Contactless Discharge-Driven Method for Separation of Oil-Water Mixtures
Abstract
:1. Introduction
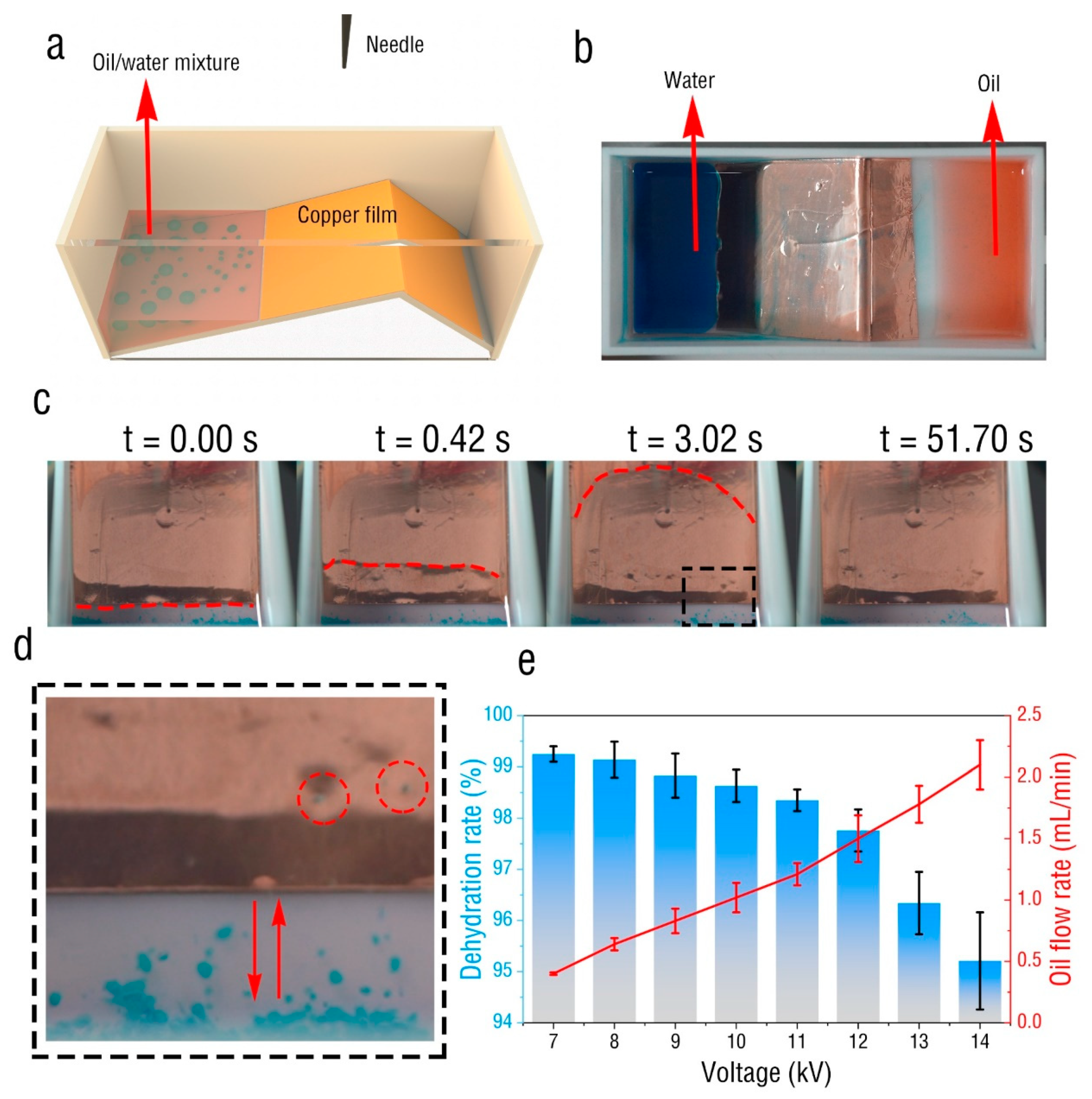
2. Experimental Setup
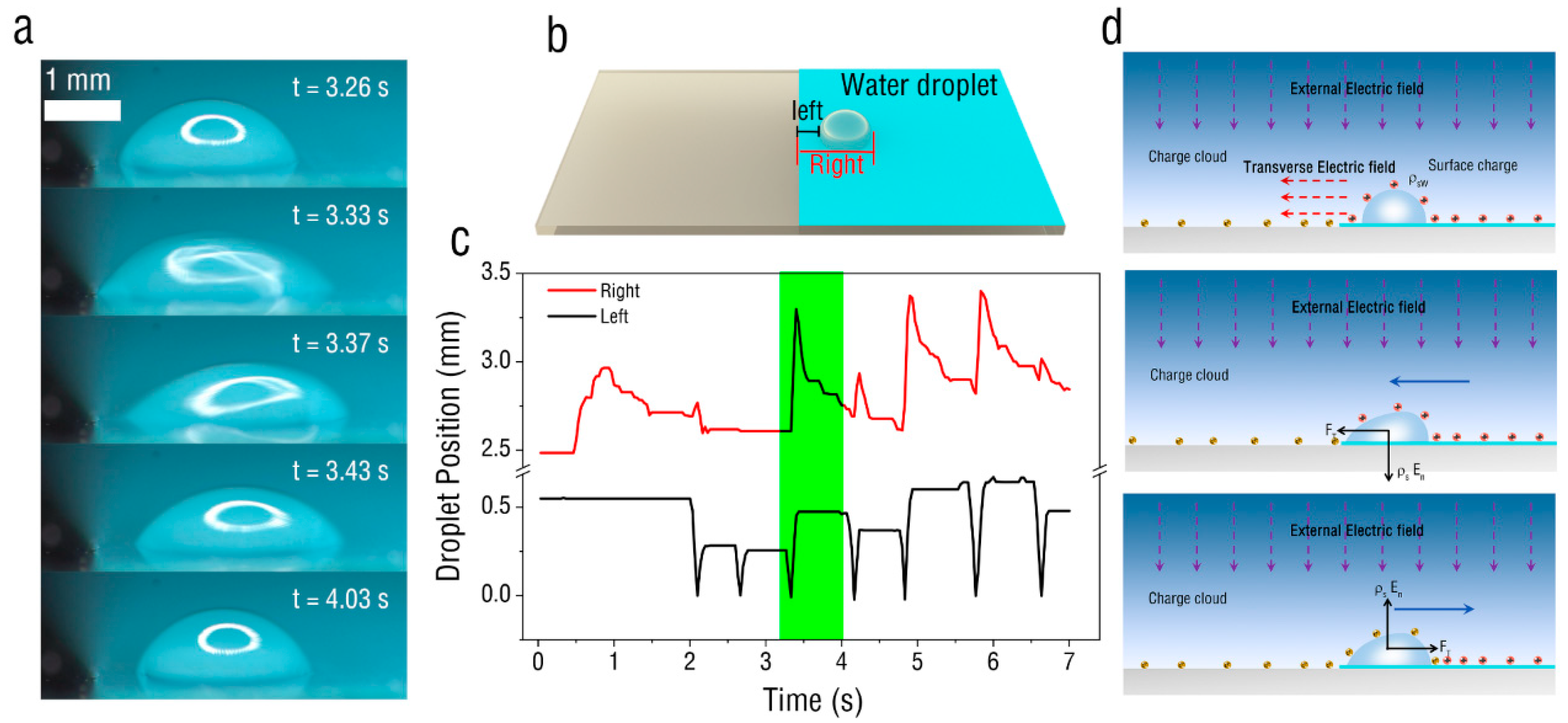
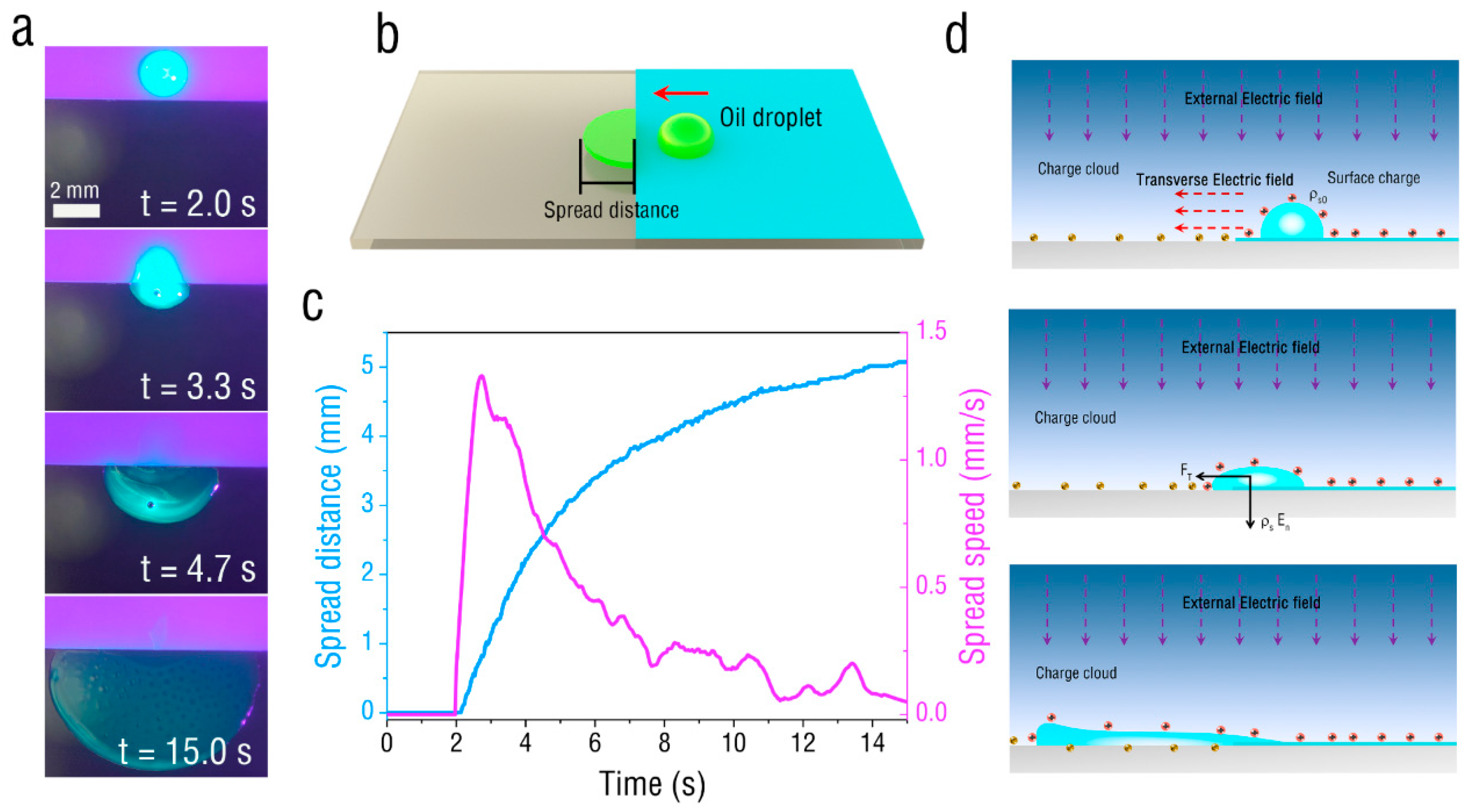
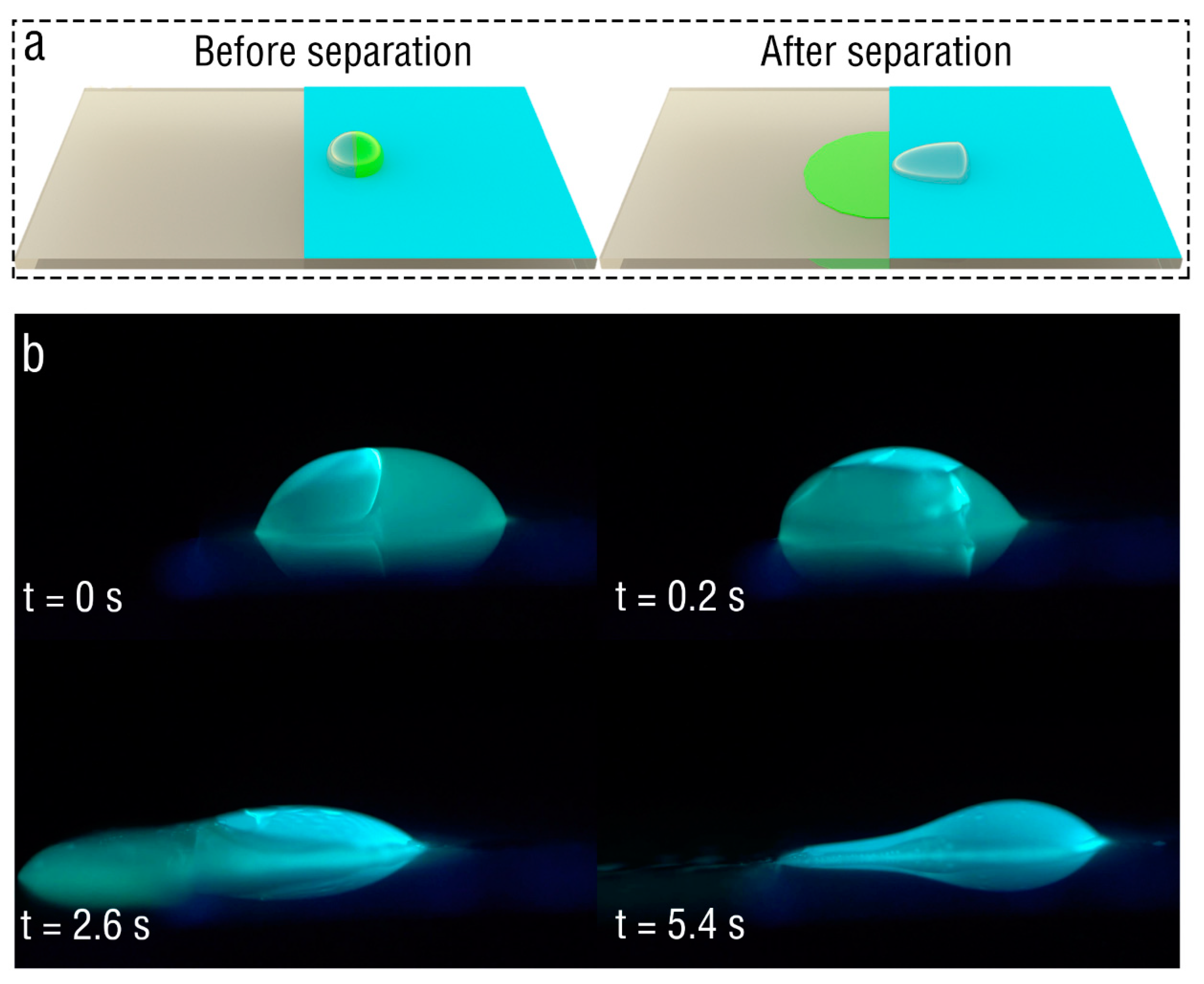
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Mei, H.; Fang, H. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar]
- Flörke, M.; Kynast, E.; Bärlund, I.; Eisner, S.; Wimmer, F.; Alcamo, J. Domestic and industrial water uses of the past 60 years as a mirror of socio-economic development: A global simulation study. Glob. Environ. Chang. 2013, 23, 144–156. [Google Scholar] [CrossRef]
- Martini, S.; Setiawati, M. Technology for Treating Oily Wastewater Derived from Various Industries: A Review Paper. Chem. J. Tek. Kim. 2021, 7, 106–116. [Google Scholar] [CrossRef]
- Ahmad, T.; Belwal, T.; Li, L.; Ramola, S.; Aadil, R.M.; Xu, Y.; Zisheng, L. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci. Technol. 2020, 99, 21–33. [Google Scholar] [CrossRef]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mubarak, N.M. Development of fruit waste derived bio-adsorbents for wastewater treatment: A review. J. Hazard. Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Frising, T.; Noïk, C.; Dalmazzone, C. The Liquid/Liquid Sedimentation Process: From Droplet Coalescence to Technologically Enhanced Water/Oil Emulsion Gravity Separators: A Review. J. Dispers. Sci. Technol. 2006, 27, 1035–1057. [Google Scholar] [CrossRef]
- Liu, G.-h.; Ye, Z.; Tong, K.; Zhang, Y.-h. Biotreatment of heavy oil wastewater by combined upflow anaerobic sludge blanket and immobilized biological aerated filter in a pilot-scale test. Biochem. Eng. J. 2013, 72, 48–53. [Google Scholar] [CrossRef]
- Binner, E.R.; Robinson, J.P.; Silvester, S.A.; Kingman, S.W.; Lester, E.H. Investigation into the mechanisms by which microwave heating enhances separation of water-in-oil emulsions. Fuel 2014, 116, 516–521. [Google Scholar] [CrossRef]
- Binner, E.R.; Robinson, J.P.; Kingman, S.W.; Lester, E.H.; Azzopardi, B.J.; Dimitrakis, G.; Briggs, J. Separation of oil/water emulsions in continuous flow using microwave heating. Energy Fuels 2013, 27, 3173–3178. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, Q.; Shen, L.; Duan, B. Reaction mechanism and kinetics of oil-bearing sewage disposal by supercritical water oxidation. J. Xi’an Jiaotong Univ. 2006, 40, 115–119. [Google Scholar]
- Moosai, R.; Dawe, R.A. Gas attachment of oil droplets for gas flotation for oily wastewater cleanup. Sep. Purif. Technol. 2003, 33, 303–314. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Manoj, S.; Luong, J.H.T.; Gedanken, A. Facile ultrasonic preparation of a polypyrrole membrane as an absorbent for efficient oil-water separation and as an antimicrobial agent. Ultrason. Sonochem. 2021, 78, 105746. [Google Scholar] [CrossRef]
- Alshaafi, E.A.; Prakash, A.; Mercer, S.M. Ultrasonic technique for tracking oil-water emulsion layer in separation vessels. J. Pet. Sci. Eng. 2020, 184, 106591. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil–water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef]
- Bai, Z.S.; Wang, H.L. Crude Oil Desalting Using Hydrocyclones. Chem. Eng. Res. Des. 2007, 85, 1586–1590. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Chen, Y.; Quan, Z.; Song, W.; Wang, Z.; Li, B.; Mu, Z.; Niu, S.; Zhang, J.; Han, Z.; Ren, L. Hierarchically structured biomimetic membrane with mechanically/chemically durability and special wettability for highly efficient oil–water separation. Sep. Purif. Technol. 2022, 300, 121860. [Google Scholar] [CrossRef]
- Ni, T.; Kong, L.; Xie, Z.; Lin, J.; Zhao, S. Flux vs. permeability: How to effectively evaluate mass transfer performance of membranes in oil-water separation. J. Water Process Eng. 2022, 49, 103119. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Huo, J.; Fang, Y.; Yang, Q.; Bian, H.; Li, W.; Wei, Y.; Dai, Y.; Hou, X. Green, biodegradable, underwater superoleophobic wood sheet for efficient oil/water separation. ACS Omega 2018, 3, 1395–1402. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, J.; Zhang, Y.; Wu, C. Molecular dynamics simulation on influence of temperature effect on electro-coalescence behavior of nano-droplets. J. Dispers. Sci. Technol. 2018, 39, 1537–1543. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M.; Sharif, A.O.; Williams, T.J. Electrostatic enhancement of coalescence of water droplets in oil: A review of the current understanding. Chem. Eng. J. 2001, 84, 173–192. [Google Scholar] [CrossRef]
- McAdie, A.G. The Disposition of Smoke. Mon. Weather Rev. 1910, 38, 1107–1108. [Google Scholar] [CrossRef]
- Gong, H.; Li, W.; Zhang, X.; Peng, Y.; Yu, B.; Mou, Y. Effects of droplet dynamic characteristics on the separation performance of a demulsification and dewatering device coupling electric and centrifugal fields. Sep. Purif. Technol. 2021, 257, 117905. [Google Scholar] [CrossRef]
- He, X.; Wang, S.-L.; Yang, Y.-R.; Wang, X.-D.; Chen, J.-Q. Electro-coalescence of two charged droplets under pulsed direct current electric fields with various waveforms: A molecular dynamics study. J. Mol. Liq. 2020, 312, 113429. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Ghadiri, M.; Buckley, M. Electro-coalescence of water drops in oils under pulsatile electric fields. Chem. Eng. Sci. 2014, 120, 130–142. [Google Scholar] [CrossRef]
- Hjartnes, T.N.; Mhatre, S.; Gao, B.; Sørland, G.H.; Simon, S.; Sjöblom, J. Demulsification of crude oil emulsions tracked by pulsed field gradient NMR. Part II: Influence of chemical demulsifiers in external AC electric field. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124188. [Google Scholar] [CrossRef]
- Li, B.; Dou, X.; Yu, K.; Zhang, W.; Xu, H.; Sun, Z.; Wang, Z.; Wang, J. Electrocoalescence of water droplet trains in sunflower oil under the coupling of Non-uniform electric and Laminar flow fields. Chem. Eng. Sci. 2022, 248, 117158. [Google Scholar] [CrossRef]
- Shahbaznezhad, M.; Dehghanghadikolaei, A.; Sojoudi, H. Optimum Operating Frequency for Electrocoalescence Induced by Pulsed Corona Discharge. ACS Omega 2020, 5, 31000–31010. [Google Scholar] [CrossRef]
- Shahbaznezhad, M.; Dehghanghadikolaei, A.; Sojoudi, H. Contactless Method for Electrocoalescence of Water in Oil. ACS Omega 2021, 6, 14298–14308. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Asai, Y.; Yamanoi, A.; Seki, Y.; Wiranata, A.; Minaminosono, A. Fluidic rolling robot using voltage-driven oscillating liquid. Smart Mater. Struct. 2022, 31, 105006. [Google Scholar] [CrossRef]
- Mao, Z.; Yoshida, K.; Kim, J.-w. Active sorting of droplets by using an ECF (Electro-conjugate Fluid) micropump. Sens. Actuators A Phys. 2020, 303, 111702. [Google Scholar] [CrossRef]
- Yang, D.; Feng, Y.; Wang, B.; Liu, Y.; Zheng, Y.; Sun, X.; Peng, J.; Feng, M.; Wang, D. An asymmetric AC electric field of triboelectric nanogenerator for efficient water/oil emulsion separation. Nano Energy 2021, 90, 106641. [Google Scholar] [CrossRef]
- Ohyama, R.; Kumeta, M.; Ueda, A.; Watson, A.; Chang, J.-S. A fundamental characteristic and image analysis of liquid flow in an AW type EHD pump. J. Vis. 2005, 8, 339–346. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Cui, X.; Hu, Z.; Lu, S.; Wang, C.; Tang, J. Contactless Discharge-Driven Method for Separation of Oil-Water Mixtures. Micromachines 2022, 13, 1652. https://doi.org/10.3390/mi13101652
Tang Q, Cui X, Hu Z, Lu S, Wang C, Tang J. Contactless Discharge-Driven Method for Separation of Oil-Water Mixtures. Micromachines. 2022; 13(10):1652. https://doi.org/10.3390/mi13101652
Chicago/Turabian StyleTang, Qiang, Xiaxia Cui, Zhibin Hu, Shaotian Lu, Chengjun Wang, and Jau Tang. 2022. "Contactless Discharge-Driven Method for Separation of Oil-Water Mixtures" Micromachines 13, no. 10: 1652. https://doi.org/10.3390/mi13101652
APA StyleTang, Q., Cui, X., Hu, Z., Lu, S., Wang, C., & Tang, J. (2022). Contactless Discharge-Driven Method for Separation of Oil-Water Mixtures. Micromachines, 13(10), 1652. https://doi.org/10.3390/mi13101652





