Soft, Conductive, Brain-Like, Coatings at Tips of Microelectrodes Improve Electrical Stability under Chronic, In Vivo Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Soft Elastomeric Interface
2.2. Mechanical Characterization
2.3. FTIR Characterization
2.4. Cell Culture Experiments
2.5. Animal Surgery and Implantation
2.6. Electrochemical Impedance Spectroscopy (EIS)
2.7. Neural Recordings
2.8. Statistical Methods
3. Results
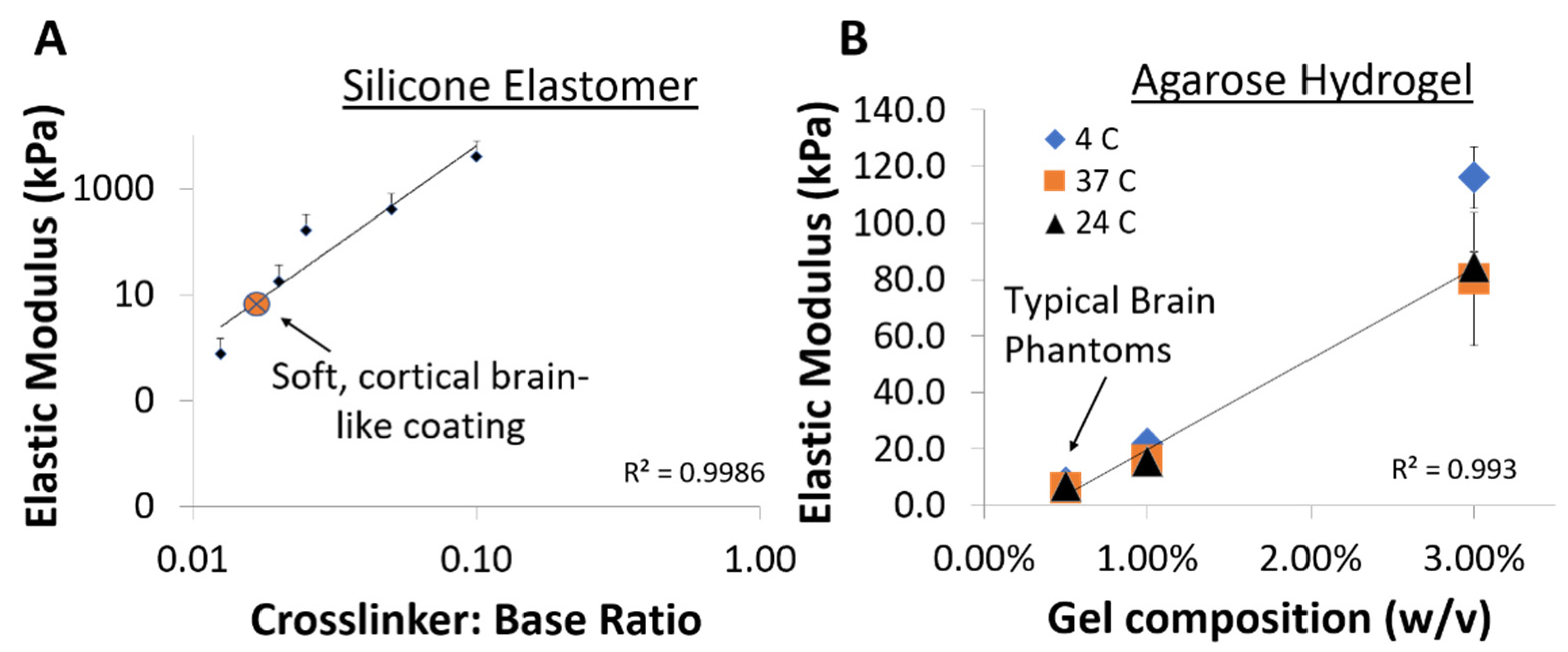
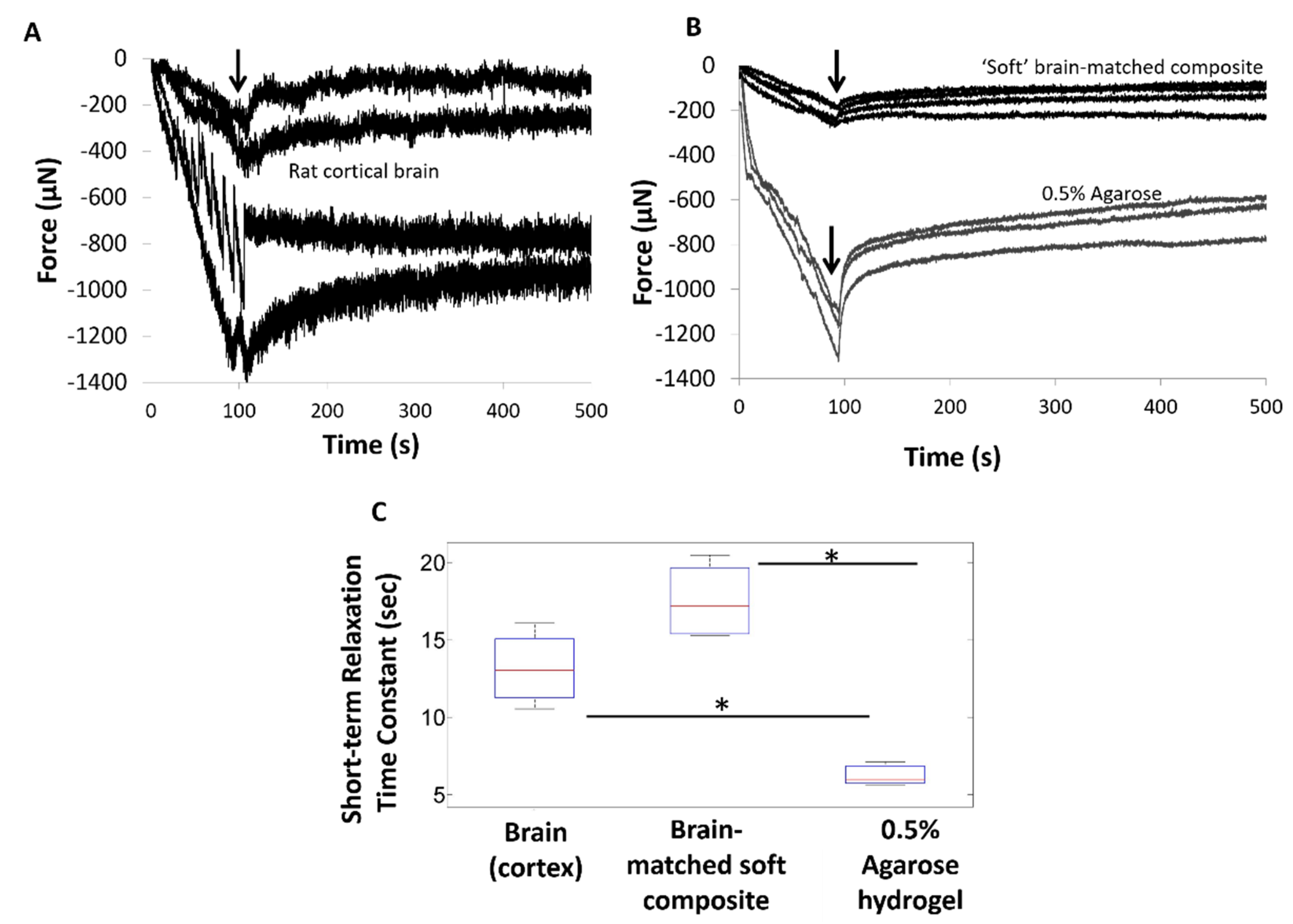
3.1. Soft Brain-Like Coatings from Silicone/CNT Composites
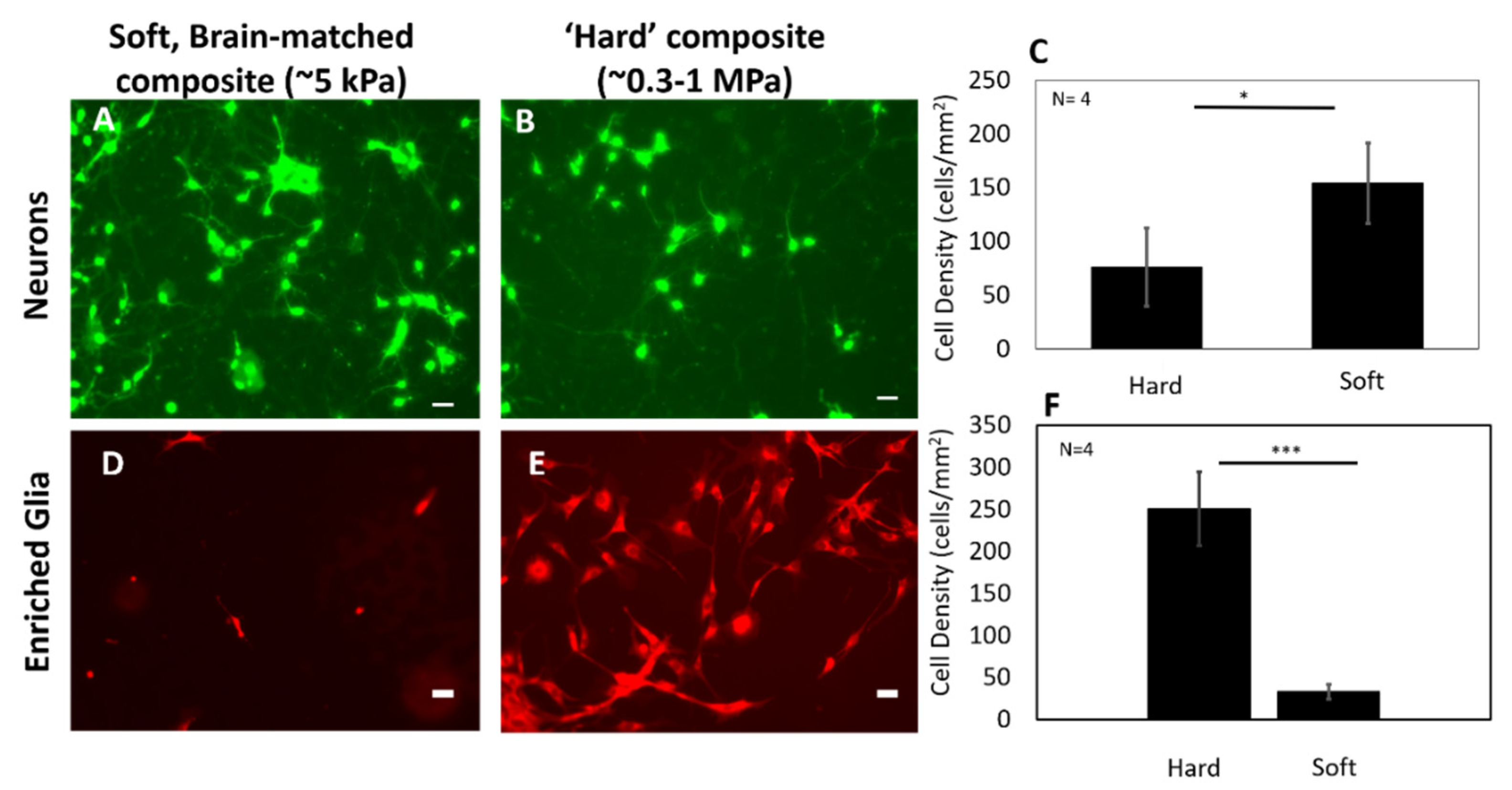
3.2. Primary Cortical Neurons Show Higher Viability on Soft Substrate in In Vitro Tests
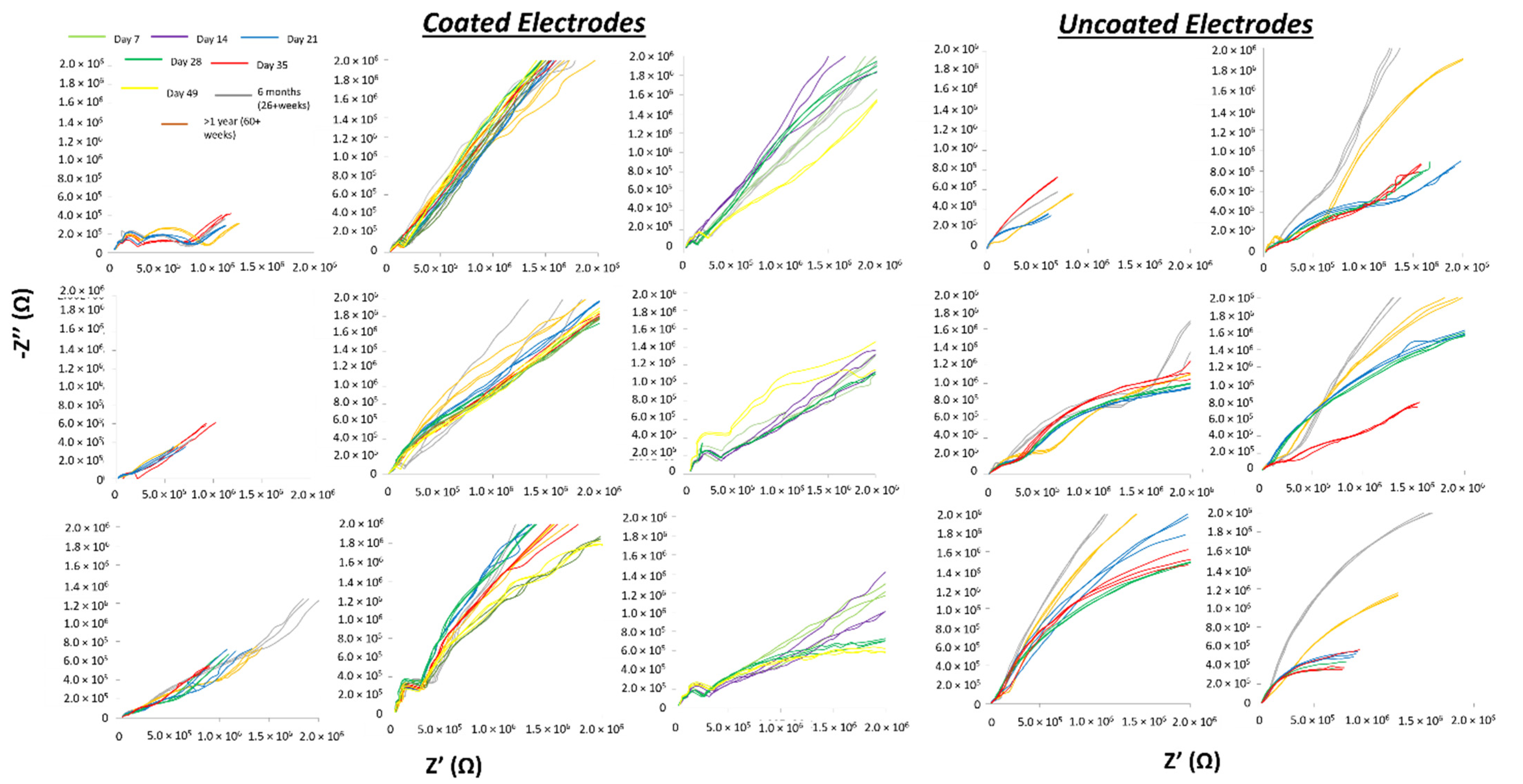
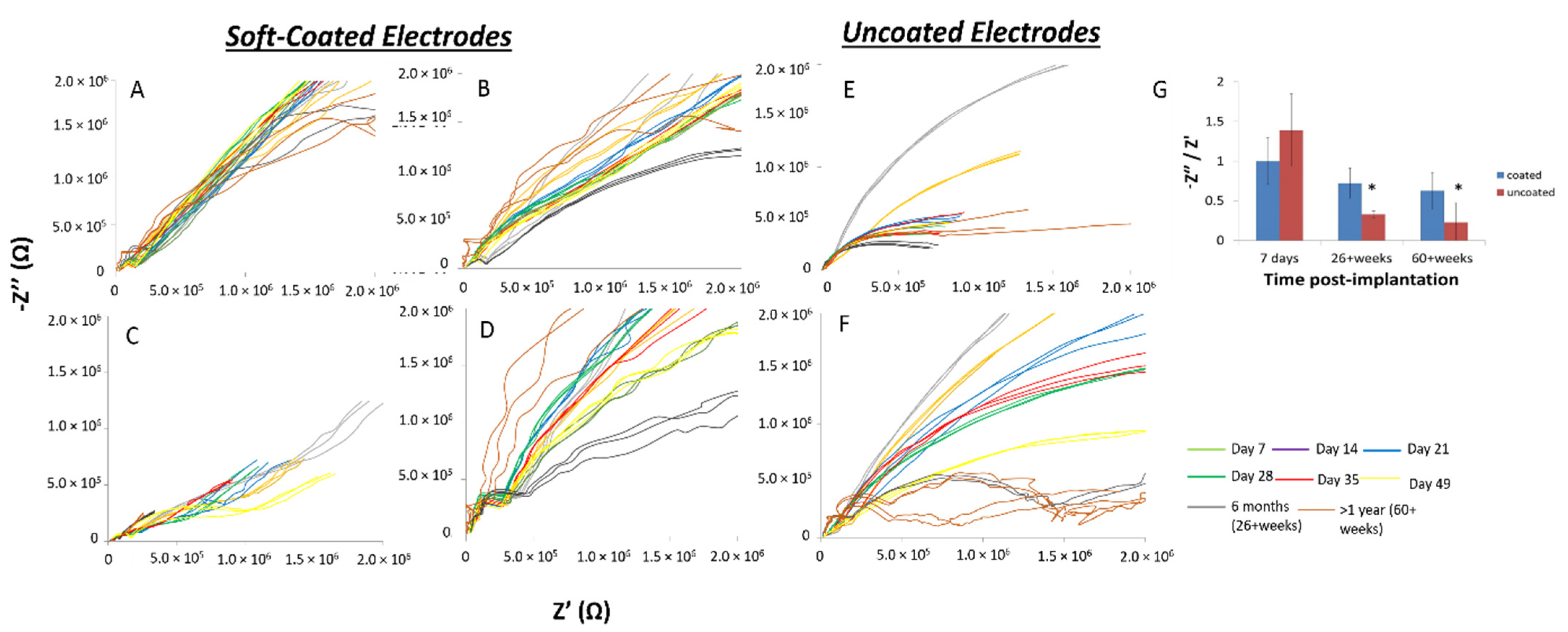
3.3. In Vivo Electrical Impedance Is More Stable For Soft-Coated Electrodes
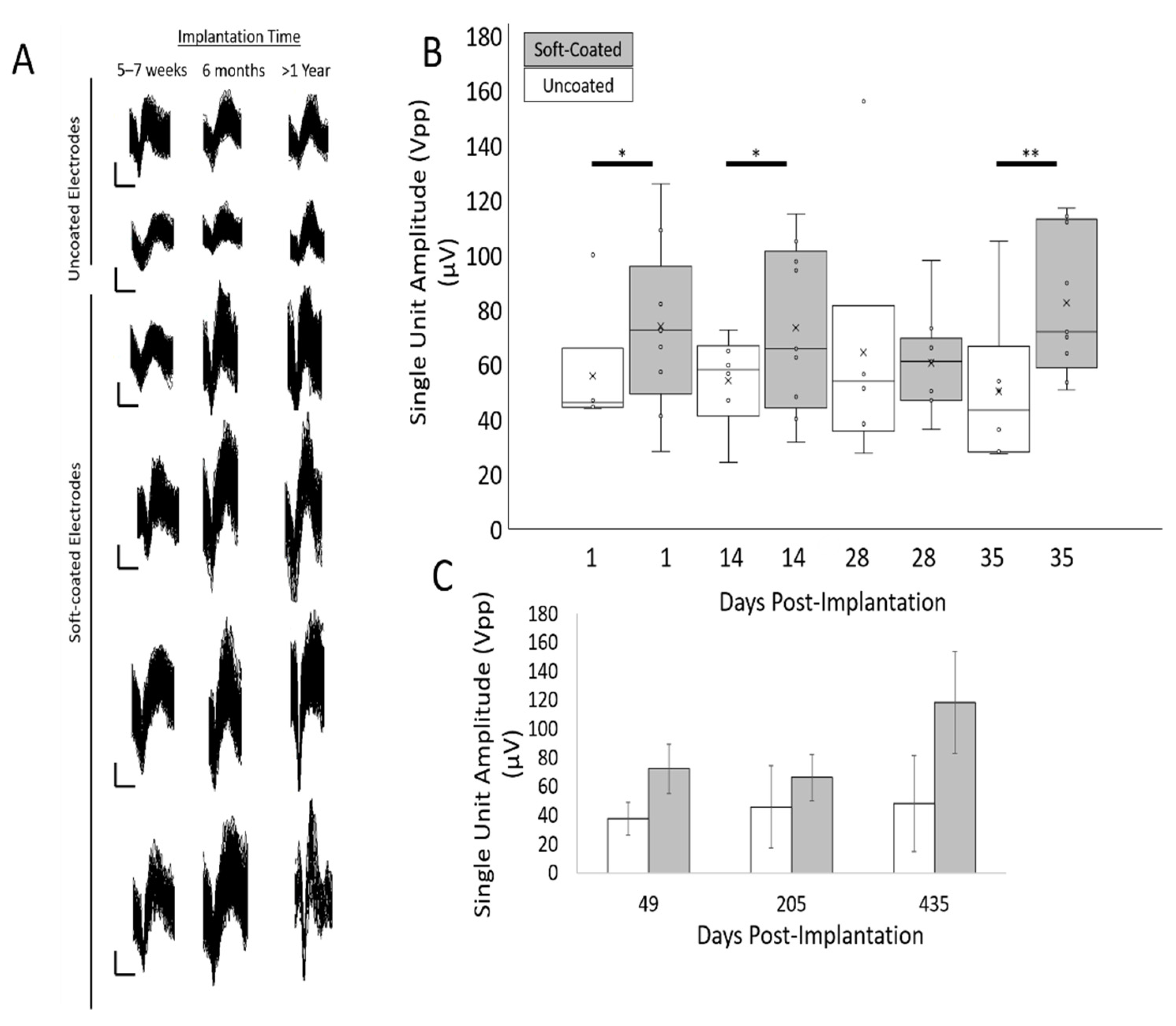
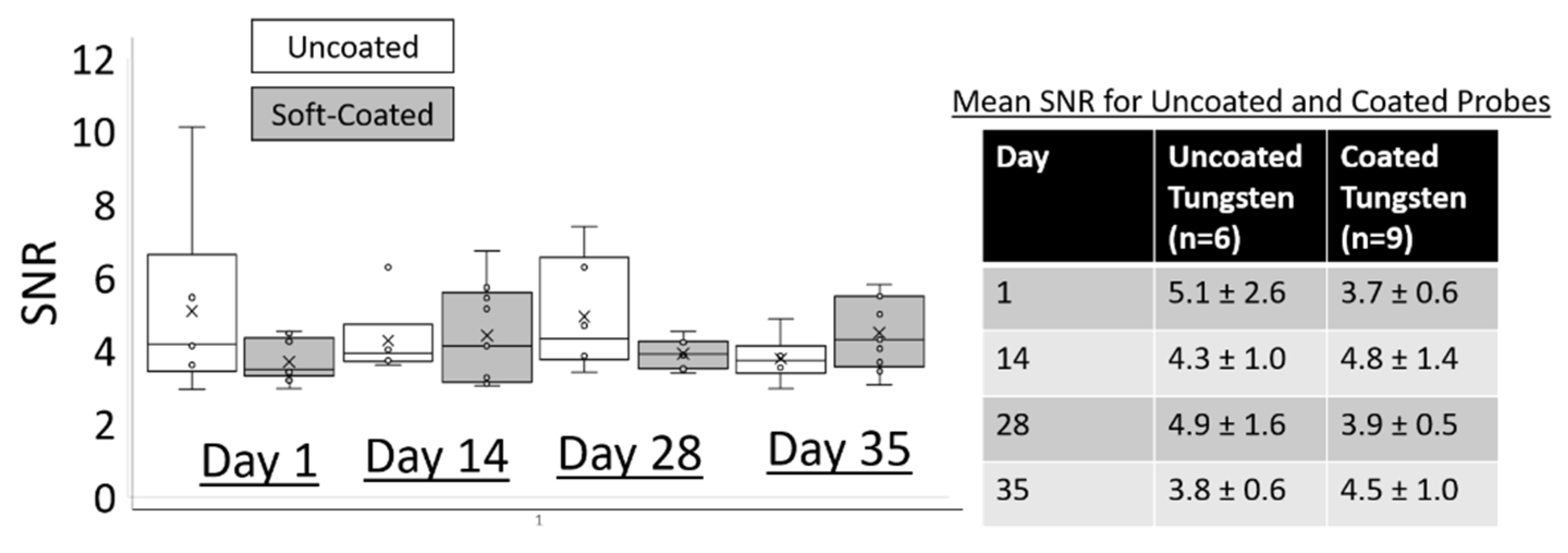
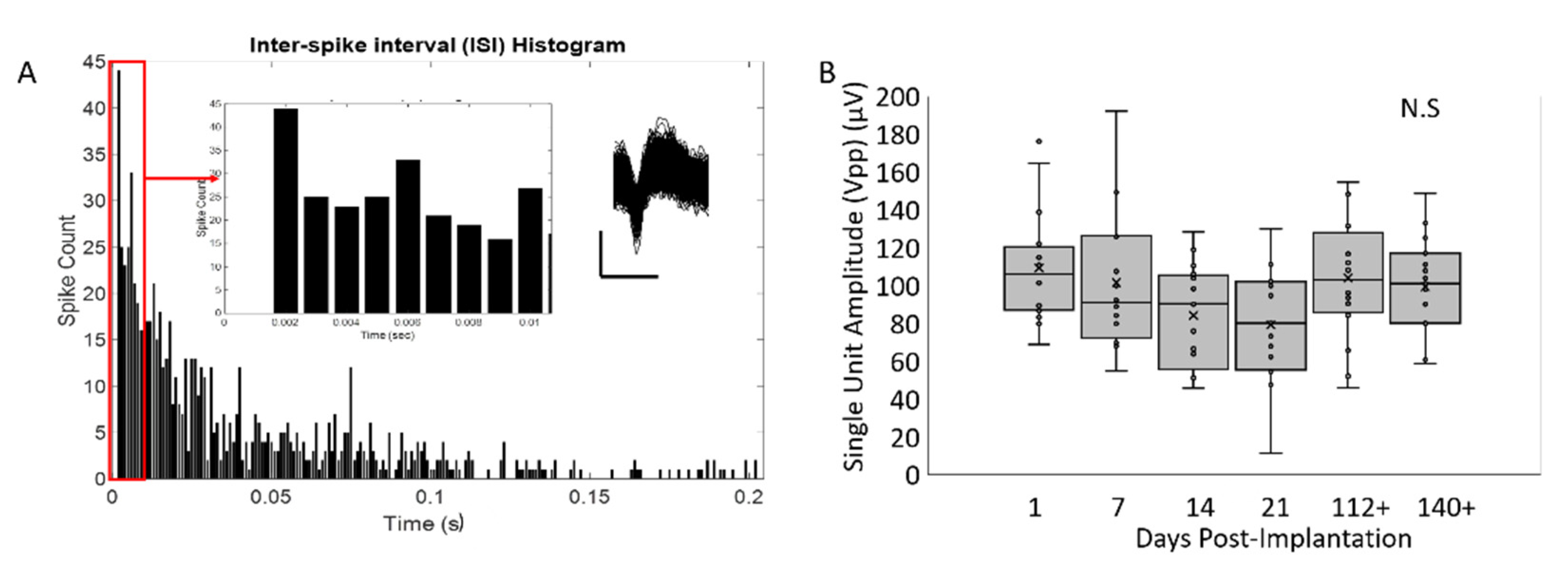
3.4. Peak-to-Peak Amplitudes for Isolated Single Units Are Larger for Soft-Coated Electrodes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunasekera, B.; Saxena, T.; Bellamkonda, R.; Karumbaiah, L. Intracortical recording interfaces: Current challenges to chronic recording function. ACS Chem. Neurosci. 2015, 6, 68–83. [Google Scholar] [CrossRef]
- Barrese, J.C.; Rao, N.; Paroo, K.; Triebwasser, C.; Vargas-Irwin, C.; Franquemont, L.; Donoghue, J.P. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Barrese, J.C.; Aceros, J.; Donoghue, J.P. Scanning electron microscopy of chronically implanted intracortical microelectrode arrays in non-human primates. J. Neural Eng. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Wu, C. Chronically implanted intracranial electrodes: Tissue reaction and electrical changes. Micromachines 2018, 9, 430. [Google Scholar] [CrossRef]
- Streit, W.J.; Xue, Q.S.; Prasad, A.; Sankar, V.; Knott, E.; Dyer, A.; Reynolds, J.R.; Nishida, T.; Shaw, G.P.; Sanchez, J.C. Electrode failure: Tissue, electrical, and material responses. IEEE Pulse 2012, 3, 30–33. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Prasad, A.; Xue, Q.S.; Sankar, V.; Nishida, T.; Shaw, G.; Streit, W.J.; Sanchez, J.C. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 2012, 9, 056015. [Google Scholar] [CrossRef]
- Prasad, A.; Xue, Q.S.; Dieme, R.; Sankar, V.; Mayrand, R.C.; Nishida, T.; Streit, W.J.; Sanchez, J.C. Abiotic-biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 2014, 7, 1–15. [Google Scholar] [CrossRef]
- Sankar, V.; Dieme, E.P.R.; Sanchez, J.C.; Prasad, A.; Nishida, T. Electrode impedance analysis of chronic tungsten microwire neural implants: Understanding abiotic vs. biotic contributions. Front. Neuroeng. 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Nolta, N.F.; Christensen, M.B.; Crane, P.D.; Skousen, J.L.; Tresco, P.A. BBB leakage, astrogliosis, and tissue loss correlate with silicon microelectrode array recording performance. Biomaterials 2015, 53, 753–762. [Google Scholar] [CrossRef]
- Gaire, J.; Lee, H.C.; Hilborn, N.; Ward, R.; Regan, M.; Otto, K.J. The role of inflammation on the functionality of intracortical microelectrodes. J. Neural Eng. 2018, 15. [Google Scholar] [CrossRef]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009, 6. [Google Scholar] [CrossRef]
- Stice, P.; Muthuswamy, J. Assessment of gliosis around moveable implants in the brain. J. Neural Eng. 2009, 6, 046004. [Google Scholar] [CrossRef]
- Bedell, H.W.; Capadona, J. Anti-inflammatory approaches to mitigate the neuroinflammatory response to brain-dwelling intracortical microelectrodes. J. Immunol. Sci. 2018, 2, 15–21. [Google Scholar] [CrossRef]
- Bennett, C.; Mohammed, F.; Álvarez-Ciara, A.; Nguyen, M.A.; Dietrich, W.D.; Rajguru, S.M.; Streit, W.J.; Prasad, A. Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response. Biomaterials 2019, 188, 144–159. [Google Scholar] [CrossRef]
- Holmkvist, A.D.; Agorelius, J.; Forni, M.; Nilsson, U.J.; Linsmeier, C.E.; Schouenborg, J. Local delivery of minocycline-loaded PLGA nanoparticles from gelatin-coated neural implants attenuates acute brain tissue responses in mice. J. Nanobiotechnol. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Potter, K.A.; Buck, A.C.; Self, W.K.; Callanan, M.E.; Sunil, S.; Capadona, J.R. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials 2013, 34, 7001–7015. [Google Scholar] [CrossRef]
- Potter, K.A.; Jorfi, M.; Householder, K.T.; Foster, E.J.; Weder, C.; Capadona, J.R. Curcumin-releasing mechanically adaptive intracortical implants improve the proximal neuronal density and blood-brain barrier stability. Acta Biomater. 2014, 10, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Potter-Baker, K.A.; Stewart, W.G.; Tomaszewski, W.H.; Wong, C.T.; Meador, W.D.; Ziats, N.P.; Capadona, J.R. Implications of chronic daily anti-oxidant administration on the inflammatory response to intracortical microelectrodes. J. Neural Eng. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Na, K.; Zhang, H.; Kozai, T.D.Y.; Kotov, N.A.; Yoon, E.; Chestek, C.A. Insertion of linear 8.4 μm diameter 16 channel carbon fiber electrode arrays for single unit recordings. J. Neural Eng. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Gugel, Z.; Li, X.; Gilgunn, P.J.; Khilwani, R.; Ozdoganlar, O.B.; Fedder, G.K.; Weber, D.J.; Cui, X.T. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials 2014, 35, 9255–9268. [Google Scholar] [CrossRef]
- He, F.; Lycke, R.; Ganji, M.; Xie, C.; Luan, L. Ultraflexible neural electrodes for long-lasting intracortical recording. iScience 2020, 23, 101387. [Google Scholar] [CrossRef]
- Shen, W.; Karumbaiah, L.; Liu, X.; Saxena, T.; Chen, S.; Patkar, R.; Bellamkonda, R.V.; Allen, M.G. Extracellular matrix-based intracortical microelectrodes: Toward a microfabricated neural interface based on natural materials. Microsyst. Nanoeng. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Gilletti, A.; Muthuswamy, J. Brain micromotion around implants in the rodent somatosensory cortex. J. Neural Eng. 2006, 3, 189–195. [Google Scholar] [CrossRef]
- Sridharan, A.; Rajan, S.D.; Muthuswamy, J. Long-term changes in the material properties of brain tissue at the implant-tissue interface. J. Neural Eng. 2013, 10, 066001. [Google Scholar] [CrossRef]
- Karumbaiah, L.; Norman, S.E.; Rajan, N.B.; Anand, S.; Saxena, T.; Betancur, M.; Patkar, R.; Bellamkonda, R. V The upregulation of specific interleukin (IL) receptor antagonists and paradoxical enhancement of neuronal apoptosis due to electrode induced strain and brain micromotion. Biomaterials 2012, 33, 5983–5996. [Google Scholar] [CrossRef]
- Hess-Dunning, A.; Tyler, D.J. A mechanically-adaptive polymer nanocomposite-based intracortical probe and package for chronic neural recording. Micromachines 2018, 9, 583. [Google Scholar] [CrossRef]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef]
- Shur, M.; Fallegger, F.; Pirondini, E.; Roux, A.; Bichat, A.; Barraud, Q.; Courtine, G.; Lacour, S.P. Soft printable electrode coating for neural interfaces. ACS Appl. Bio Mater. 2020, 3, 4388–4397. [Google Scholar] [CrossRef]
- Minev, I.R.; Wenger, N.; Courtine, G.; Lacour, S.P. Research update: Platinum-elastomer mesocomposite as neural electrode coating. APL Mater. 2015, 3. [Google Scholar] [CrossRef]
- Harris, J.P.; Hess, A.E.; Rowan, S.J.; Weder, C.; Zorman, C.A.; Tyler, D.J.; Capadona, J.R. In vivo deployment of mechanically adaptive nanocomposites for intracortical microelectrodes. J. Neural Eng. 2011, 8. [Google Scholar] [CrossRef]
- Spencer, K.C.; Sy, J.C.; Ramadi, K.B.; Graybiel, A.M.; Langer, R.; Cima, M.J. Erratum: Characterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implants. Sci. Rep. 2017, 7, 12812. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Du, M.; Wang, L.; Li, S.; Wang, G.; Yang, X.; Zhang, L.; Fang, Y.; Zheng, W.; Yang, G.; et al. Bacterial cellulose as a supersoft neural interfacing substrate. ACS Appl. Mater. Interfaces 2018, 10, 33049–33059. [Google Scholar] [CrossRef] [PubMed]
- Kolarcik, C.L.; Luebben, S.D.; Sapp, S.A.; Hanner, J.; Snyder, N.; Kozai, T.D.Y.; Chang, E.; Nabity, J.A.; Nabity, S.T.; Lagenaur, C.F.; et al. Elastomeric and soft conducting microwires for implantable neural interfaces. Soft Matter 2015, 11, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Lim, H.K.; Kim, N.H.; Park, J.K.; Kang, E.S.; Kim, Y.T.; Heo, C.; Lee, O.S.; Kim, S.G.; Yun, W.S.; et al. Supramolecular peptide hydrogel-based soft neural interface augments brain signals through a three-dimensional electrical network. ACS Nano 2020, 14, 664–675. [Google Scholar] [CrossRef]
- Stiller, A.; Black, B.; Kung, C.; Ashok, A.; Cogan, S.; Varner, V.; Pancrazio, J. A meta-analysis of intracortical device stiffness and its correlation with histological outcomes. Micromachines 2018, 9, 443. [Google Scholar] [CrossRef]
- Stiller, A.M.; Usoro, J.; Frewin, C.L.; Danda, V.R.; Ecker, M.; Joshi-Imre, A.; Musselman, K.C.; Voit, W.; Modi, R.; Pancrazio, J.J.; et al. Chronic intracortical recording and electrochemical stability of thiol-ene/acrylate shape memory polymer electrode arrays. Micromachines 2018, 9, 500. [Google Scholar] [CrossRef]
- Zhang, E.N.; Clément, J.P.; Alameri, A.; Ng, A.; Kennedy, T.E.; Juncker, D. Mechanically matched silicone brain implants reduce brain foreign body response. Adv. Mater. Technol. 2021, 6, 1–11. [Google Scholar] [CrossRef]
- Jung, W.; Heo, C.; Kim, J.U.; Jeong, C.; Ryu, H.; Park, B.; Suh, M.; Kim, T. Il Design and material for a patternable polysiloxane acrylate-based penetrating intracortical neural probe. J. Micromech. Microeng. 2021, 31. [Google Scholar] [CrossRef]
- Lee, C.D.; Hara, S.A.; Yu, L.; Kuo, J.T.W.; Kim, B.J.; Hoang, T.; Pikov, V.; Meng, E. Matrigel coatings for Parylene sheath neural probes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 357–368. [Google Scholar] [CrossRef]
- Shoffstall, A.J.; Ecker, M.; Danda, V.; Joshi-Imre, A.; Stiller, A.; Yu, M.; Paiz, J.E.; Mancuso, E.; Bedell, H.W.; Voit, W.E.; et al. Characterization of the neuroinflammatory response to thiol-ene shape memory polymer coated intracortical microelectrodes. Micromachines 2018, 9, 486. [Google Scholar] [CrossRef]
- Sridharan, A.; Nguyen, J.K.; Capadona, J.R.; Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. J. Neural Eng. 2015, 12. [Google Scholar] [CrossRef]
- Lee, H.; Bellamkonda, R.V.; Sun, W.; Levenston, M.E. Biomechanical analysis of silicon microelectrode-induced strain in the brain. J. Neural Eng. 2005, 2, 81–89. [Google Scholar] [CrossRef]
- Subbaroyan, J.; Martin, D.C.; Kipke, D.R. A finite-element model of the mechanical effects of implantable microelectrodes in the cerebral cortex. J. Neural Eng. 2005, 2, 103–113. [Google Scholar] [CrossRef]
- Polanco, M.; Yoon, H.; Bawab, S. Micromotion-induced dynamic effects from a neuron probe and brain tissue interface. In Nanosensors, Biosensors, and Info-Tech Sensors and Systems 2013; Varadan, V.K., Ed.; SPIE: Bellingham, WA, USA, 2013; Volume 8691. [Google Scholar] [CrossRef]
- Polanco, M.; Bawab, S.; Yoon, H. Computational assessment of neural probe and brain tissue interface under transient motion. Biosensors 2016, 6, 27. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Catt, K.; Li, X.; Gugel, Z.V.; Olafsson, V.T.; Vazquez, A.L.; Cui, X.T. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials 2015, 37, 25–39. [Google Scholar] [CrossRef]
- Black, B.J.; Kanneganti, A.; Joshi-Imre, A.; Rihani, R.; Chakraborty, B.; Abbott, J.; Pancrazio, J.J.; Cogan, S.F. Chronic recording and electrochemical performance of Utah microelectrode arrays implanted in rat motor cortex. J. Neurophysiol. 2018, 120, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Kodibagkar, V.D.; Muthuswamy, J. Penetrating microindentation of hyper-soft, conductive silicone neural interfaces in vivo reveals significantly lower mechanical stresses. MRS Adv. 2019, 4, 2551–2558. [Google Scholar] [CrossRef]
- Garrett, A.; Soler, G.J.; Diluna, M.L.; Grant, R.A.; Zaveri, H.P.; Hoshino, K. A passive, biocompatible microfluidic flow sensor to assess flows in a cerebral spinal fluid shunt. Sens. Actuators A Phys. 2020, 312, 112110. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, D.S.; Kim, I.G.; Sohn, D.W.; Park, J.Y.; Choi, B.K.; Kim, S.W. Evaluation of the biocompatibility of a coating material for an implantable bladder volume sensor. Kaohsiung J. Med. Sci. 2012, 28, 123–129. [Google Scholar] [CrossRef]
- McIlvain, G.; Ganji, E.; Cooper, C.; Killian, M.L.; Ogunnaike, B.A.; Johnson, C.L. Reliable preparation of agarose phantoms for use in quantitative magnetic resonance elastography. J. Mech. Behav. Biomed. Mater. 2019, 97, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Gandhi, D.; Krishnan, S.; Saggere, L.; Rousche, P.J. A benchtop system to assess cortical neural interface micromechanics. IEEE Trans. Biomed. Eng. 2007, 54, 1089–1096. [Google Scholar] [CrossRef]
- Pomfret, R.; Miranpuri, G.; Sillay, K. The substitute brain and the potential of the gel model. Ann. Neurosci. 2013, 20, 118–122. [Google Scholar] [CrossRef][Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Prasad, A.; Sanchez, J.C. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J. Neural Eng. 2012, 9. [Google Scholar] [CrossRef]
- Lempka, S.F.; Miocinovic, S.; Johnson, M.D.; Vitek, J.L.; McIntyre, C.C. In vivo impedance spectroscopy of deep brain stimulation electrodes. J. Neural Eng. 2009, 6. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Uram, J.D.; Yang, J.; Martin, D.C.; Kipke, D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 2006, 3, 59–70. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface modification of Sylgard-184 poly(dimethyl siloxane) networks by ultraviolet and ultraviolet/ozone treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.K.; Park, D.J.; Skousen, J.L.; Hess-Dunning, A.E.; Tyler, D.J.; Rowan, S.J.; Weder, C.; Capadona, J.R. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng. 2014, 11. [Google Scholar] [CrossRef]
- Arreaga-Salas, D.E.; Avendaño-Bolívar, A.; Simon, D.; Reit, R.; Garcia-Sandoval, A.; Rennaker, R.L.; Voit, W. Integration of high-charge-injection-capacity electrodes onto polymer softening neural interfaces. ACS Appl. Mater. Interfaces 2015, 7, 26614–26623. [Google Scholar] [CrossRef]
- Teixeira, A.I.; Ilkhanizadeh, S.; Wigenius, J.A.; Duckworth, J.K.; Inganäs, O.; Hermanson, O. The promotion of neuronal maturation on soft substrates. Biomaterials 2009, 30, 4567–4572. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef]
- Spedden, E.; White, J.D.; Naumova, E.N.; Kaplan, D.L.; Staii, C. Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy. Biophys. J. 2012, 103, 868–877. [Google Scholar] [CrossRef]
- Harris, J.P.; Capadona, J.R.; Miller, R.H.; Healy, B.C.; Shanmuganathan, K.; Rowan, S.J.; Weder, C.; Tyler, D.J. Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies. J. Neural Eng. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Khraiche, M.L.; Jackson, N.; Muthuswamy, J. Early onset of electrical activity in developing neurons cultured on carbon nanotube immobilized microelectrodes. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: “Engineering the Future of Biomedicine” EMBC 2009, Minneapolis, MN, USA, 2–6 September 2009; pp. 777–780. [Google Scholar] [CrossRef]
- Kim, J.; Chaudhury, M.K.; Owen, M.J.; Orbeck, T. The mechanisms of hydrophobic recovery of polydimethylsiloxane elastomers exposed to partial electrical discharges. J. Colloid Interface Sci. 2001, 244, 200–207. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Beltsios, K.; Gogolides, E. Effect of surface nanostructuring of PDMS on wetting properties, hydrophobic recovery and protein adsorption. Microelectron. Eng. 2009, 86, 1321–1324. [Google Scholar] [CrossRef]
- Williams, J.C.; Hippensteel, J.A.; Dilgen, J.; Shain, W.; Kipke, D.R. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 2007, 4, 410–423. [Google Scholar] [CrossRef]
- Chestek, C.A.; Gilja, V.; Nuyujukian, P.; Foster, J.D.; Fan, J.M.; Kaufman, M.T.; Churchland, M.M.; Rivera-Alvidrez, Z.; Cunningham, J.P.; Ryu, S.I.; et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural Eng. 2011, 8. [Google Scholar] [CrossRef]
- Mccreery, D.; Cogan, S.; Kane, S.; Pikov, V. Correlations between histology and neuronal activity recorded by microelectrodes implanted chronically in the cerebral cortex. J. Neural Eng. 2016, 13, 036012. [Google Scholar] [CrossRef]
- Jackson, N.; Sridharan, A.; Anand, S.; Baker, M.; Okandan, M.; Muthuswamy, J. Long-term neural recordings using MEMS based movable microelectrodes in the brain. Front. Neuroeng. 2010, 3. [Google Scholar] [CrossRef]
- Karumbaiah, L.; Saxena, T.; Carlson, D.; Patil, K.; Patkar, R.; Gaupp, E.A.; Betancur, M.; Stanley, G.B.; Carin, L.; Bellamkonda, R.V. Relationship between intracortical electrode design and chronic recording function. Biomaterials 2013, 34, 8061–8074. [Google Scholar] [CrossRef] [PubMed]

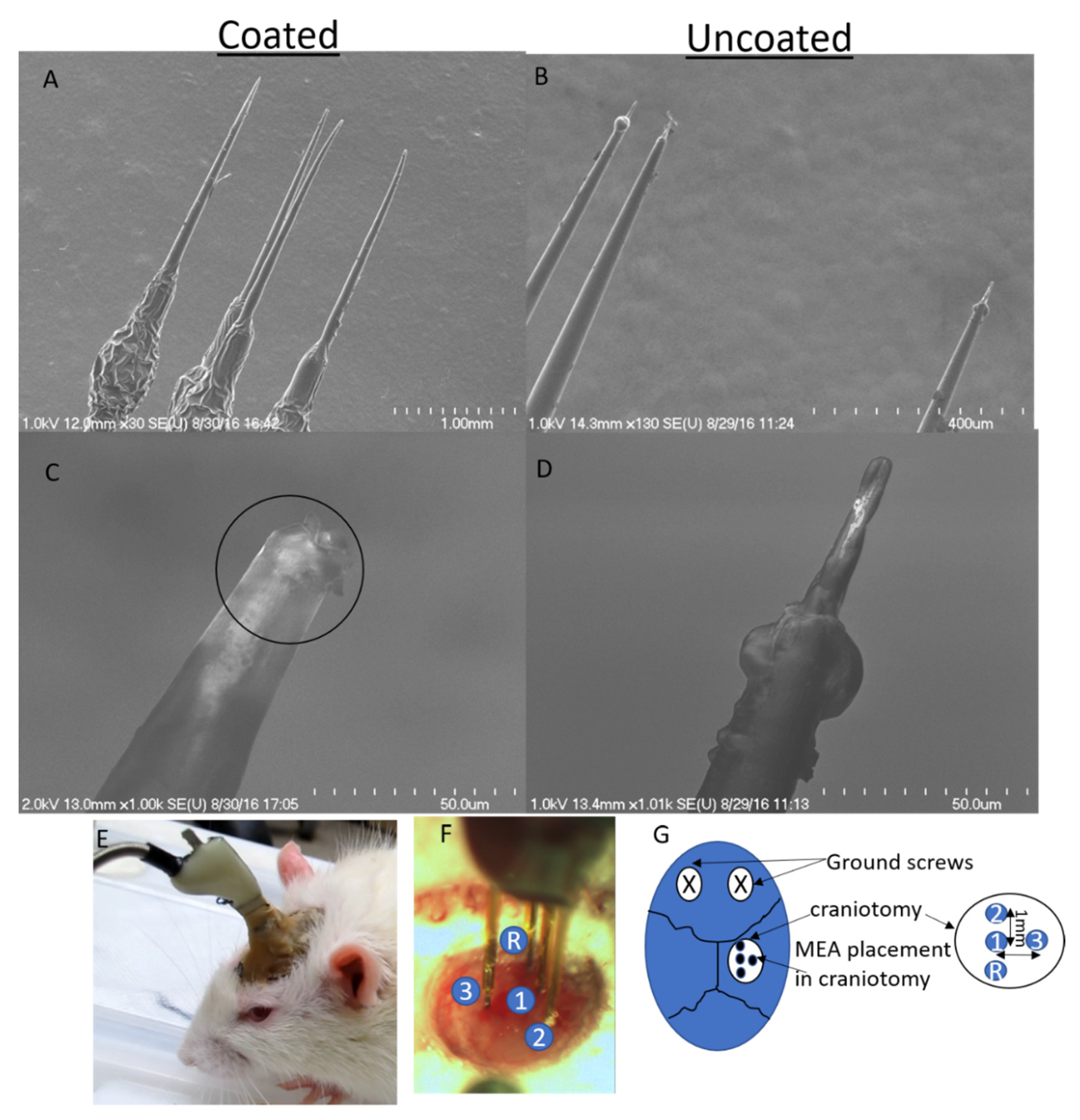





| Rat# | Days Implanted |
|---|---|
| #1 (3 uncoated tungsten electrodes) | 35 |
| #2 (3 coated tungsten electrodes) | 437 |
| #3 (1 coated, 2 uncoated tungsten electrodes) | 432 |
| #4 (2 coated, 1 uncoated tungsten electrodes) | 35 |
| #5 (3 coated tungsten electrodes) | 49 |
| #6 (16 coated, platinum–iridium electrodes) | 140 |
| Timepoint (Day) | Uncoated Tungsten (Mean No. of Units) | Coated Tungsten (Mean No. of Units) |
|---|---|---|
| 1 | 3.7 ± 0.4 | 2.7 ± 0.9 |
| 14 | 2.7 ± 0.5 | 3.3 ± 0.5 |
| 28 | 2.5 ± 1.0 | 3.8 ± 0.7 |
| 35 | 1.7 ± 1.0 | 4.4 ± 0.7 |
| 49 | 2.0 ± 1.4 | 4.3 ± 0.5 |
| 180+ | 1.5 ± 0.7 | 3.3 ± 0.5 |
| >1 year | 1 ± 0 | 4.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sridharan, A.; Muthuswamy, J. Soft, Conductive, Brain-Like, Coatings at Tips of Microelectrodes Improve Electrical Stability under Chronic, In Vivo Conditions. Micromachines 2021, 12, 761. https://doi.org/10.3390/mi12070761
Sridharan A, Muthuswamy J. Soft, Conductive, Brain-Like, Coatings at Tips of Microelectrodes Improve Electrical Stability under Chronic, In Vivo Conditions. Micromachines. 2021; 12(7):761. https://doi.org/10.3390/mi12070761
Chicago/Turabian StyleSridharan, Arati, and Jit Muthuswamy. 2021. "Soft, Conductive, Brain-Like, Coatings at Tips of Microelectrodes Improve Electrical Stability under Chronic, In Vivo Conditions" Micromachines 12, no. 7: 761. https://doi.org/10.3390/mi12070761
APA StyleSridharan, A., & Muthuswamy, J. (2021). Soft, Conductive, Brain-Like, Coatings at Tips of Microelectrodes Improve Electrical Stability under Chronic, In Vivo Conditions. Micromachines, 12(7), 761. https://doi.org/10.3390/mi12070761






