, S. Acquisition of EMG signals to recognize multiple hand gestures for prosthesis robotic hand—A review. Int. J. Curr. Eng. Technol. 2014, 4, 65–70. [Google Scholar]
- Oweis, R.J.; Rihani, R.; Alkhawaja, A. ANN-based EMG classification for myoelectric control. Int. J. Med. Eng. Inform. 2014, 6, 365–380. [Google Scholar] [CrossRef]
- Hudgins, B.; Parker, P.; Scott, R.N. A new strategy for multifunction myoelectric control. IEEE Trans. Biomed. Eng. 1993, 40, 82–94. [Google Scholar] [CrossRef]
- Rastogi, N.; Mehra, R. Analysis of butterworth and chebyshev filters for ECG denoising using wavelets. IOSR J. Electron. Commun. Eng. (IOSR-JECE) 2013, 6, 37–44. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Misal, S.B.; Mishra, A.; Murugkar, A. Designing of stepped impedance Butterworth and Chebyshev filters for wireless communication. In Proceedings of the 2017 IEEE Applied Electromagnetics Conference (AEMC), Aurangabad, India, 19–22 December 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–2. [Google Scholar]
- Li, J.; Zhang, Z.; Zhu, X.; Zhao, Y.; Ma, Y.; Zang, J.; Li, B.; Cao, X.; Xue, C. Automatic Classification Framework of Tongue Feature Based on Convolutional Neural Networks. Micromachines 2022, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, M.A.B.; Johnson, C. Object detection at level crossing using deep learning. Micromachines 2020, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Egmont-Petersen, M.; de Ridder, D.; Handels, H. Image processing with neural networks—A review. Pattern Recognit. 2002, 35, 2279–2301. [Google Scholar] [CrossRef]
- Barreda-Luna, A.A.; Rodríguez-Reséndiz, J.; Flores Rangel, A.; Rodríguez-Abreo, O. Neural Network and Spatial Model to Estimate Sustainable Transport Demand in an Extensive Metropolitan Area. Sustainability 2022, 14, 4872. [Google Scholar] [CrossRef]
- Rodríguez-Abreo, O.; Rodríguez-Reséndiz, J.; Fuentes-Silva, C.; Hernández-Alvarado, R.; Falcón, M.D.C.P.T. Self-tuning neural network PID with dynamic response control. IEEE Access 2021, 9, 65206–65215. [Google Scholar] [CrossRef]
- Cabrera-Rufino, M.A.; Ramos-Arreguín, J.M.; Rodríguez-Reséndiz, J.; Gorrostieta-Hurtado, E.; Aceves-Fernandez, M.A. Implementation of ANN-Based Auto-Adjustable for a Pneumatic Servo System Embedded on FPGA. Micromachines 2022, 13, 890. [Google Scholar] [CrossRef]
- Sanchez-Reyes, L.M.; Rodriguez-Resendiz, J.; Salazar-Colores, S.; Avecilla-Ramírez, G.N.; Pérez-Soto, G.I. A High-accuracy mathematical morphology and multilayer perceptron-based approach for melanoma detection. Appl. Sci. 2020, 10, 1098. [Google Scholar] [CrossRef]
- Gao, W.; Fang, G.; Zhao, D.; Chen, Y. A Chinese sign language recognition system based on SOFM/SRN/HMM. Pattern Recognit. 2004, 37, 2389–2402. [Google Scholar] [CrossRef]
- Liu, K.; Kehtarnavaz, N. Real-time robust vision-based hand gesture recognition using stereo images. J. Real-Time Image Process. 2016, 11, 201–209. [Google Scholar] [CrossRef]
- Benalcázar, M.E.; Motoche, C.; Zea, J.A.; Jaramillo, A.G.; Anchundia, C.E.; Zambrano, P.; Segura, M.; Palacios, F.B.; Pérez, M. Real-time hand gesture recognition using the Myo armband and muscle activity detection. In Proceedings of the 2017 IEEE Second Ecuador Technical Chapters Meeting (ETCM), Salinas, Ecuador, 16–20 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Toledo-Perez, D.; Rodríguez-Reséndiz, J.; Gómez-Loenzo, R.A. A study of computing zero crossing methods and an improved proposal for EMG signals. IEEE Access 2020, 8, 8783–8790. [Google Scholar] [CrossRef]
- Jamal, M.Z. Signal acquisition using surface EMG and circuit design considerations for robotic prosthesis. Comput. Intell. Electromyogr. —Anal. —Perspect. Curr. Appl. Future Chall. 2012, 18, 427–448. [Google Scholar]
- Ahsan, M.R.; Ibrahimy, M.I.; Khalifa, O.O. Electromygraphy (EMG) signal based hand gesture recognition using artificial neural network (ANN). In Proceedings of the 2011 4th International Conference on Mechatronics (ICOM), Kuala Lumpur, Malaysia, 17–19 May 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1–6. [Google Scholar]
- Asif, A.R.; Waris, A.; Gilani, S.O.; Jamil, M.; Ashraf, H.; Shafique, M.; Niazi, I.K. Performance evaluation of convolutional neural network for hand gesture recognition using EMG. Sensors 2020, 20, 1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, K.; Qian, J.; Zhang, L. Real-time surface EMG pattern recognition for hand gestures based on an artificial neural network. Sensors 2019, 19, 3170. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimy, M.I.; Ahsan, M.R.; Khalifa, O.O. Design and optimization of Levenberg-Marquardt based neural network classifier for EMG signals to identify hand motions. Meas. Sci. Rev. 2013, 13, 142–151. [Google Scholar] [CrossRef]
- Abu, M.A.; Rosleesham, S.; Suboh, M.Z.; Yid, M.S.M.; Kornain, Z.; Jamaluddin, N.F. Classification of EMG signal for multiple hand gestures based on neural network. Indones. J. Electr. Eng. Comput. Sci. 2020, 17, 256–263. [Google Scholar]
- Lee, K.H.; Min, J.Y.; Byun, S. Electromyogram-based classification of hand and finger gestures using artificial neural networks. Sensors 2021, 22, 225. [Google Scholar] [CrossRef]
- Manimegalai, E.H.S.D.P. Hand gesture recognition based on EMG signals using ANN. Int. J. Comput. Appl. 2013, 3, 31–39. [Google Scholar]
- Poliakov, A.; Pakhaliuk, V. Fast User Activity Phase Recognition for the Safety of Transfemoral Prosthesis Control. Facta Univ. Ser. Mech. Eng. 2020, 18, 135–151. [Google Scholar] [CrossRef]



| 20 Characteristics Vector |
|---|
| Mean absolute value |
| Zero crossing |
| Slope sign change |
| Waveform length |
| Autoregressive model AR (5): first, second, and third coefficients |
| Fast Fourier Transform: Energy, periodogram, and mean power |
| Short time Fourier transform, Window 1: Energy, spectrogram, and mean power |
| Short time Fourier transform, Window 2: Energy, spectrogram, and mean power |
| Short time Fourier transform, Window 3: Energy, spectrogram, and mean power |
| Wavelet transform approximation: Coefficient 1 and variance; coefficient 2 and variance; coefficient 3 and variance; coefficient 4 and variance; coefficient 5, variance |
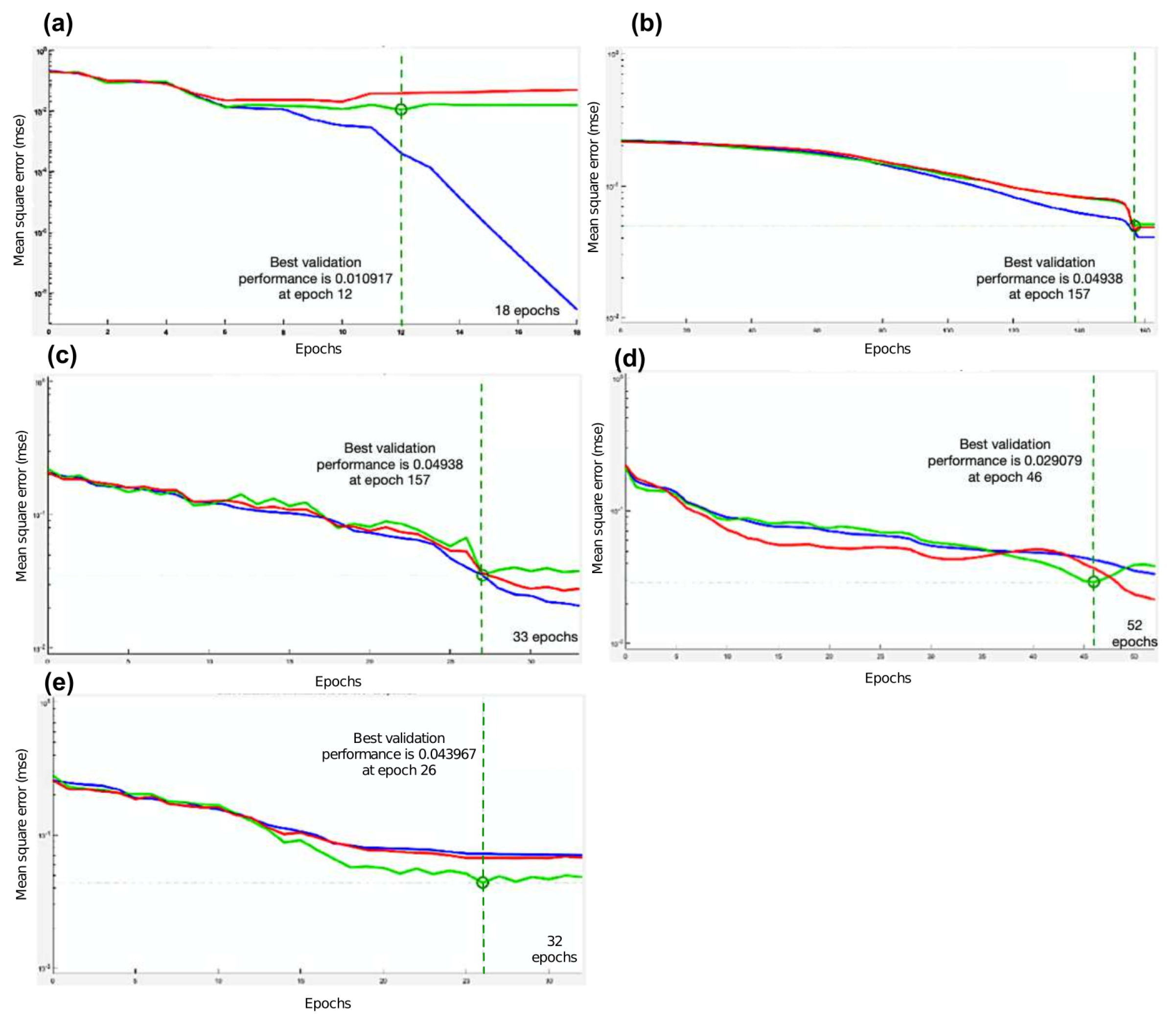
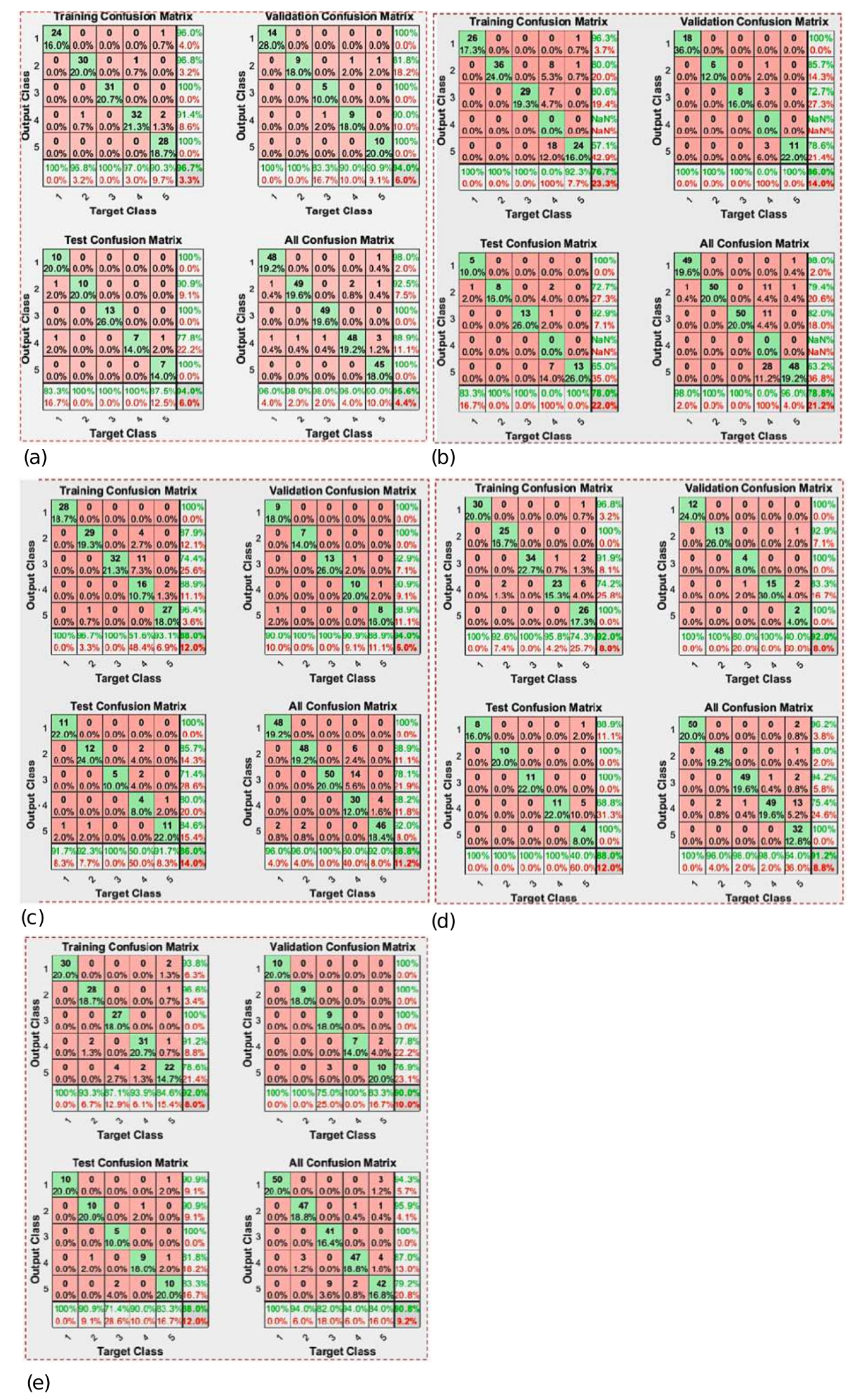
| TF from MATLAB | Algorithm | Best Performance of Mean Square Error | Epoch of the Best Validated Performance | Accuracy |
|---|---|---|---|---|
| trainlm | Levenberg-Marquardt | 0.010917 | 12 | 95.6% |
| trainbfg | BFGS Quasi-Newton | 0.04938 | 157 | 78.8% |
| traincgp | Polak-Ribiére Conjugate Gradient | 0.035416 | 27 | 88.8% |
| trainoss | One Step Secant | 0.029079 | 48 | 91.2% |
| traingdx | Variable Learning Rate Backpropagation | 0.043967 | 26 | 90.8% |
| Reference | Acquisition Device | Hand Gestures | Algorithm Characterization | Extracted Features or Training Data | Acquisition Frequency | Success Average Rate |
|---|---|---|---|---|---|---|
| [35] | Biopac MP100 data acquisition system | Left, right, up, down | Back propagation with Levenberg-Marquardt | 7 features: MAV, RMS, VAR, SD, ZC, SSC, WT, and db2 with 4 levels | 1 kHz | Between 88.4% and 89.2% |
| [36] | ° | Close hand, flex hand, extend the hand and fine grip | Convolutional NN | Images | 8 kHz | 83.7% ± 13.5%, 71.2% ± 20.2%, 82.6% ± 13.9% and 74.6% ± 15 % for each movement |
| [37] | MYO armband | Fist, Wave In, Wave Out, Fingers Spread, and Double Tap | feedforward ANN | 5 features: MAV, SSC, WL, RMS, and Hjorth parameter (HP) | ° | 98.7 % |
| [38] | BIOPAC-MP100C data acquisition system | Left, Right, Up and Down | Levenberg-Marquardt and scaled conjugate gradient based back-propagation | 7 features: Moving Average, RMS, VAR, SD, ZC, SSC and WL. | 1 kHz | 88.4% |
| [39] | MyoWare Muscle Sensor (AT-04-001) | Cylindrical Grasp, Supination (Twist Left), Pronation (Twist Right), Resting Hand and Open Hand | Back propagation used Lavenberg-Marquardt | 4 features: MAV, median, WL and RMS | ° | 80% |
| [40] | MyoWare Muscle Sensor | Rock, scissors, paper, one, three, four, good, okay, finger gun, and rest | Multilayer perceptron, support vector machine (SVM), random forest (RF), and a logistic regression (LR). | 6 features: RMS, VAR, MAV, SSC, ZC, and WL. | ° | Maximum 94%, depending on the methodology |
| [41] | labVIEW and Biokit | Open and closing, thumb flexion, index flexion, middle and ring finger flexion. | Levenberg-Marquardt | 6 features: MAV, RMS, MNF, ZC, SSC and SD. | 1 kHz | ° |
| Our project | Own | Closed hand, grip, index, middle, ring. | Back propagation with Lavenberg-Marquardt | 20 features: explained above. | 1 kHz | 95.2% and 93% in real test. |
| Movement | Online Execution Time [s] | Offline Execution Time [s] |
|---|---|---|
| Trial 1: Index | 1.095399 | 2.352354 |
| Trial 2: Grip | 0.874528 | 2.300595 |
| Trial 3: Closed hand | 1.015186 | 2.208586 |
| Trial 4: Ring finger | 1.043519 | 2.342219 |
| Trial 5: Middle | 0.953655 | 2.816689 |
| Trial 6: Ring finger | 1.008239 | 2.234787 |
| Trial 7: Closed hand | 1.069840 | 2.185594 |
| Trial 8: Grip | 1.003771 | 2.324529 |
| Trial 9: Index | 0.893527 | 2.065108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinoco-Varela, D.; Ferrer-Varela, J.A.; Cruz-Morales, R.D.; Padilla-García, E.A. Design and Implementation of a Prosthesis System Controlled by Electromyographic Signals Means, Characterized with Artificial Neural Networks. Micromachines 2022, 13, 1681. https://doi.org/10.3390/mi13101681
Tinoco-Varela D, Ferrer-Varela JA, Cruz-Morales RD, Padilla-García EA. Design and Implementation of a Prosthesis System Controlled by Electromyographic Signals Means, Characterized with Artificial Neural Networks. Micromachines. 2022; 13(10):1681. https://doi.org/10.3390/mi13101681
Chicago/Turabian StyleTinoco-Varela, David, Jose Amado Ferrer-Varela, Raúl Dalí Cruz-Morales, and Erick Axel Padilla-García. 2022. "Design and Implementation of a Prosthesis System Controlled by Electromyographic Signals Means, Characterized with Artificial Neural Networks" Micromachines 13, no. 10: 1681. https://doi.org/10.3390/mi13101681
APA StyleTinoco-Varela, D., Ferrer-Varela, J. A., Cruz-Morales, R. D., & Padilla-García, E. A. (2022). Design and Implementation of a Prosthesis System Controlled by Electromyographic Signals Means, Characterized with Artificial Neural Networks. Micromachines, 13(10), 1681. https://doi.org/10.3390/mi13101681






