Lateral Deformation of Human Red Blood Cells by Optical Tweezers
Abstract
:1. Introduction
2. Materials and Methods
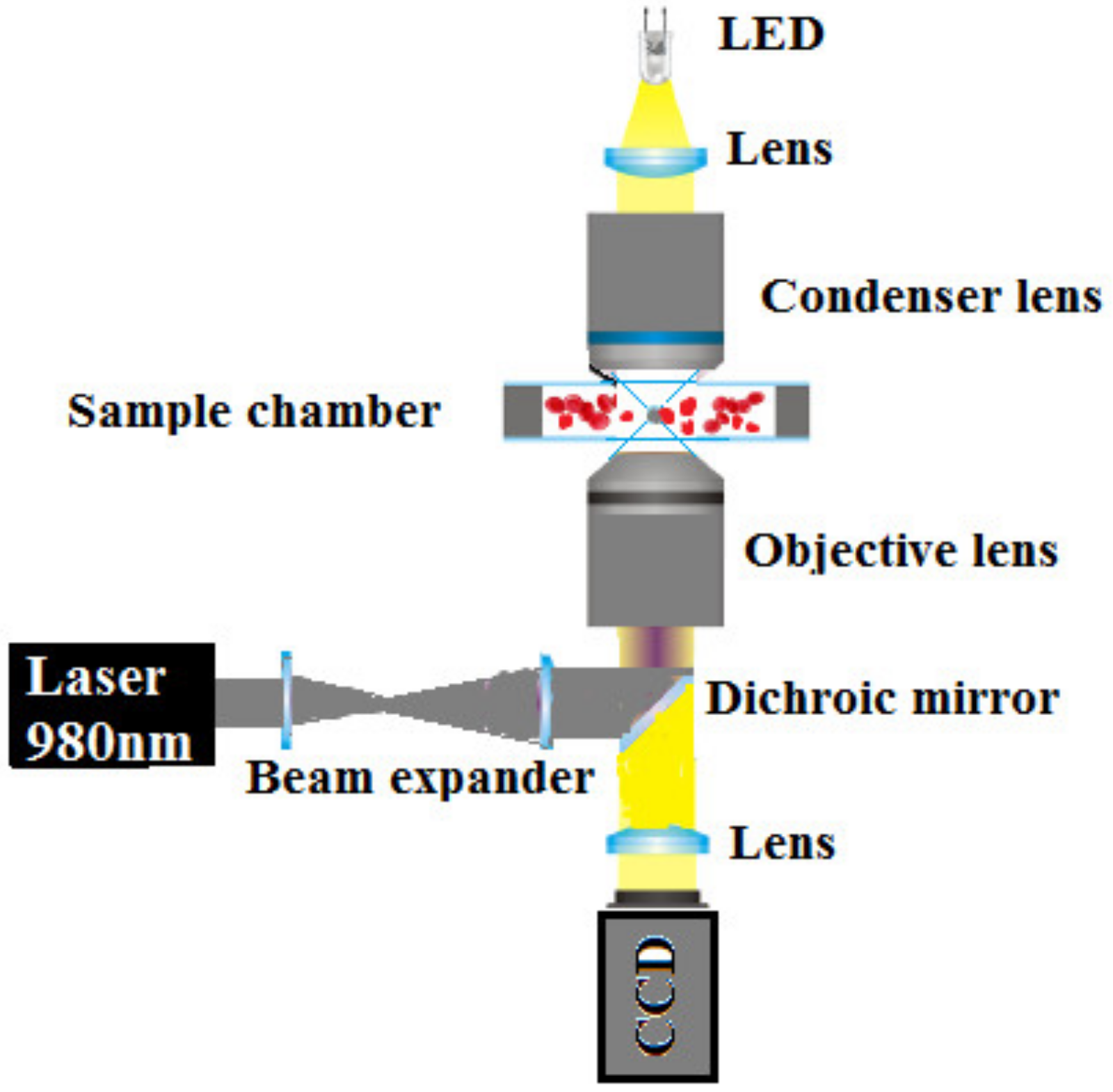
2.1. Experimental Setup
2.2. Method and Force Calibration
2.3. Elastic Stiffness and Shear Stiffness Calculation
3. Results
3.1. Mechanical Properties of Biconcave RBCs
3.2. Measurements Obtained from Spherical and Crenelated RBCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashkin, A.; Dziedzic, J.M.; Yamane, T. Optical trapping and manipulation of single cells using infrared laser beams. Nature 1987, 330, 769. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Ananthakrishnan, R.; Mahmood, H.; Moon, T.; Cunningham, J.C.; Kâs, J. The Optical Stretcher: A Novel Laser Tool to Micromanipulate Cells. Biophys. J. 2001, 81, 767–784. [Google Scholar] [CrossRef] [Green Version]
- Adamo, M.; Naveiras, O.; Wenzel, P.; McKinney-Freeman, S.; Mack, P.; Gracia-Sancho, J.; Suchy-Dicey, A.; Yoshimoto, M.; Lensch, M.; Yoder, M. Biomechanical forces promote embryonic haematopoiesis. Nature 2009, 459, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, G.; Suresh, S. Cell and molecular mechanics of biological materials. Nat. Mater. 2003, 2, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Sung, K.L.P.; Skalak, R.; Usami, S.; Zern, A.T. Theoretical and experimental studies on scoelastic properties of erythrocyte membrane. Biophys. J. 1978, 24, 463. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.; Limb, C.T.; Suresha, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Park, Y.K.; Best, C.A.; Gov, T.; Auth, N.S.; Safrane, S.A.; Popescug, G.; Suresh, S.; Feld, M.S. Metabolic remodelling of the human red blood cell membrane. Proc. Natl. Acad. Sci. USA 2010, 107, 6731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, M.-C.; Wei1, X.-B.; Zhou, J.-H.; Wang, Z.-Q.; Li, Y.-M. Trapping red blood cells in living animals using optical tweezers. Nature 2013, 4, 2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Kinnunen, M.; Khokhlova, M.D.; Lyubin, E.V.; Priezzhev, A.V.; Meglinski, I.; Fedyaninc, A.A. Optical tweezers study of red blood cell aggregation and disaggregation in plasma and protein solutions. J. Biomed. Opt. 2016, 21, 035001. [Google Scholar] [CrossRef] [PubMed]
- Yousafzai, M.S.; Ndoye, F.; Coceano, G.; Niemela, J.; Bonin, S.; Scoles, G.; Cojoc, D. Substrate-dependent cell elasticity measured by optical tweezers indentation. Opt. Lasers Eng. 2015, 76, 27–33. [Google Scholar] [CrossRef]
- Konin, J.-M.E.; Yale, P.; Megnassan, E.; Kouacou, M.A.; Zoueu, J.T. Dynamics Study of the Deformation of Red Blood Cell by Optical Tweezers. Open J. Biophys. 2017, 7, 59–69. [Google Scholar]
- Yale, P.; Konin, J.-M.E.; Kouacou, M.A.; Zoueu, J.T. New Detector Sensitivity Calibration and the Calculation of the Interaction Force between Particles Using an Optical Tweezer. Micromachines 2018, 9, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterman, N. TweezPal—Optical tweezers analysis and calibration software. Comput. Phys. Commun. 2010, 181, 1911–1916. [Google Scholar] [CrossRef]
- Jun, Y.; Tripathy, S.K.; Narayanareddy, B.R.J.; Mattson-Hoss, M.K.; Gross, S.P. Calibration of Optical Tweezers for In Vivo Force Measurements: How do Different Approaches Compare? Biophys. J. 2014, 107, 1474–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, S.; Sanchez, P.; Bodensiek, K.; Li, S.; Simons, M.; Schaap, I.A. Cell visco-elasticity measured with AFM and optical trapping at sub-micrometer deformations. PLoS ONE 2012, 7, 45297. [Google Scholar]
- Danon, D.; Marikovsky, Y. The aging of the red blood cell. A multifactor process. Blood Cells 1988, 14, 7–15. [Google Scholar] [PubMed]
- Evans, E.A. New membrane concept applied to the analysis of fluid shear- and micropipette deformed red blood cells. Biophys. J. 1973, 13, 941–954. [Google Scholar] [CrossRef] [Green Version]
- Lingyao, Y.; Chiou, H.Y.A.; Sheng, Y. Deformation of biconcave red blood cell in the dual-beam optical tweezers. In Proceedings of the 2011 COMSOL Conference, Boston, MA, USA, 25 February 2011. [Google Scholar]
- Hénon, S.; Lenormand, G.; Richert, A.; Gallet, F. A New Determination of the Shear Modulus of the Human Erythrocyte Membrane Using Optical Tweezers. Biophys. J. 1999, 76, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- Alderman, E.M.; Fudenberg, H.H.; Lovins, R.E. Isolation and characterization of an age-related antigen present on senescent human red blood cell. Blood 1981, 58, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]

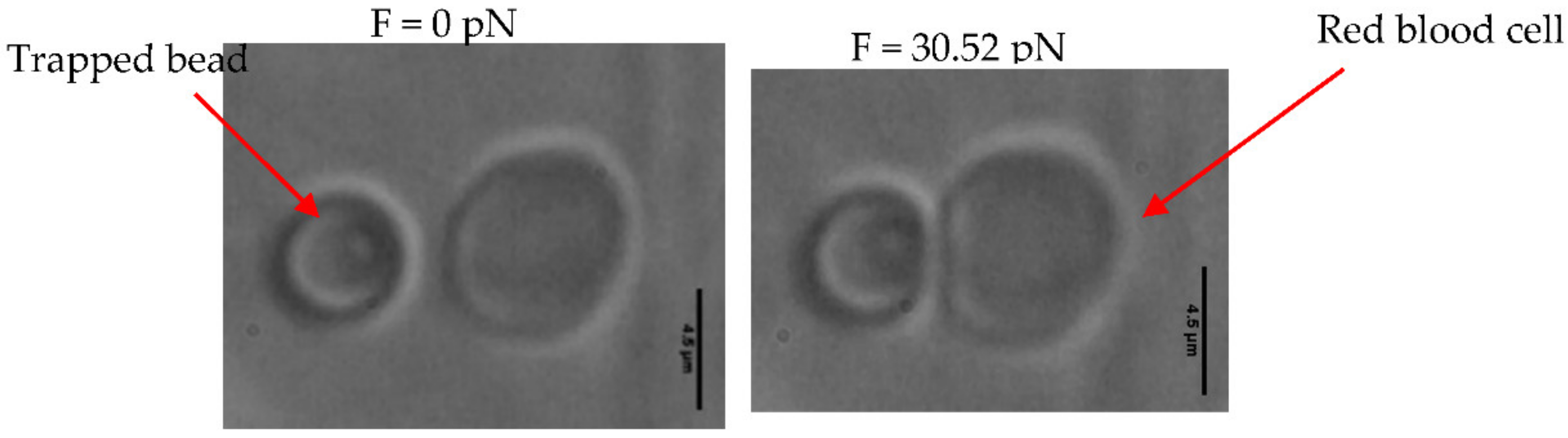

| Parameters | Biconcave RBCs | Spherical RBCs | Crenelated RBCs |
|---|---|---|---|
| Elastic stiffness (µN/m) | 10.11 ± 1.20 | 10.46 ± 0.70 | 11.40 ± 0.67 |
| Shear stiffness (µN/m) | 3.37 ± 0.40 | 3.48 ± 0.23 | 3.80 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yale, P.; Kouacou, M.A.; Konin, J.-M.E.; Megnassan, E.; Zoueu, J.T. Lateral Deformation of Human Red Blood Cells by Optical Tweezers. Micromachines 2021, 12, 1024. https://doi.org/10.3390/mi12091024
Yale P, Kouacou MA, Konin J-ME, Megnassan E, Zoueu JT. Lateral Deformation of Human Red Blood Cells by Optical Tweezers. Micromachines. 2021; 12(9):1024. https://doi.org/10.3390/mi12091024
Chicago/Turabian StyleYale, Pavel, Michel A. Kouacou, Jean-Michel E. Konin, Eugène Megnassan, and Jérémie T. Zoueu. 2021. "Lateral Deformation of Human Red Blood Cells by Optical Tweezers" Micromachines 12, no. 9: 1024. https://doi.org/10.3390/mi12091024
APA StyleYale, P., Kouacou, M. A., Konin, J.-M. E., Megnassan, E., & Zoueu, J. T. (2021). Lateral Deformation of Human Red Blood Cells by Optical Tweezers. Micromachines, 12(9), 1024. https://doi.org/10.3390/mi12091024







