Microfluidic Device for Droplet Pairing by Combining Droplet Railing and Floating Trap Arrays
Abstract
:1. Introduction
2. Materials and Methods
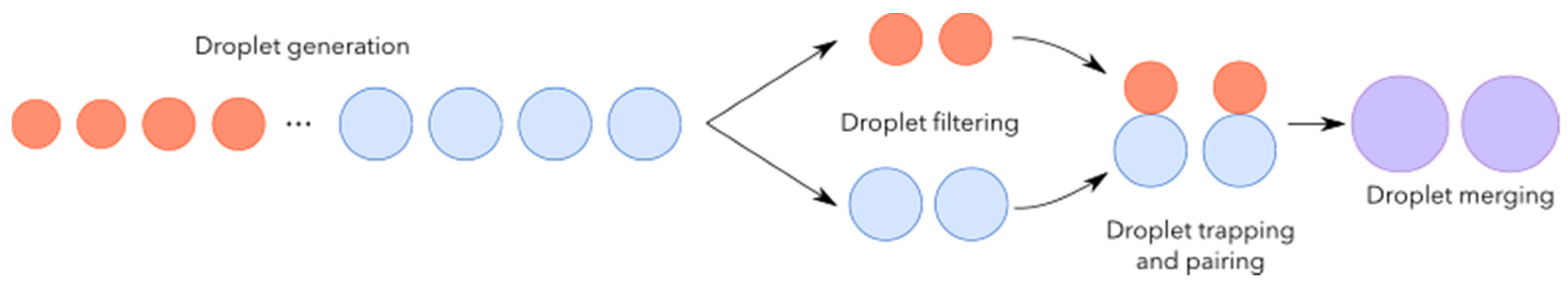
2.1. Working Principle
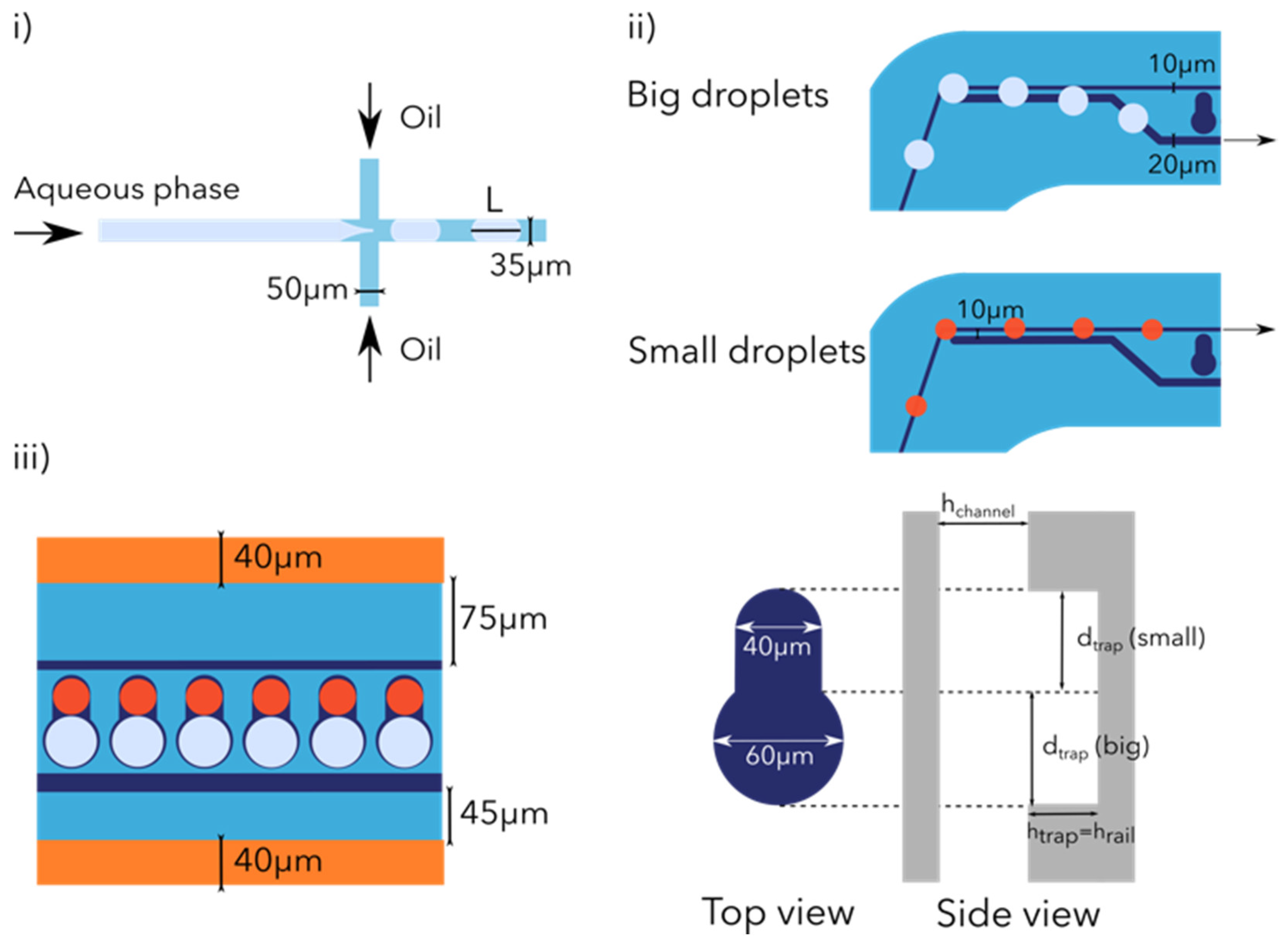
2.1.1. Droplet Generation
2.1.2. Droplet Railing
Droplet Size Filtering
Droplet Trapping and Pairing
Droplet Merging
2.2. Design Droplet Device
2.3. Device Fabrication
2.4. Microfluidic Chip Priming
2.5. Aqueous Phase Suspension
2.6. Experimental Setup and Device Operation
3. Results
3.1. Droplet Size-Based Filtering
3.2. Droplet Pairing
3.3. Droplet Merging
4. Conclusions
- generate droplets of different sizes,
- pair droplets of different size in traps and
- merge droplet pairs confined in traps.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agresti, J.J.; Antipov, E.; Abate, A.R.; Ahn, K.; Rowat, A.C.; Baret, J.C.; Marquez, M.; Klibanov, A.M.; Griffiths, A.D.; Weitz, D.A. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 4004–4009. [Google Scholar] [CrossRef] [Green Version]
- Kok, M.P.; Segers, T.; Versluis, M. Bubble sorting in pinched microchannels for ultrasound contrast agent enrichment. Lab Chip 2015, 15, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Ramji, R.; Warkiani, M.E.; Han, J.; Lim, C.T.; Chen, C.H. Jetting microfluidics with size-sorting capability for single-cell protease detection. Biosens. Bioelectron. 2015, 66, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Maenaka, H.; Yamada, M.; Yasuda, M.; Seki, M. Continuous and size-dependent sorting of emulsion droplets using hydrodynamics in pinched microchannels. Langmuir 2008, 24, 4405–4410. [Google Scholar] [CrossRef] [PubMed]
- Joensson, H.N.; Uhlén, M.; Svahn, H.A. Droplet size based separation by deterministic lateral displacement—Separating droplets by cell-induced shrinking. Lab Chip 2011, 11, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Lloyd Ung, W.; Heyman, J.A.; Weitz, D.A. Sensitive and predictable separation of microfluidic droplets by size using in-line passive filter. Biomicrofluidics 2017, 11, 014114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, D.H.; Numakunai, S.; Nakahara, A.; Sekiguchi, T.; Shoji, S. Hydrodynamic on-rail droplet pass filter for fully passive sorting of droplet-phase samples. RSC Adv. 2014, 4, 37721–37725. [Google Scholar] [CrossRef]
- Rehman, A.U.; Coskun, U.C.; Rashid, Z.; Morova, B.; Jonáš, A.; Erten, A.; Kiraz, A. Size-Based Sorting of Emulsion Droplets in Microfluidic Channels Patterned with Laser-Ablated Guiding Tracks. Anal. Chem. 2020, 92, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Tanaka, D.; Sekiguchi, T.; Shoji, S. Size-dependent and property-independent passive microdroplet sorting by droplet transfer on dot rails. Micromachines 2018, 9, 513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guzman, A.R.; Wippold, J.A.; Li, Y.; Dai, J.; Huang, C.; Han, A. An ultra high-efficiency droplet microfluidics platform using automatically synchronized droplet pairing and merging. Lab Chip 2020, 20, 3948–3959. [Google Scholar] [CrossRef]
- Qiao, Y.; Fu, J.; Yang, F.; Duan, M.; Huang, M.; Tu, J.; Lu, Z. An efficient strategy for a controllable droplet merging system for digital analysis. RSC Adv. 2018, 8, 34343–34349. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Kim, J.; Lee, D. Static array of droplets and on-demand recovery for biological assays. Biomicrofluidics 2020, 14, 051302. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.T.; Nunez, D.; Cai, D.; Kurabayashi, K. Sort’N merge: A deterministic microfluidic platform for co-encapsulating distinct particles in microdroplets. In Proceedings of the 2018 IEEE Micro Electro Mechanical Systems (MEMS), Belfast, UK, 21–25 January 2018; Volume 2018, pp. 265–268. [Google Scholar] [CrossRef]
- Labanieh, L.; Nguyen, T.N.; Zhao, W.; Kang, D.-K. Floating Droplet Array: An Ultrahigh-Throughput Device for Droplet Trapping, Real-time Analysis and Recovery Louai. Micromachines 2015, 6, 1469–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangla, R.; Lee, S.; Baroud, C.N. Trapping microfluidic drops in wells of surface energy. Phys. Rev. Lett. 2011, 107, 124501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbyad, P.; Dangla, R.; Alexandrou, A.; Baroud, C.N. Rails and anchors: Guiding and trapping droplet microreactors in two dimensions. Lab Chip 2011, 11, 813–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, A.J.T.; Tan, S.H.; Nguyen, N.T. On-Demand Droplet Merging with an AC Electric Field for Multiple-Volume Droplet Generation. Anal. Chem. 2020, 92, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.B.; Ray, A.; Wang, Z.M.; Wang, Z.P.; Ramanujan, R.V. Droplet merging on a lab-on-a-chip platform by uniform magnetic fields. Sci. Rep. 2016, 6, 37671. [Google Scholar] [CrossRef]
- Tomasi, R.F.X.; Sart, S.; Champetier, T.; Baroud, C.N. Individual Control and Quantification of 3D Spheroids in a High-Density Microfluidic Droplet Array. Cell Rep. 2020, 31, 107670. [Google Scholar] [CrossRef]
- Mazutis, L.; Baret, J.C.; Griffiths, A.D. A fast and efficient microfluidic system for highly selective one-to-one droplet fusion. Lab Chip 2009, 9, 2665–2672. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef]
- Simon, M.G.; Lin, R.; Fisher, J.S.; Lee, A.P. A Laplace pressure based microfluidic trap for passive droplet trapping and controlled release. Biomicrofluidics 2012, 6, 014110. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.-T.; Núñez, D.; Cai, D.; Kurabayashi, K. Deterministic droplet-based co-encapsulation and pairing of microparticles via active sorting and downstream merging. Lab Chip 2017, 17, 3664–3671. [Google Scholar] [CrossRef]
- Xu, J.; Ahn, B.; Lee, H.; Xu, L.; Lee, K.; Panchapakesan, R.; Oh, K.W. Droplet-based microfluidic device for multiple-droplet clustering. Lab Chip 2012, 12, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, C.L. A microfluidic chip integrated with droplet generation, pairing, trapping, merging, mixing and releasing. RSC Adv. 2017, 7, 16738–16750. [Google Scholar] [CrossRef] [Green Version]
- Akartuna, I.; Aubrecht, D.M.; Kodger, T.E.; Weitz, D.A. Chemically induced coalescence in droplet-based microfluidics. Lab Chip 2015, 15, 1140–1144. [Google Scholar] [CrossRef]
- Segaliny, A.I.; Li, G.; Kong, L.; Ren, C.; Chen, X.; Wang, J.K.; Baltimore, D. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip 2018, 18, 3733–3749. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Nguyen, N.-T.; Neng Wong, T. Droplet Coalescence in Microfluidic Systems. Micro Nanosyst. 2012, 3, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.C.; Fisher, J.S.; Lee, A.I.; Cristini, V.; Lee, A.P. Design of microfluidic channel geometries for the control of droplet volume, chemical concentration, and sorting. Lab Chip 2004, 4, 292–298. [Google Scholar] [CrossRef]
- Fidalgo, L.M.; Abell, C.; Huck, W.T.S. Surface-induced droplet fusion in microfluidic devices. Lab Chip 2007, 7, 984–986. [Google Scholar] [CrossRef]
- Niu, X.; Gielen, F.; DeMello, A.J.; Edel, J.B. Electro-coalescence of digitally controlled droplets. Anal. Chem. 2009, 81, 7321–7325. [Google Scholar] [CrossRef]
- Köhler, J.M.; Henkel, T.; Grodrian, A.; Kirner, T.; Roth, M.; Martin, K.; Metze, J. Digital reaction technology by micro segmented flow—Components, concepts and applications. Chem. Eng. J. 2004, 101, 201–216. [Google Scholar] [CrossRef]
- Chabert, M.; Dorfman, K.D.; Viovy, J.L. Droplet fusion by alternating current (AC) field electrocoalescence in microchannels. Electrophoresis 2005, 26, 3706–3715. [Google Scholar] [CrossRef] [PubMed]
- Zagnoni, M.; Cooper, J.M. On-chip electrocoalescence of microdroplets as a function of voltage, frequency and droplet size. Lab Chip 2009, 9, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.J. A Model for Bilayer Membrane Electroporation Based on Resultant Electromechanical Stress. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 769–777. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchamp, M.; Arnaud, M.; Bobisse, S.; Coukos, G.; Harari, A.; Renaud, P. Microfluidic Device for Droplet Pairing by Combining Droplet Railing and Floating Trap Arrays. Micromachines 2021, 12, 1076. https://doi.org/10.3390/mi12091076
Duchamp M, Arnaud M, Bobisse S, Coukos G, Harari A, Renaud P. Microfluidic Device for Droplet Pairing by Combining Droplet Railing and Floating Trap Arrays. Micromachines. 2021; 12(9):1076. https://doi.org/10.3390/mi12091076
Chicago/Turabian StyleDuchamp, Margaux, Marion Arnaud, Sara Bobisse, George Coukos, Alexandre Harari, and Philippe Renaud. 2021. "Microfluidic Device for Droplet Pairing by Combining Droplet Railing and Floating Trap Arrays" Micromachines 12, no. 9: 1076. https://doi.org/10.3390/mi12091076
APA StyleDuchamp, M., Arnaud, M., Bobisse, S., Coukos, G., Harari, A., & Renaud, P. (2021). Microfluidic Device for Droplet Pairing by Combining Droplet Railing and Floating Trap Arrays. Micromachines, 12(9), 1076. https://doi.org/10.3390/mi12091076





