Feasibility of Implant Strain Measurement for Assessing Mandible Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
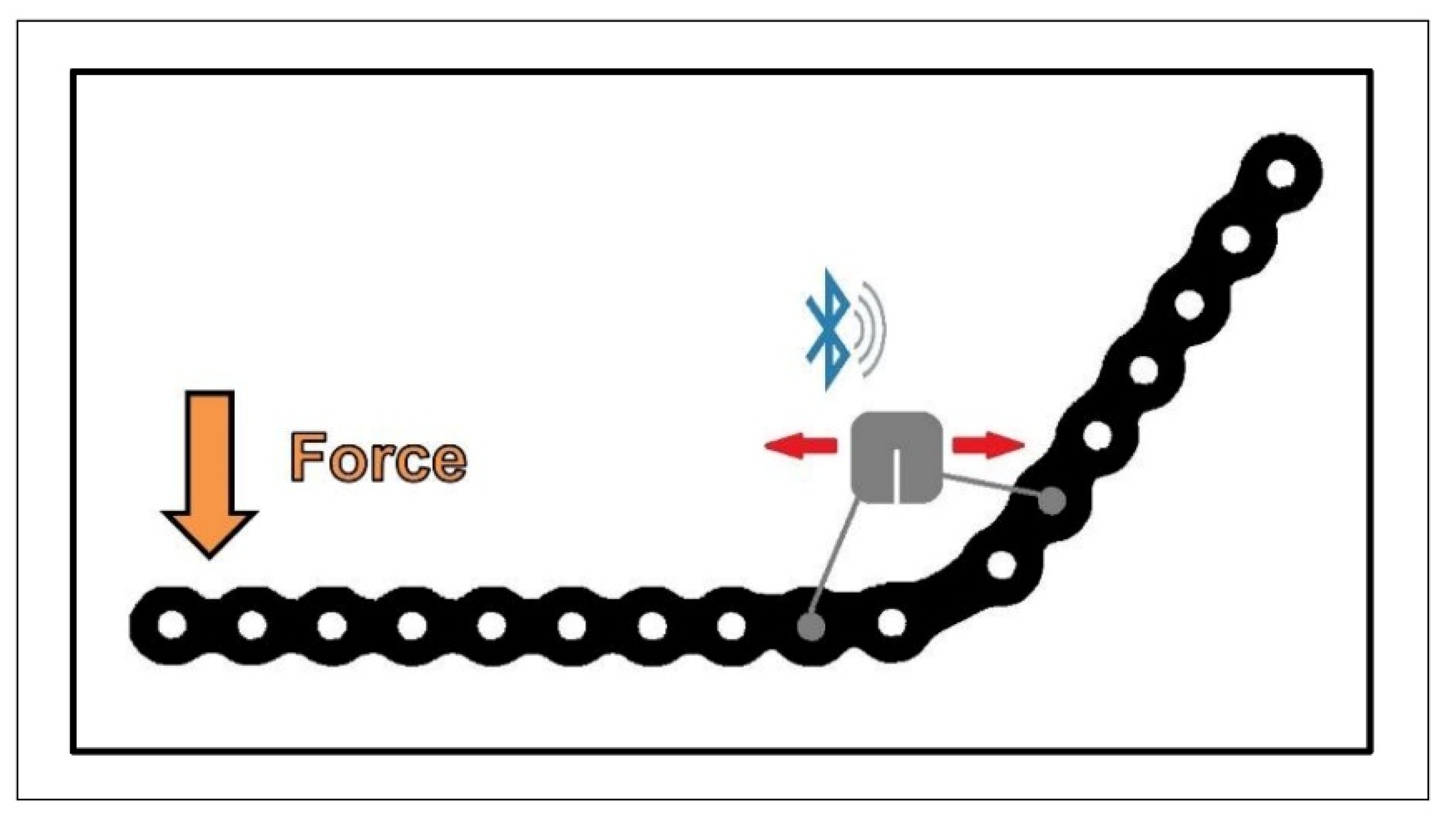
2.1. Strain Sensor
2.2. Data Processing
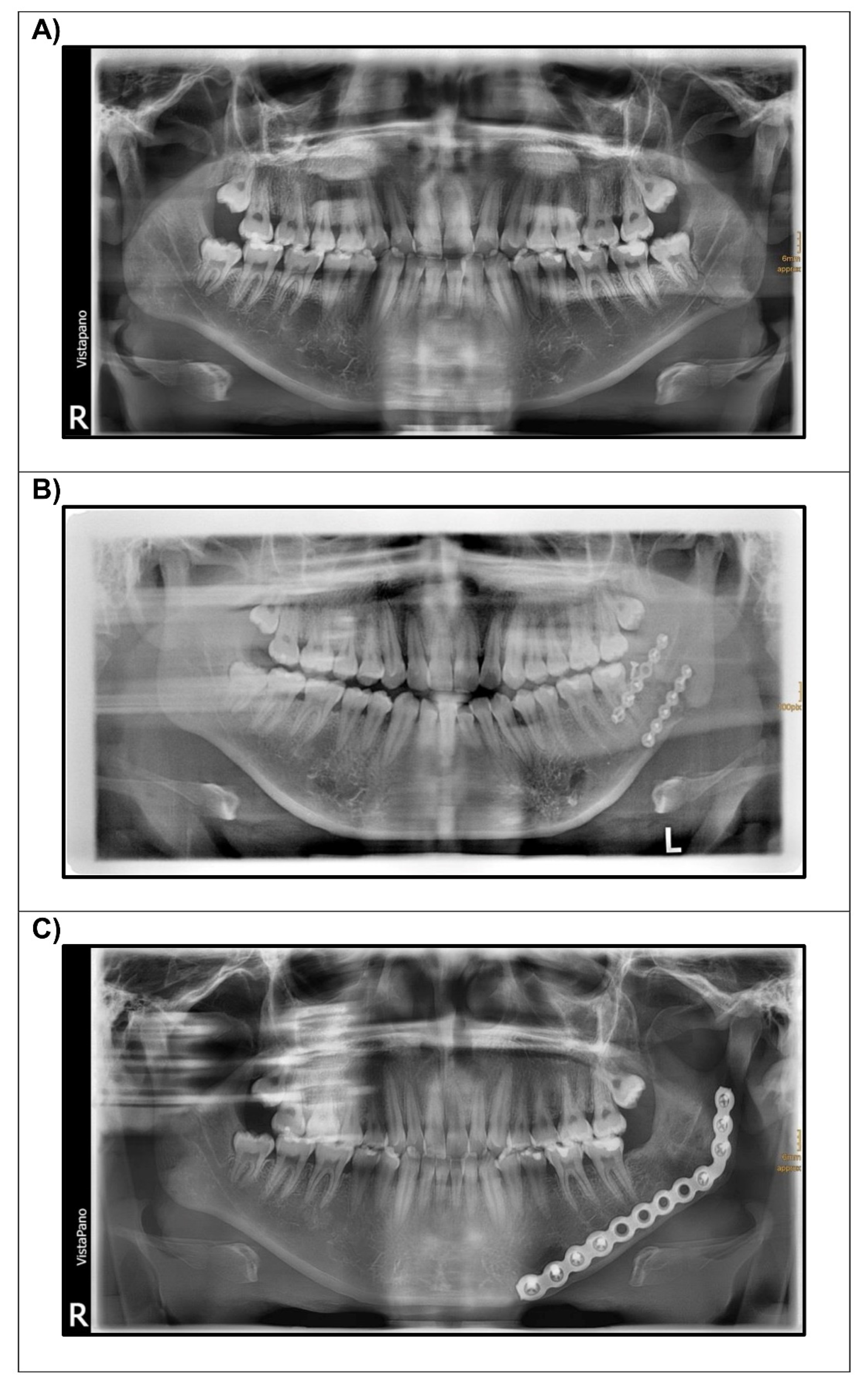
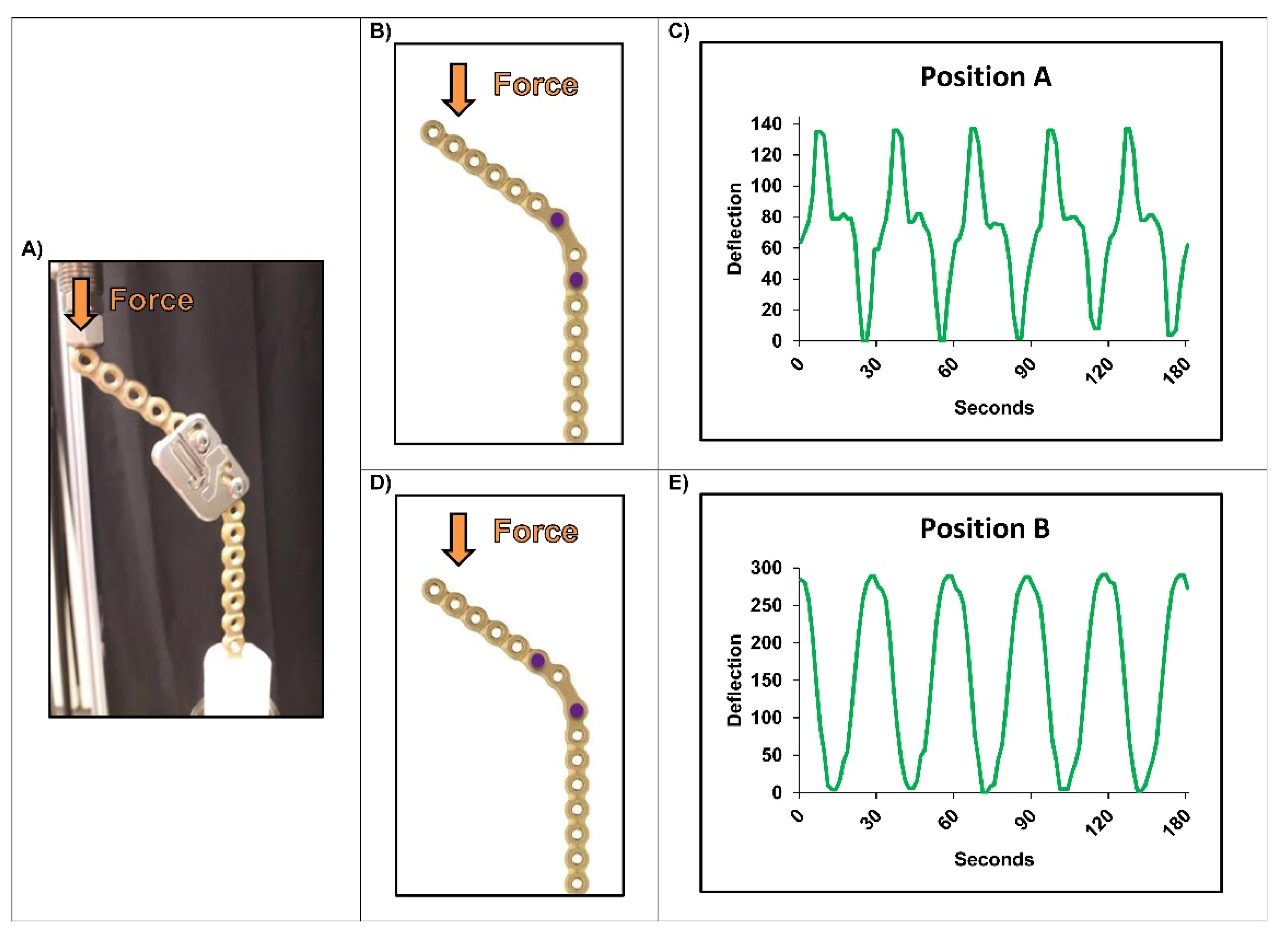
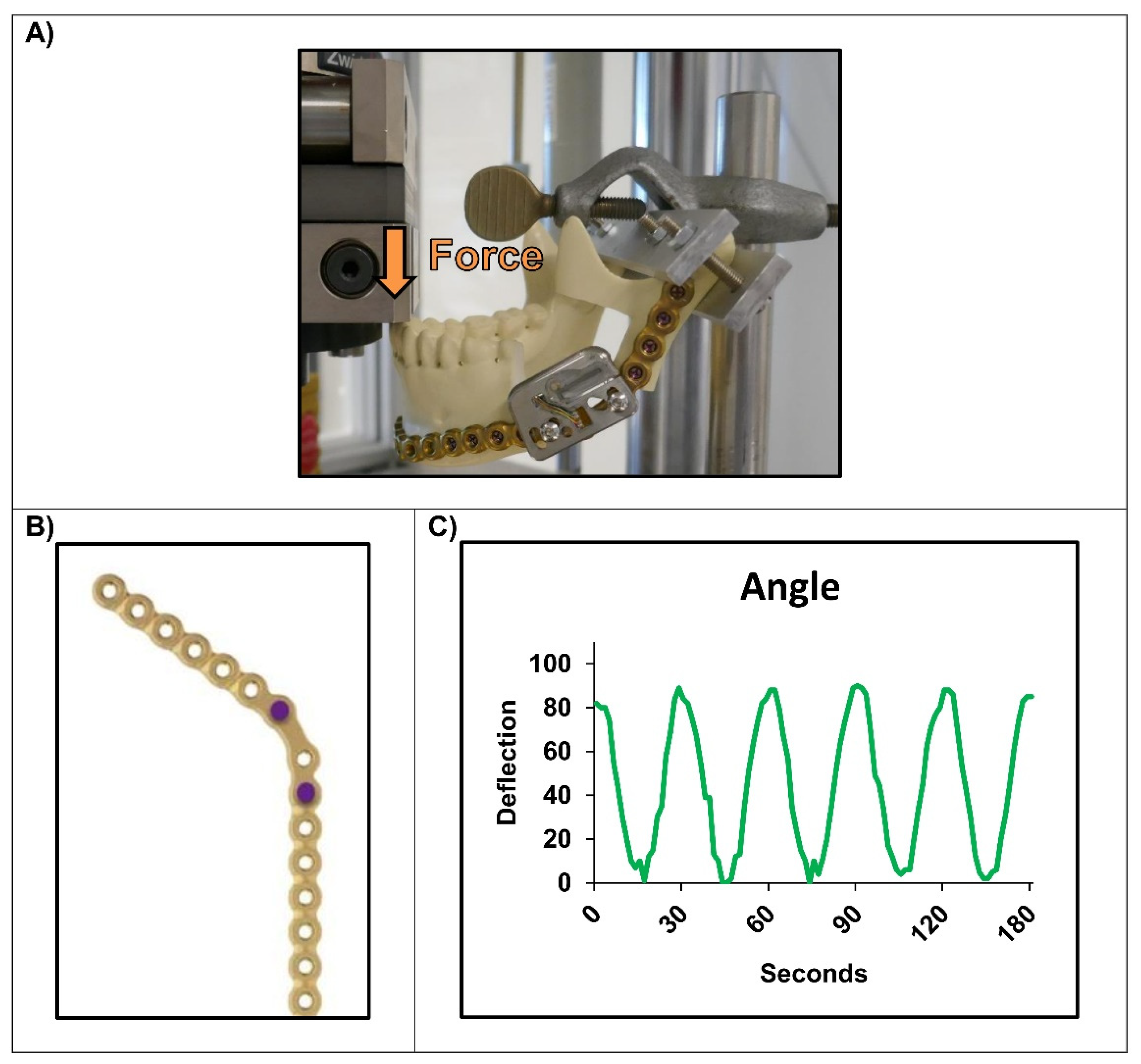
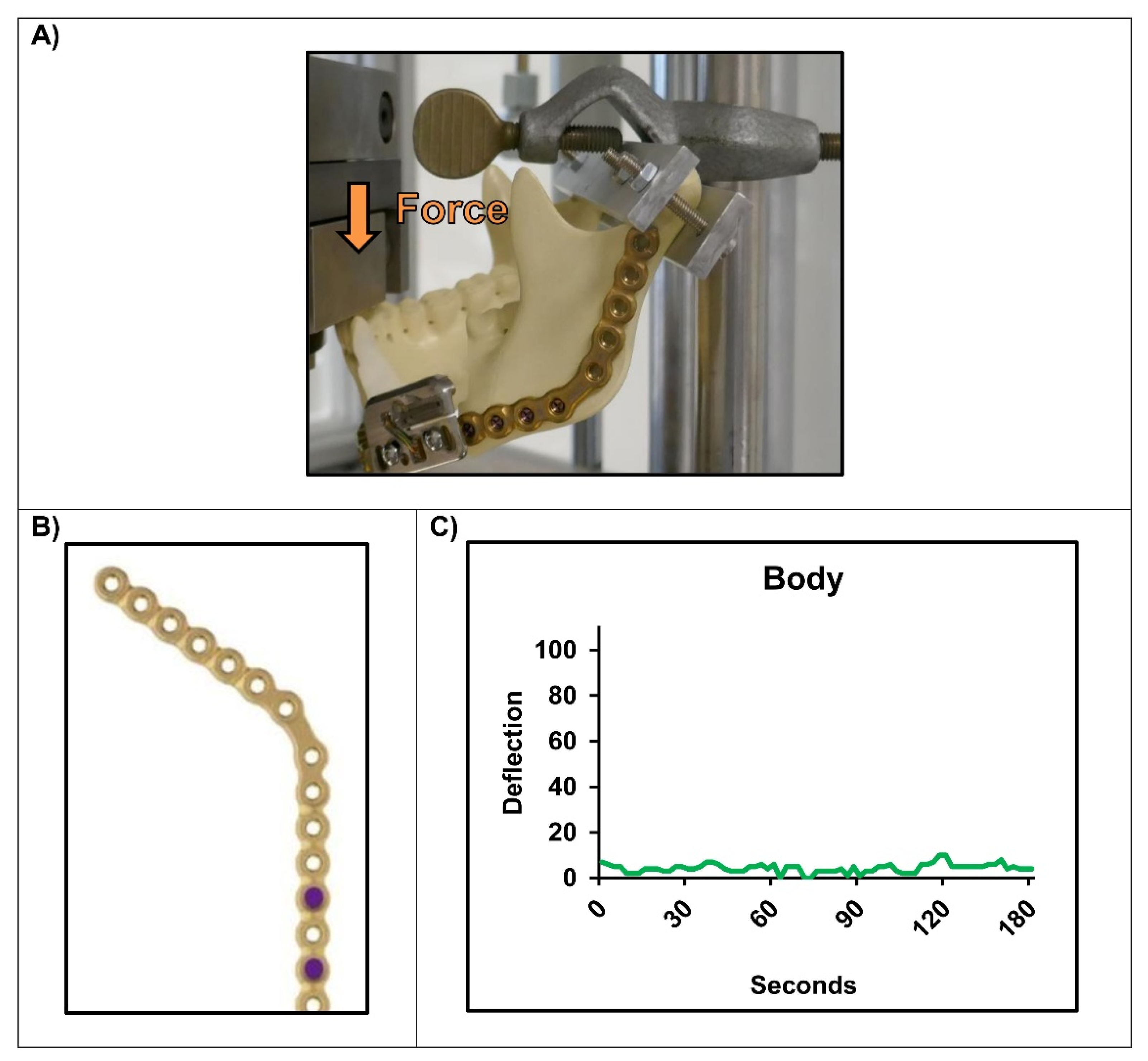
2.3. Experimental Setups
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathog, R.H.; Boies, L.R. Nonunion of the Mandible. Laryngoscope 1976, 86, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.J.; Schwope, R.B.; Theler, J.M. Postoperative CT of the Mandible Following Trauma: Review of Normal Appearances and Common Complications. Acad. Radiol. 2019, 26, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Lowe, D.; Kanatas, A.; Schache, A. Mandibular Reconstruction with Vascularised Bone Flaps: A Systematic Review over 25 Years. Br. J. Oral Maxillofac. Surg. 2017, 55, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, M.J.L.; David, M.C.; Batstone, M.D. A Prospective Study Examining the Effects of Treatment Timing in the Management of Mandible Fractures. Int. J. Oral Maxillofac. Surg. 2018, 47, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Rothweiler, R.; Bayer, J.; Zwingmann, J.; Suedkamp, N.P.; Kalbhenn, J.; Schmelzeisen, R.; Gutwald, R. Outcome and Complications after Treatment of Facial Fractures at Different Times in Polytrauma Patients. J. Craniomaxillofac. Surg. 2018, 46, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.A.; Souza, G.M.; Silva de Rezende, V.; Al-Sharani, H.M.; Douglas-de-Oliveira, D.W.; Galvão, E.L.; Falci, S.G.M. Effect of Third Molars in the Line of Mandibular Angle Fractures on Postoperative Complications: Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Jain, A.; Datarkar, A. Comparison of Single versus Two Non-Compression Miniplates in the Management of Unfavourable Angle Fracture of the Mandible: A Prospective Randomized Clinical Study. Oral Maxillofac. Surg. 2018, 22, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R. Fixation of Mandibular Angle Fractures: Clinical Studies. Oral Maxillofac. Surg. 2014, 18, 123–152. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Salmon, B.; Codari, M.; Hassan, B.; Bornstein, M.M. Cone Beam Computed Tomography in Implant Dentistry: Recommendations for Clinical Use. BMC Oral Health 2018, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Avery, C.M.E.; Clifford, N.; Niamat, J.; Vaidhyanath, R. Early Detection of Bone Union with Transcutaneous Ultrasound in the Management of Non-Union of the Mandible. Br. J. Oral Maxillofac. Surg. 2011, 49, 661–663. [Google Scholar] [CrossRef]
- Burny, F.; Donkerwolcke, M.; Moulart, F.; Bourgois, R.; Puers, R.; Van Schuylenbergh, K.; Barbosa, M.; Paiva, O.; Rodes, F.; Bégueret, J.B.; et al. Concept, Design and Fabrication of Smart Orthopedic Implants. Med. Eng. Phys. 2000, 22, 469–479. [Google Scholar] [CrossRef]
- Ernst, M.; Richards, R.G.; Windolf, M. Smart Implants in Fracture Care-Only Buzzword or Real Opportunity? Injury 2020, 52, S101–S105. [Google Scholar] [CrossRef] [PubMed]
- Windolf, M.; Heumann, M.; Varjas, V.; Constant, C.; Ernst, M.; Richards, R.G.; Wilke, H.-J.; Benneker, L.M. Continuous Rod Load Monitoring to Assess Spinal Fusion Status–Pilot In Vivo Data in Sheep. Medicina 2022, 58, 899. [Google Scholar] [CrossRef] [PubMed]
- Windolf, M.; Varjas, V.; Gehweiler, D.; Schwyn, R.; Arens, D.; Constant, C.; Zeiter, S.; Richards, R.G.; Ernst, M. Continuous Implant Load Monitoring to Assess Bone Healing Status—Evidence from Animal Testing. Medicina 2022, 58, 858. [Google Scholar] [CrossRef]
- Windolf, M.; Ernst, M.; Schwyn, R.; Perren, S.M.; Mathis, H.; Wilke, M.; Richards, R.G. A Biofeedback System for Continuous Monitoring of Bone Healing. In Proceedings of the BIODEVICES; Sience and Technology Publications, Lda.: Setúbal, Portugal, 2014. [Google Scholar]
- Ernst, M.; Baumgartner, H.; Döbele, S.; Höntzsch, D.; Pohlemann, T.; Windolf, M. Clinical Feasibility of Fracture Healing Assessment through Continuous Monitoring of Implant Load. J. Biomech. 2021, 116, 110188. [Google Scholar] [CrossRef] [PubMed]
- Haug, R.H.; Fattahi, T.T.; Goltz, M. A Biomechanical Evaluation of Mandibular Angle Fracture Plating Techniques. J. Oral Maxillofac. Surg. 2001, 59, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Schupp, W.; Arzdorf, M.; Linke, B.; Gutwald, R. Biomechanical Testing of Different Osteosynthesis Systems for Segmental Resection of the Mandible. J. Oral Maxillofac. Surg. 2007, 65, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Fountain, S.; Windolf, M.; Henkel, J.; Tavakoli, A.; Schuetz, M.A.; Hutmacher, D.W.; Epari, D.R. Monitoring Healing Progression and Characterizing the Mechanical Environment in Preclinical Models for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2016, 22, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Knoll, W.-D.; Gaida, A.; Maurer, P. Analysis of Mechanical Stress in Reconstruction Plates for Bridging Mandibular Angle Defects. J. Craniomaxillofac. Surg. 2006, 34, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Gutwald, R.; Jaeger, R.; Lambers, F.M. Customized Mandibular Reconstruction Plates Improve Mechanical Performance in a Mandibular Reconstruction Model. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 426–435. [Google Scholar] [CrossRef]
- Daegling, D.J.; Hotzman, J.L. Functional significance of cortical bone distribution in anthropoid mandibles: An in vitro assessment of bone strain under combined loads. Am. J. Phys. Anthropol. 2003, 122, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Gröning, F.; Fagan, M.; O’higgins, P. Comparing the Distribution of Strains with the Distribution of Bone Tissue in a Human Mandible: A Finite Element Study. Anat. Rec. 2013, 296, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, P.; Ma, Z.; Li, S.; Gao, X.; Wu, R.; Pan, L.; Shi, Y. Near-Field Communication Sensors. Sensors 2019, 19, 3947. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Y.; Wikner, J.J.; Zötterman, J.; Rydén, L.; Farnebo, S. NFC Powered Implantable Temperature Sensor. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4359–4362. [Google Scholar]
- Lin, R.; Kim, H.-J.; Achavananthadith, S.; Kurt, S.A.; Tan, S.C.C.; Yao, H.; Tee, B.C.K.; Lee, J.K.W.; Ho, J.S. Wireless Battery-Free Body Sensor Networks Using near-Field-Enabled Clothing. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Kinra, P.K.; Jayakumar, K.; Soumithran, C.S.; Michael, M.J.; Passi, D.; Singh, M. Comparative Evaluation of Bite Force Analytical Study Following Mandibular Osteosysthesis Using Three-Dimensional and Conventional Locking Miniplates. Natl. J. Maxillofac. Surg. 2017, 8, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Umesh, S.; Padma, S.; Asokan, S.; Srinivas, T. Fiber Bragg Grating Based Bite Force Measurement. J. Biomech. 2016, 49, 2877–2881. [Google Scholar] [CrossRef]
- Agarwal, M.; Mohammad, S.; Singh, R.K.; Singh, V. Prospective Randomized Clinical Trial Comparing Bite Force in 2-Mm Locking Plates versus 2-Mm Standard Plates in Treatment of Mandibular Fractures. J. Oral Maxillofac. Surg. 2011, 69, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Tatara, A.M.; Kretlow, J.D.; Spicer, P.P.; Lu, S.; Lam, J.; Liu, W.; Cao, Y.; Liu, G.; Jackson, J.D.; Yoo, J.J.; et al. Autologously Generated Tissue-Engineered Bone Flaps for Reconstruction of Large Mandibular Defects in an Ovine Model. Tissue Eng. Part A 2015, 21, 1520–1528. [Google Scholar] [CrossRef]
- Tatara, A.M.; Shah, S.R.; Demian, N.; Ho, T.; Shum, J.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Reconstruction of Large Mandibular Defects Using Autologous Tissues Generated from in Vivo Bioreactors. Acta Biomater. 2016, 45, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Russmueller, G.; Moser, D.; Spassova, E.; Plasenzotti, R.; Poeschl, P.W.; Seemann, R.; Becker, S.; Pirklbauer, K.; Eder-Czembirek, C.; Czembirek, C.; et al. Tricalcium Phosphate-Based Biocomposites for Mandibular Bone Regeneration--A Histological Study in Sheep. J. Craniomaxillofac. Surg. 2015, 43, 696–704. [Google Scholar] [CrossRef]
| Type of Sensor | Active; Strain-Gauge Wheatstone Half-Bridge |
| Battery | Li-Ion |
| Housing | Titanium grade 5 |
| Sampling rate | 10 Hz |
| Transmission protocol | Bluetooth |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rothweiler, R.M.; Zankovic, S.; Brandenburg, L.S.; Fuessinger, M.-A.; Gross, C.; Voss, P.J.; Metzger, M.-C. Feasibility of Implant Strain Measurement for Assessing Mandible Bone Regeneration. Micromachines 2022, 13, 1602. https://doi.org/10.3390/mi13101602
Rothweiler RM, Zankovic S, Brandenburg LS, Fuessinger M-A, Gross C, Voss PJ, Metzger M-C. Feasibility of Implant Strain Measurement for Assessing Mandible Bone Regeneration. Micromachines. 2022; 13(10):1602. https://doi.org/10.3390/mi13101602
Chicago/Turabian StyleRothweiler, René Marcel, Sergej Zankovic, Leonard Simon Brandenburg, Marc-Anton Fuessinger, Christian Gross, Pit Jacob Voss, and Marc-Christian Metzger. 2022. "Feasibility of Implant Strain Measurement for Assessing Mandible Bone Regeneration" Micromachines 13, no. 10: 1602. https://doi.org/10.3390/mi13101602
APA StyleRothweiler, R. M., Zankovic, S., Brandenburg, L. S., Fuessinger, M.-A., Gross, C., Voss, P. J., & Metzger, M.-C. (2022). Feasibility of Implant Strain Measurement for Assessing Mandible Bone Regeneration. Micromachines, 13(10), 1602. https://doi.org/10.3390/mi13101602





