Toward Development of a Higher Flow Rate Hemocompatible Biomimetic Microfluidic Blood Oxygenator
Abstract
:1. Introduction
2. Design and Experimental Methods
2.1. Device Design
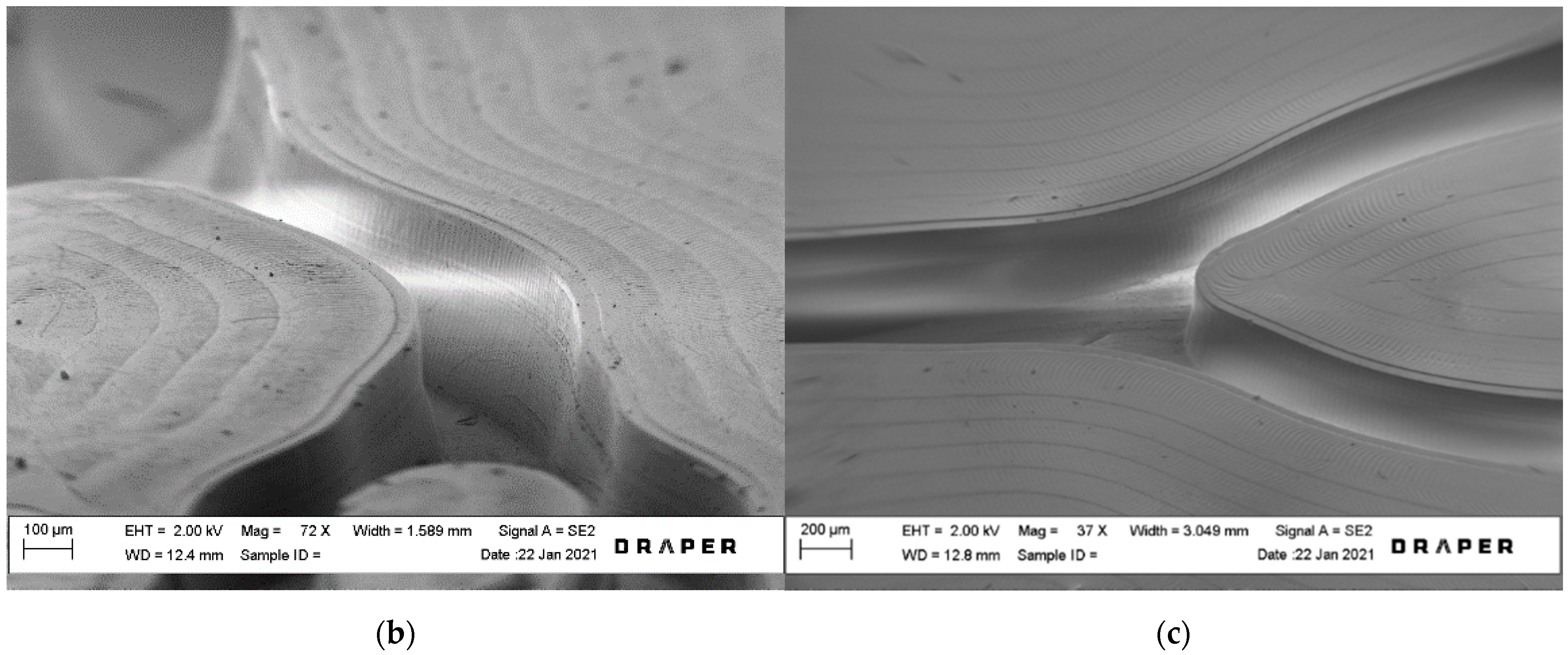
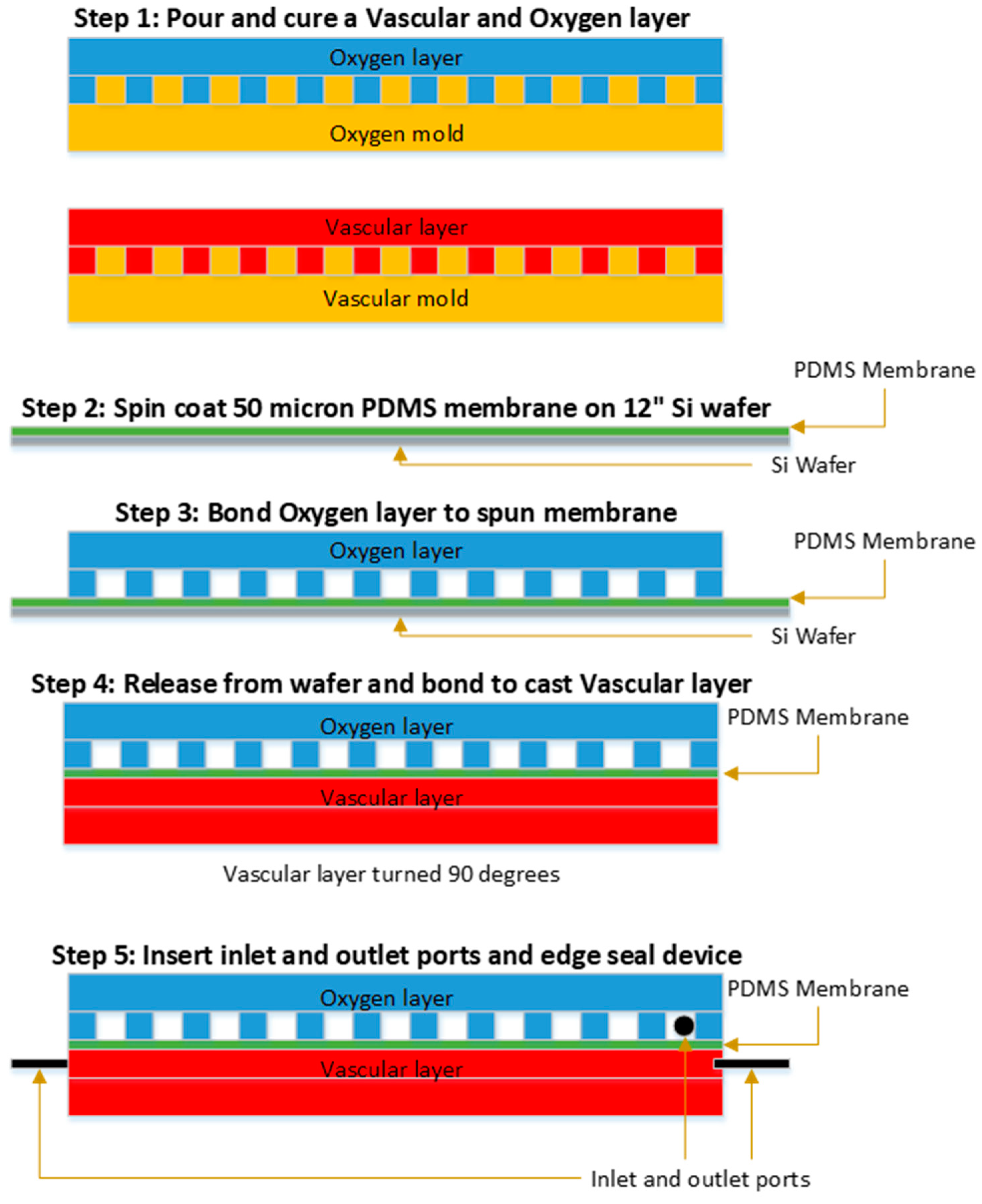
2.2. Layer Fabrication
2.3. Membrane Fabrication
2.4. Device Assembly
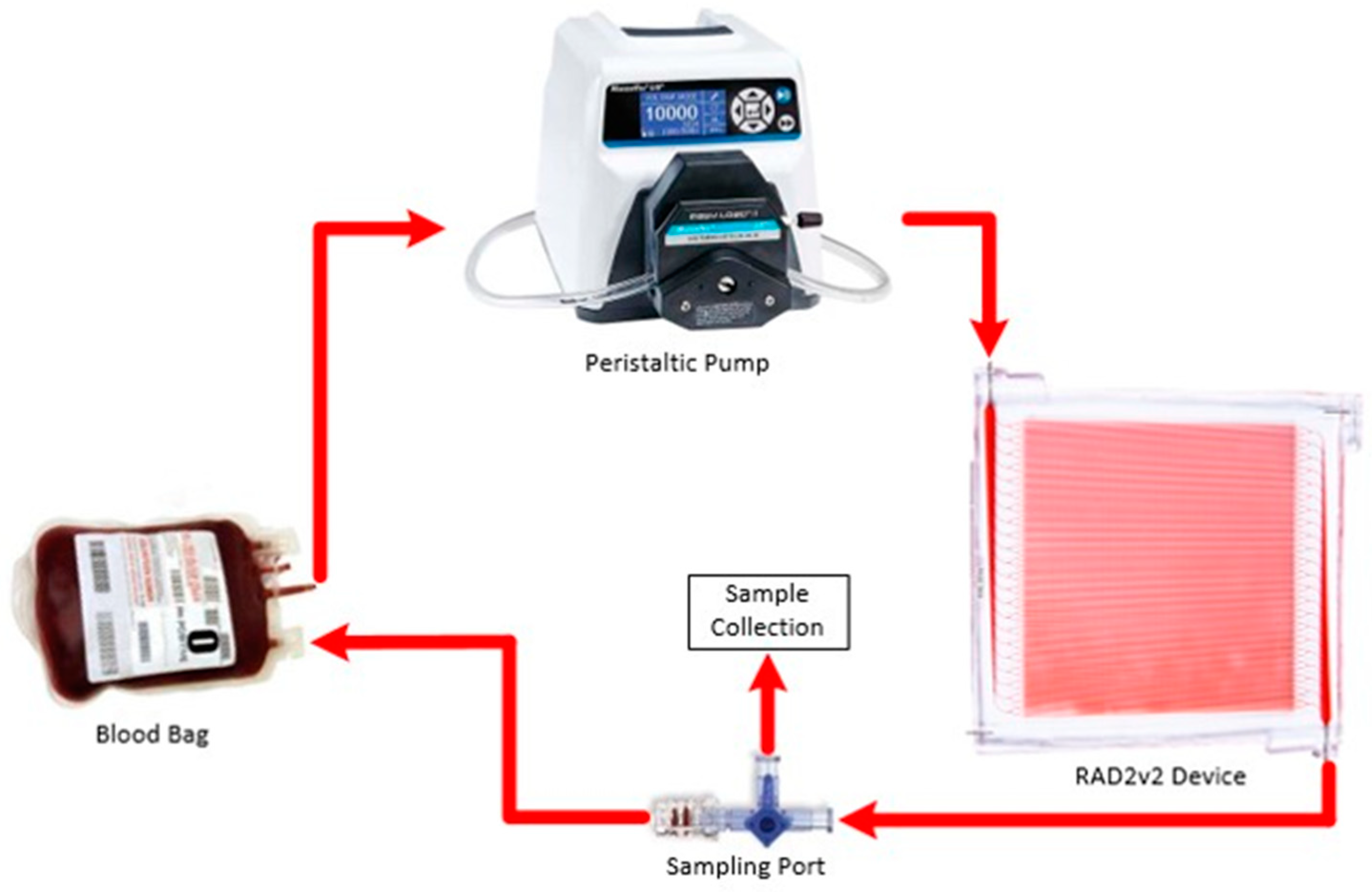
2.5. Oxygen Transfer Testing
2.6. Hemocompatibility Studies
3. Results and Discussion
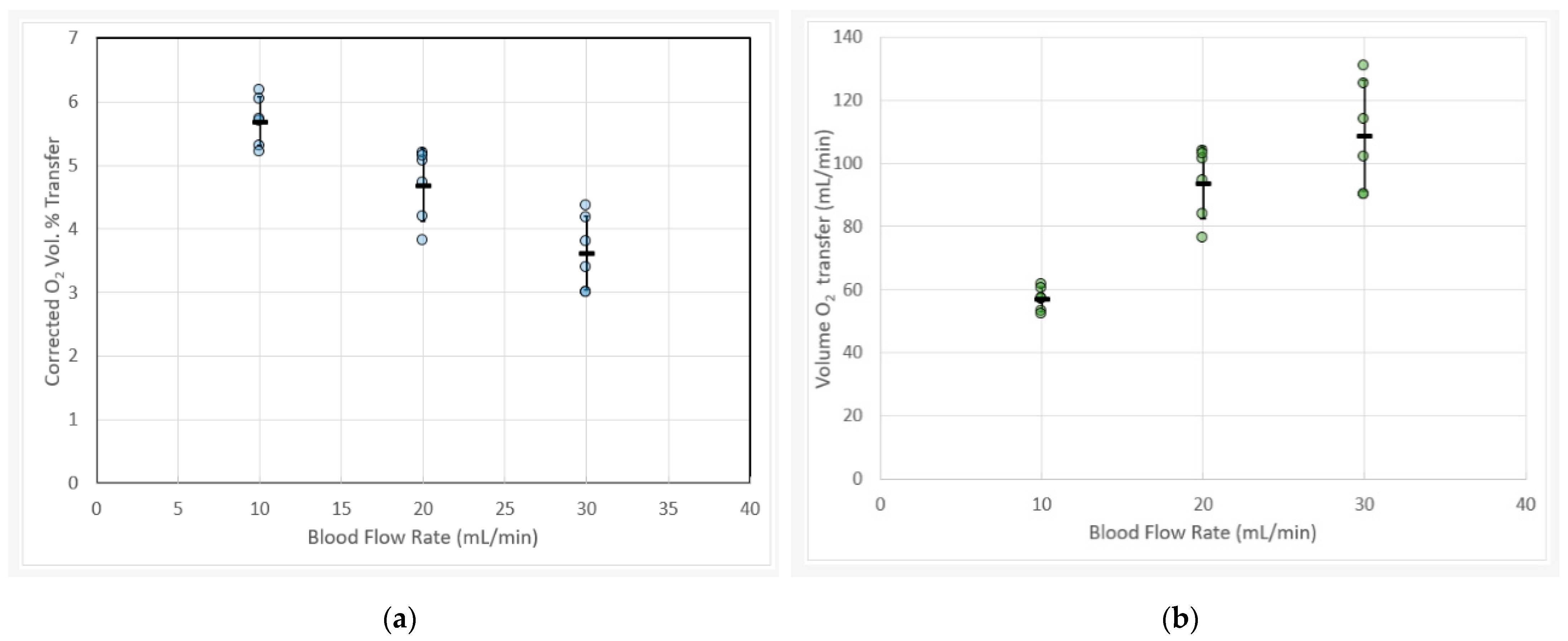
3.1. Blood Gas Oxygen Transfer
3.2. Hemocompatibility Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalhan, R.; Mannino, D.; Rosenberg, S.R. Epidemiology of Chronic Obstructive Pulmonary Disease: Prevalence, Morbidity, Mortality, and Risk Factors. Semin. Respir. Crit. Care Med. 2015, 36, 457–469. [Google Scholar] [CrossRef]
- Herold, S.; Becker, C.; Ridge, K.M.; Budinger, G.S. Influenza virus-induced lung injury: Pathogenesis and implications for treatment. Eur. Respir. J. 2015, 45, 1463–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Mechanical Ventilation during Extracorporeal Support for Acute Respiratory Distress Syndrome. For Now, a Necessary Evil. Am. J. Respir. Crit. Care Med. 2017, 195, 1137–1139. [Google Scholar] [CrossRef]
- Federspiel, W.; Henchir, K. Lung, Artificial: Basic Principles and Current Applications. In Encyclopedia of Biomaterials and Biomedical Engineering, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; Volume 4. [Google Scholar]
- Auld, S.C.; Caridi-Scheible, M.; Blum, J.M.; Robichaux, C.; Kraft, C.; Jacob, J.T.; Jabaley, C.S.; Carpenter, D.; Kaplow, R.; Hernandez-Romieu, A.C.; et al. ICU and Ventilator Mortality among Critically Ill Adults with Coronavirus Disease 2019. Crit. Care Med. 2020, 48, e799–e804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, Z.; Li, B.; Zhang, X.; Tian, R.; Wu, W.; Zhang, Z.; Lu, Y.; Chen, N.; Clifford, S.P.; et al. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019 in Shanghai, China. ASAIO J. 2020, 66, 475–481. [Google Scholar] [CrossRef]
- Gray, B.W.; Haft, J.W.; Hirsch, J.C.; Annich, G.M.; Hirschl, R.B.; Bartlett, R.H. Extracorporeal Life Support: Experience with 2000 patients. ASAIO J. 2015, 61, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenough, A.; Agha, M.; Smith, A.P.R. Extracorporeal membrane oxygenation for neonates. In Neonatology: A Practical Approach to Neonatal Diseases; Springer: New York, NY, USA, 2012. [Google Scholar]
- Bartlett, R.H. Esperanza: The First Neonatal ECMO Patient. ASAIO J. 2017, 63, 832–843. [Google Scholar] [CrossRef]
- Cornish, J.D.; Carter, J.M.; Gerstmann, D.R.; Null, D.M. Extracorporeal membrane oxygenation as a means of stabilizing and transporting high risk neonates. ASAIO Trans. 1991, 37, 564–568. [Google Scholar]
- Schoberer, M.; Arens, J.; Lohr, A.; Seehase, M.; Jellema, R.; Collins, J.; Kramer, B.W.; Schmitz-Rode, T.; Steinseifer, U.; Orlikowsky, T. Fifty Years of Work on the Artificial Placenta: Milestones in the History of Extracorporeal Support of the Premature Newborn. Artif. Organs 2012, 36, 512–516. [Google Scholar] [CrossRef]
- Noah, A.M.; Peek, G.J.; Finney, S.J.; Griffiths, M.J.; Harrison, D.; Grieve, R.; Sadique, Z.; Sekhon, J.S.; McAuley, D.; Firmin, R.K.; et al. Referral to an Extracorporeal Membrane Oxygenation Center and Mortality among Patients with Severe 2009 Influenza A(H1N1). JAMA 2011, 306, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Brodie, D.; Bacchetta, M. Extracorporeal Membrane Oxygenation for ARDS in Adults. N. Engl. J. Med. 2011, 365, 1905–1914. [Google Scholar] [CrossRef] [Green Version]
- Lund, L.W.; Federspiel, W.J. Removing extra CO2 in COPD patients. Curr. Respir. Care Rep. 2013, 2, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Potkay, J.A. The promise of microfluidic artificial lungs. Lab Chip 2014, 14, 4122–4138. [Google Scholar] [CrossRef]
- Burgess, K.A.; Hu, H.-H.; Wagner, W.; Federspiel, W.J. Towards microfabricated biohybrid artificial lung modules for chronic respiratory support. Biomed. Microdevices 2009, 11, 117–127. [Google Scholar] [CrossRef]
- Kniazeva, T.; Epshteyn, A.A.; Hsiao, J.C.; Kim, E.S.; Kolachalama, V.B.; Charest, J.L.; Borenstein, J.T. Performance and scaling effects in a multilayer microfluidic extracorporeal lung oxygenation device. Lab Chip 2012, 12, 1686–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabaghi, M.; Saraei, N.; Fusch, G.; Rochow, N.; Brash, J.L.; Fusch, C.; Selvaganapathy, P.R. An ultra-thin highly flexible microfluidic device for blood oxygenation. Lab Chip 2018, 18, 3780–3789. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Saraei, N.; Fusch, G.; Rochow, N.; Brash, J.L.; Fusch, C.; Selvaganapathy, P.R. An ultra-thin, all PDMS-based microfluidic lung assist device with high oxygenation capacity. Biomicrofluidics 2019, 13, 034116. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Saraei, N.; Fusch, G.; Rochow, N.; Brash, J.L.; Fusch, C.; Selvaganapathy, P.R. Microfluidic blood oxygenators with integrated hollow chambers for enhanced air exchange from all four sides. J. Membr. Sci. 2020, 596, 117741. [Google Scholar] [CrossRef]
- Gimbel, A.A.; Hsiao, J.C.; Kim, E.S.; Lewis, D.J.; Risoleo, T.F.; Urban, J.N.; Borenstein, J.T. A high gas transfer efficiency microfluidic oxygenator for extracorporeal respiratory assist applications in critical care medicine. Artif. Organs 2021. [Google Scholar] [CrossRef] [PubMed]
- Page, T.C.; Light, W.R. Oxygen transport in 10 microns artificial capillaries. In Oxygen Transport to Tissue XXI; Springer International: New York, NY, USA, 1999. [Google Scholar]
- Kniazeva, T.; Hsiao, J.C.; Charest, J.L.; Borenstein, J.T. A microfluidic respiratory assist device with high gas permeance for artificial lung applications. Biomed. Microdevices 2011, 13, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Hoganson, D.M.; Ii, H.I.P.; Bassett, E.K.; Spool, I.D.; Vacanti, J.P. Lung assist device technology with physiologic blood flow developed on a tissue engineered scaffold platform. Lab Chip 2011, 11, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Potkay, J.A.; Magnetta, M.; Vinson, A.; Cmolik, B. Bio-inspired, efficient, artificial lung employing air as the ventilating gas. Lab Chip 2011, 11, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Buchan, S.; Carr, B.; Poling, C.; Hayes, M.; Fernando, U.P.; Kaesler, A.; Schlanstein, P.; Hesselmann, F.; Arens, J.; et al. Low-Resistance, Concentric-Gated Pediatric Artificial Lung for End-Stage Lung Failure. ASAIO J. 2020, 66, 423–432. [Google Scholar] [CrossRef]
- Dharia, A.; Abada, E.; Feinberg, B.; Yeager, T.; Moses, W.; Park, J.; Blaha, C.; Wright, N.; Padilla, B.; Roy, S. Silicon Micropore-Based Parallel Plate Membrane Oxygenator. Artif. Organs 2018, 42, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Rochow, N.; Manan, A.; Wu, W.-I.; Fusch, G.; Monkman, S.; Leung, J.; Chan, E.; Nagpal, D.; Predescu, D.; Brash, J.; et al. An Integrated Array of Microfluidic Oxygenators as a Neonatal Lung Assist Device: In Vitro Characterization and In Vivo Demonstration. Artif. Organs 2014, 38, 856–866. [Google Scholar] [CrossRef]
- Dabaghi, M.; Rochow, N.; Saraei, N.; Fusch, G.; Monkman, S.; Da, K.; Shahin-Shamsabadi, A.; Brash, J.L.; Predescu, D.; Delaney, K.; et al. A Pumpless Microfluidic Neonatal Lung Assist Device for Support of Preterm Neonates in Respiratory Distress. Adv. Sci. 2020, 7, 2001860. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Gimbel, A.A.; Peppas, A.; Truslow, J.G.; Lang, D.; Sukavaneshvar, S.; Solt, D.; Mulhern, T.; Markoski, A.; Kim, E.; et al. Design and Construction of Three-Dimensional Physiologically-Based Vascular Branching Networks for Respiratory Assist Devices. Lab Chip 2021. in review. [Google Scholar]
- Wagner, G.; Kaesler, A.; Steinseifer, U.; Schmitz-Rode, T.; Arens, J. Comment on “The promise of microfluidic artificial lungs” by J. A. Potkay, Lab Chip, 2014, 14, 4122–4138. Lab Chip 2016, 16, 1272–1273. [Google Scholar] [CrossRef]
- Potkay, J.A. Reply to the ‘Comment on “the promise of microfluidic artificial lungs”’ by G. Wagner, A. Kaesler, U. Steinseifer, T. Schmitz-Rode and J. Arens, Lab Chip, 2016, 16, doi:10.1039/C5LC01508A. Lab Chip 2016. [Google Scholar] [CrossRef]
- Murray, C.D. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc. Natl. Acad. Sci. USA 1926, 12, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Emerson, D.R.; Cieślicki, K.; Gu, X.; Barber, R.W. Biomimetic design of microfluidic manifolds based on a generalised Murray’s law. Lab Chip 2006, 6, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Noe, D.A.; Weedn, V.; Bell, W.R. Direct spectrophotometry of serum hemoglobin: An Allen correction compared with a three-wavelength polychromatic analysis. Clin. Chem. 1984, 30, 627–630. [Google Scholar] [CrossRef]
- Prat, N.J.; Meyer, A.D.; Langer, T.; Montgomery, R.K.; Parida, B.K.; Batchinsky, A.I.; Cap, A.P. Low-Dose Heparin Anticoagulation During Extracorporeal Life Support for Acute Respiratory Distress Syndrome in Conscious Sheep. Shock 2015, 44, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Omar, H.R.; Mirsaeidi, M.; Socias, S.; Sprenker, C.; Caldeira, C.; Camporesi, E.M.; Mangar, D. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS ONE 2015, 10, e0124034. [Google Scholar] [CrossRef] [Green Version]
- Sukavaneshvar, S. Device thrombosis and pre-clinical blood flow models for assessing antithrombogenic efficacy of drug-device combinations. Adv. Drug Deliv. Rev. 2017, 112, 24–34. [Google Scholar] [CrossRef]
- Betit, P. Technical Advances in the Field of ECMO. Respir. Care 2018, 63, 1162–1173. [Google Scholar] [CrossRef]
- Ronco, C.; Brendolan, A.; Crepaldi, C.; Rodighiero, M.; Scabardi, M. Blood and Dialysate Flow Distributions in Hollow-Fiber Hemodialyzers Analyzed by Computerized Helical Scanning Technique. J. Am. Soc. Nephrol. 2002, 13, S53–S61. [Google Scholar] [CrossRef]
- Runyon, M.K.; Kastrup, C.J.; Johnson-Kerner, B.L.; Van Ha, T.G.; Ismagilov, R.F. Effects of Shear Rate on Propagation of Blood Clotting Determined Using Microfluidics and Numerical Simulations. J. Am. Chem. Soc. 2008, 130, 3458–3464. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, E.J.; Borenstein, J.T.; Kaazempur-Mofrad, M.R.; Orrick, B.; Vacanti, J.P. Design and fabrication of a constant shear microfluidic network for tissue engineering. Mater. Res. Soc. Symp. Proc. 2004, 823, W9.4/O5.4. [Google Scholar] [CrossRef]



| Variable | Group | Baseline | 3H | 6H |
|---|---|---|---|---|
| Blood Flow Rate (mL/min) | RAD2v2 | 25 ± 1 | 25 ± 0 | 25 ± 0 |
| Control | 26 ± 1 | 25 ± 1 | 25 ± 1 | |
| Blood Pump (revolutions/min) | RAD2v2 | 24 ± 1 | 25 ± 1 | 25 ± 1 |
| Control | 24 ± 0 | 23 ± 1 | 23 ± 1 | |
| WBC Count (×103/µL) | RAD2v2 | 11 ± 3 | 9 ± 3 | 6 ± 2 |
| Control | 10 ± 1 | 10 ± 2 | 8 ± 2 | |
| RBC Count (×106/µL) | RAD2v2 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| Control | 4 ± 1 | 4 ± 1 | 4 ± 1 | |
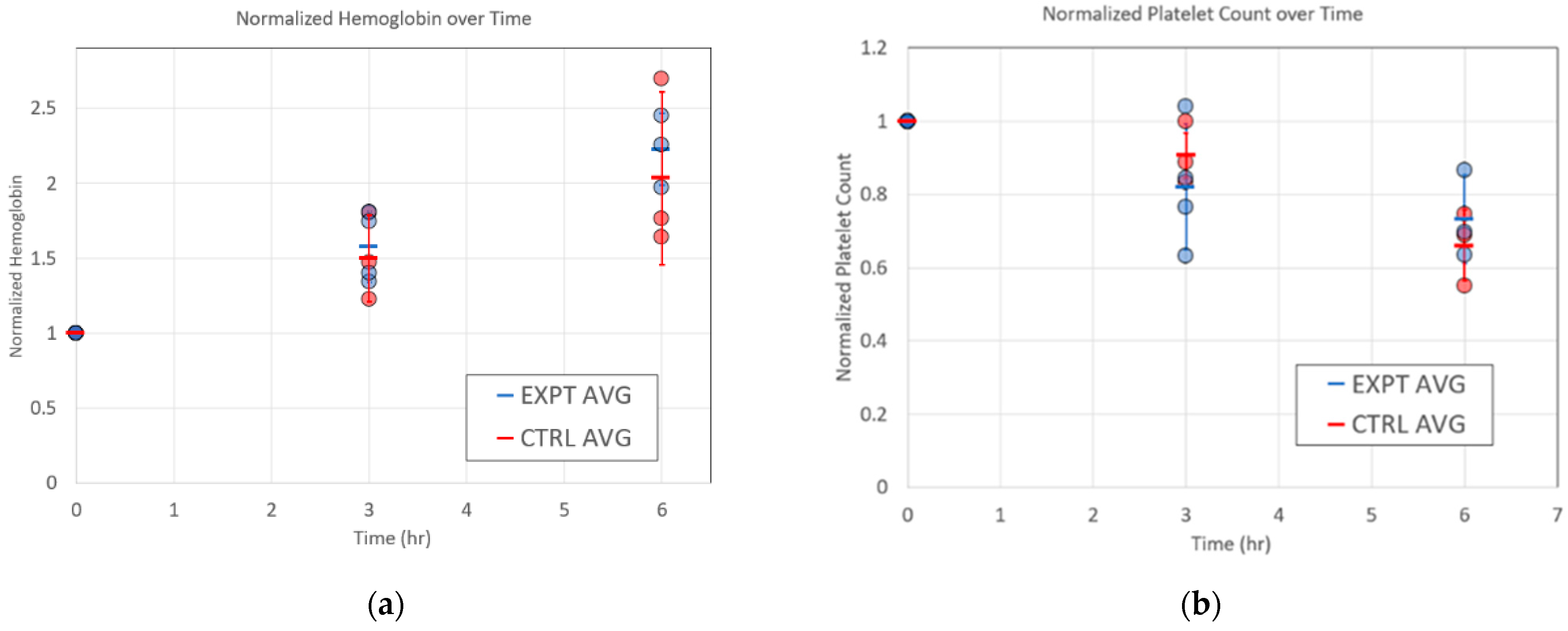
| Hgb (g/dL) | RAD2v2 | 7 ± 2 | 7 ± 1 | 7 ± 1 |
| Control | 8 ± 2 | 7 ± 1 | 7 ± 1 | |
| TEG Reaction Time (R, min) | RAD2v2 | 3.3 ± 0.2 *,† | 2.7 ± 0.1 * | 2.3 ± 0.2 * |
| Control | 4.0 ± 0.2 * | 3.4 ± 0.5 * | 3.0 ± 0.4 * | |
| TEG Initial Clot Formation Rate (Alpha, deg) | RAD2v2 | 0.8 ± 0.0 † | 0.8 ± 0.0 † | 0.8 ± 0.0 † |
| Control | 1.1 ± 0.1 | 1.3 ± 0.0 | 1.5 ± 0.4 | |
| TEG Clot Strength (MA, mm) | RAD2v2 | 82 ± 6 | 81 ± 6 | 78 ± 7 |
| Control | 71 ± 8 | 72 ± 12 | 68 ± 13 | |
| Collagen-induced platelet aggregation (AUC) | RAD2v2 | 67 ± 36 | 43 ± 30 * | 15 ± 18 * |
| Control | 58 ± 24 | 43 ± 18 | 21 ± 11 | |
| %P-Selectin Positive Platelets | RAD2v2 | 3 ± 1 | 5 ± 2 | 9 ± 4 |
| Control | 2 ± 1 | 3 ± 1 | 2 ± 1 | |
| % PS Positive Platelets | RAD2v2 | 4 ± 2 | 6 ± 3 | 9 ± 5 |
| Control | 2 ± 1 | 3 ± 1 | 5 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Vedula, E.M.; Lai, W.; Isenberg, B.C.; Lewis, D.J.; Lang, D.; Sutherland, D.; Roberts, T.R.; Harea, G.T.; Wells, C.; et al. Toward Development of a Higher Flow Rate Hemocompatible Biomimetic Microfluidic Blood Oxygenator. Micromachines 2021, 12, 888. https://doi.org/10.3390/mi12080888
Santos J, Vedula EM, Lai W, Isenberg BC, Lewis DJ, Lang D, Sutherland D, Roberts TR, Harea GT, Wells C, et al. Toward Development of a Higher Flow Rate Hemocompatible Biomimetic Microfluidic Blood Oxygenator. Micromachines. 2021; 12(8):888. https://doi.org/10.3390/mi12080888
Chicago/Turabian StyleSantos, Jose, Else M. Vedula, Weixuan Lai, Brett C. Isenberg, Diana J. Lewis, Dan Lang, David Sutherland, Teryn R. Roberts, George T. Harea, Christian Wells, and et al. 2021. "Toward Development of a Higher Flow Rate Hemocompatible Biomimetic Microfluidic Blood Oxygenator" Micromachines 12, no. 8: 888. https://doi.org/10.3390/mi12080888
APA StyleSantos, J., Vedula, E. M., Lai, W., Isenberg, B. C., Lewis, D. J., Lang, D., Sutherland, D., Roberts, T. R., Harea, G. T., Wells, C., Teece, B., Karandikar, P., Urban, J., Risoleo, T., Gimbel, A., Solt, D., Leazer, S., Chung, K. K., Sukavaneshvar, S., ... Borenstein, J. T. (2021). Toward Development of a Higher Flow Rate Hemocompatible Biomimetic Microfluidic Blood Oxygenator. Micromachines, 12(8), 888. https://doi.org/10.3390/mi12080888








